Bioactive Hydrogels Based on Tyramine and Maleimide Functionalized Dextran for Tissue Engineering Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of DexTA–Mal
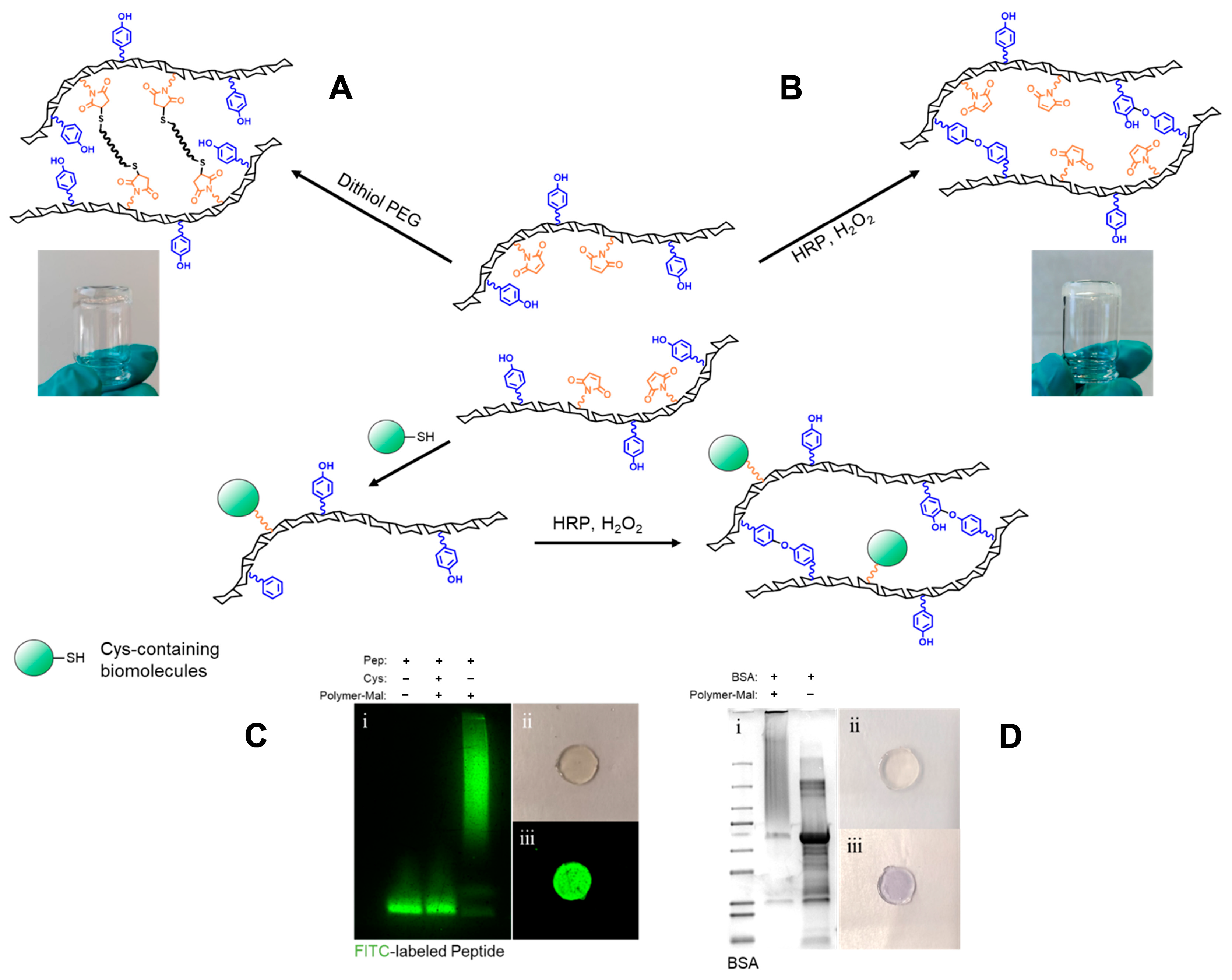
2.2. Hydrogel (Bio-)Functionalization
2.3. Hydrogel Formation and Gelation Time
2.4. Mechanical Properties of Selected Compositions
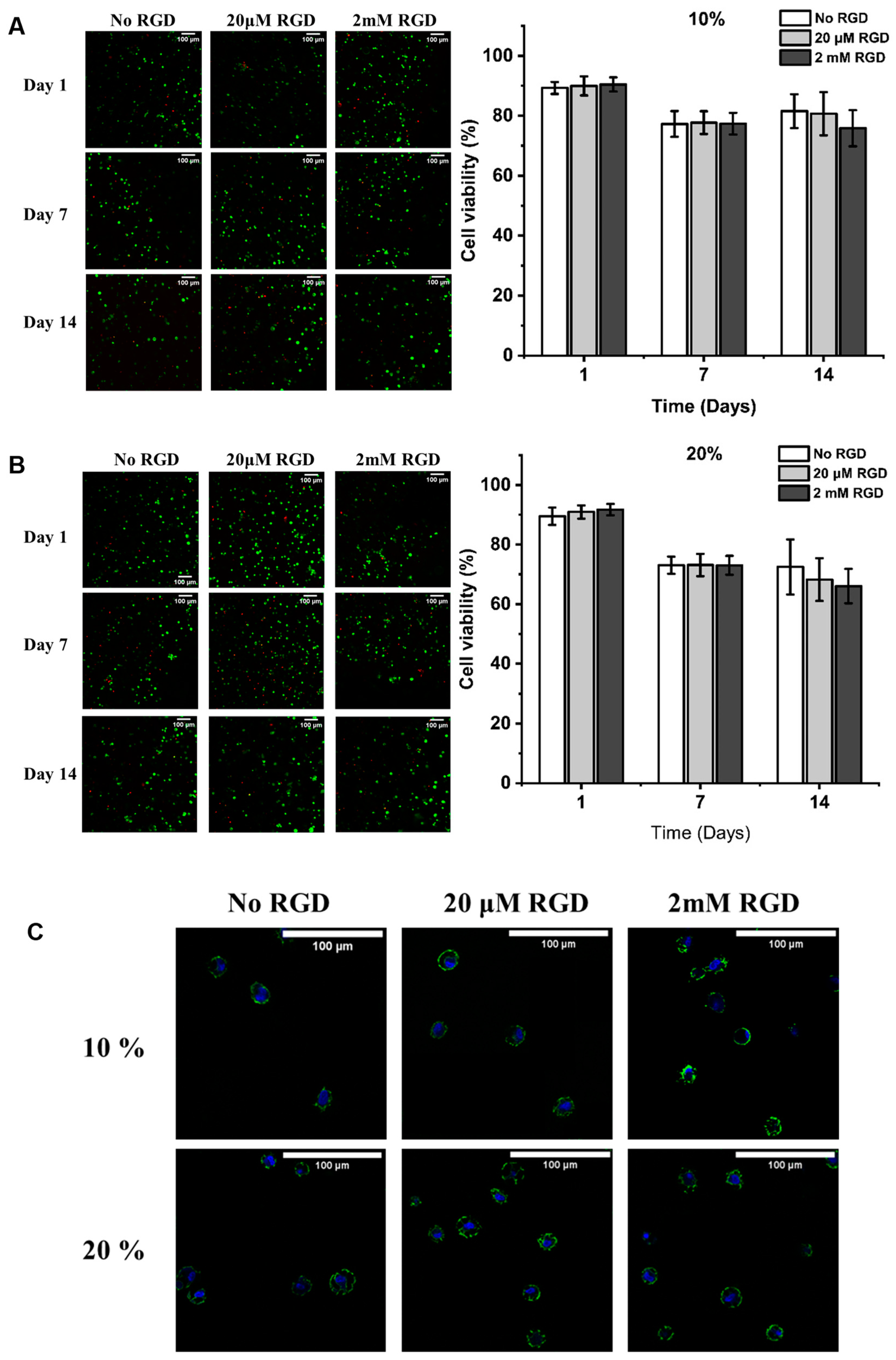
2.5. Viability and Morphology of hMSCs in the Different Hydrogels
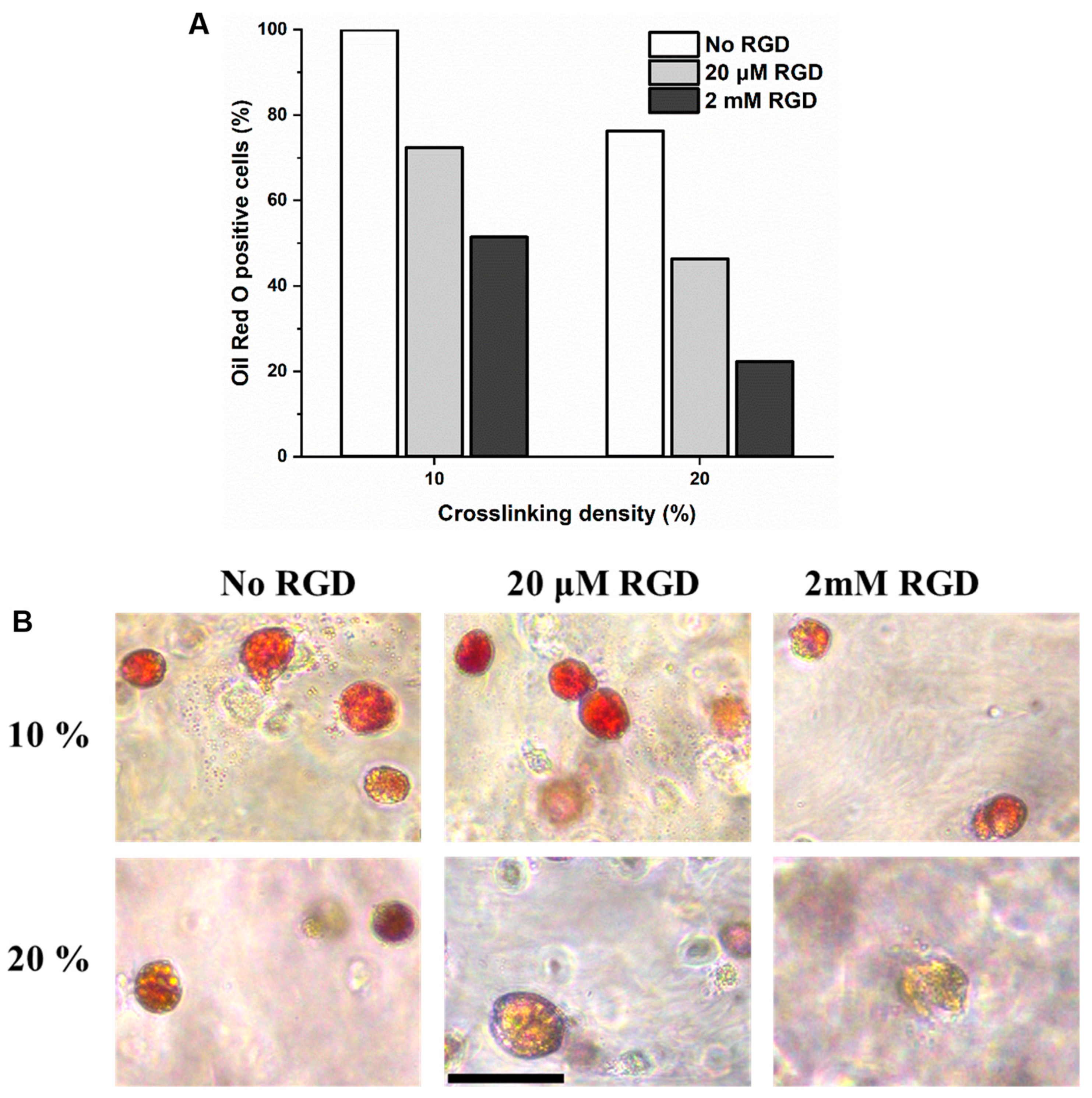
2.6. Evaluation of In Vitro Adipogenesis of hMSCs Encapsulated in DexTA–Mal Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Dextran-Tyramine-Maleimide
- Dextran-p-nitrophenyl carbonate (Dex–PNC)
- b.
- Dextran–tyramine–butylamine (DexTA–bNH2)
- c.
- Dextran–tyramine–(butylamine)maleimide (DexTA–Mal)
4.3. Hydrogel (Bio-)Functionalization
4.4. Hydrogel Formation and Gelation Time
4.5. Hydrogel Swelling
4.6. Mechanical Properties
4.7. Compression Test
4.8. Cell Culture and Expansion
- Cell encapsulation in DexTA–Mal Hydrogels
- b.
- Live/Dead Cell Viability Assay
- c.
- Influence of Hydrogel Formulations on Morphology in the DexTA–Mal Hydrogels
- d.
- Differentiation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AMAS | n-(α-maleimidoacetoxy) succinimide ester |
| BSA | Bovine serum albumin |
| Cys | Cysteine |
| EC | Enzymatic crosslinking |
| Dex | Dextran |
| DexTA | Dextran–tyramine |
| DexTA–Mal | Dextran–tyramine–maleimide |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DS | Degree of substitution |
| ECM | Extracellular matrix |
| hMSCs | Human mesenchymal stem cells |
| HRP | Horseradish peroxidase |
| H2O2 | Hydrogen peroxide |
| 1H-NMR | Proton nuclear magnetic resonance |
| LVE | Linear viscoelastic range |
| MW | Molecular weight |
| MWCO | Molecular weight cut-off |
| PBS | Phosphate-buffered saline |
| PEG | Polyethylene glycol |
| PNC | p-nitrophenyl chloroformate |
| PTFE | Polytetrafluoroethylene |
| RGD | Arginylglycylaspartic acid |
| TA | Tyramine |
References
- Khalikova, E.; Susi, P.; Korpela, T. Microbial dextran-hydrolyzing enzymes: Fundamentals and applications. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef]
- Wang, R. Macromolecular Engineering of In Situ Forming Hydrogels. Ph.D. Thesis, Department of Develomental BioEngineering, University of Twente, Enschede, The Netherlands, 2016. [Google Scholar]
- Estevanato, L.L.C.; Lacava, L.M.; Carvalho, L.C.; Azevedo, R.B.; Silva, O.; Pelegrini, F.; Báo, S.N.; Morais, P.C.; Lacava, Z.G.M. Long-term biodistribution and biocompatibility investigation of dextran-coated magnetite nanoparticle using mice as the animal model. J. Biomed. Nanotechnol. 2012, 8, 301–308. [Google Scholar] [CrossRef]
- Hifumi, H.; Yamaoka, S.; Tanimoto, A.; Akatsu, T.; Shindo, Y.; Honda, A.; Citterio, D.; Oka, K.; Kuribayashib, S.; Suzuki, K. Dextran Coated Gadolinium Phosphate Nanoparticles for Magnetic Resonance Tumor Imaging. J. Mater. Chem. 2009, 19, 6393–6399. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Gerecht, S.; Fuller, J.; Shieh, H.F.; Vunjak-Novakovic, G.; Langer, R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 2007, 28, 2706–2717. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, D. Dextran and haemostasis. A review. Acta Chir. Scand. 1982, 148, 633–640. [Google Scholar] [PubMed]
- Van Tomme, S.R.; Hennink, W.E. Biodegradable dextran hydrogels for protein delivery applications. Expert Rev. Med. Devices 2007, 4, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J. Dextran conjugates in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.; Tan, F.E.; Langer, R. Synthesis and characterization of dextran-peptide-methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjugate Chem. 2004, 15, 931–941. [Google Scholar] [CrossRef]
- Baudys, M.; Letourneur, D.; Liu, F.; Mix, D.; Jozefonvicz, J.; Kim, S.W. Extending insulin action in vivo by conjugation to carboxymethyl dextran. Bioconjugate Chem. 1998, 9, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gedda, L.; Olsson, P.; Ponten, J.; Carlsson, J. Development and in vitro studies of epidermal growth factor-dextran conjugates for boron neutron capture therapy. Bioconjugate Chem. 1996, 7, 584–591. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Sofia, S.J.; Singh, A.; Kaplan, D.L. Peroxidase-catalyzed crosslinking of functionalized polyaspartic acid polymers. J. Macromol. Sci. Part A 2002, 39, 1151–1181. [Google Scholar] [CrossRef]
- Darr, A.; Calabro, A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J. Mater. Sci. Mater. Med. 2009, 20, 33–44. [Google Scholar] [CrossRef]
- Jin, R.; Hiemstra, C.; Zhong, Z.; Feijen, J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials 2007, 28, 2791–2800. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gao, S.J.; Uyama, H. Injectable biodegradable hydrogels composed of hyaluronic acid–tyramine conjugates for drug delivery and tissue engineering. Chem. Commun. 2005, 34, 4312–4314. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.; Chung, J.E.; Kurisawa, M. An injectable hyaluronic acid–tyramine hydrogel system for protein delivery. J. Control. Release 2009, 134, 186–193. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. J. Control. Release 2011, 152, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Schwartz, M.P.; Durney, A.R.; Anseth, K.S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008, 7, 816–823. [Google Scholar] [CrossRef]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chemistry 2019, 25, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pong, K.; Tomatsu, I.; Korobko, A.V.; Kros, A. Cyclodextrin–dextran based in situ hydrogel formation: A carrier for hydrophobic drugs. Soft Matter 2010, 6, 85–87. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brondsted, H. Dextran hydrogels for colon-specific drug delivery. J. Control. Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- de Jong, S.J.; van Eerdenbrugh, B.; van Nostrum, C.F.; den Bosch, J.J.K.-v.; Hennink, W.E. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: Degradation and protein release behavior. J. Control. Release 2001, 71, 261–275. [Google Scholar] [CrossRef]
- Morimoto, J.; Sarkar, M.; Kenrick, S.; Kodadek, T. Dextran as a Generally Applicable Multivalent Scaffold for Improving Immunoglobulin-Binding Affinities of Peptide and Peptidomimetic Ligands. Bioconjugate Chem. 2014, 25, 1479–1491. [Google Scholar] [CrossRef]
- Richter, M.; Chakrabarti, A.; Ruttekolk, I.R.; Wiesner, B.; Beyermann, M.; Brock, R.; Rademann, J. Multivalent Design of Apoptosis-Inducing Bid-BH3 Peptide–Oligosaccharides Boosts the Intracellular Activity at Identical Overall Peptide Concentrations. Chem.-A Eur. J. 2012, 18, 16708–16715. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, X.; Zhang, R. Characterization and Analysis of Collective Cellular Behaviors in 3D Dextran Hydrogels with Homogenous and Clustered RGD Compositions. Materials 2019, 12, 3391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, X.; Yin, X. Quantitatively Designed Cross-Linker-Clustered Maleimide-Dextran Hydrogels for Rationally Regulating the Behaviors of Cells in a 3D Matrix. ACS Appl. Bio Mater. 2020, 3, 5759–5774. [Google Scholar] [CrossRef]
- Jansen, L.E.; Negron-Pineiro, L.J.; Galarza, S.; Peyton, S.R. Control of thiol-maleimide reaction kinetics in PEG hydrogel networks. Acta Biomater. 2018, 70, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Jiang, Y.; Zhang, J.; Klok, H.; Zhong, Z. pH-Sensitive Coiled-Coil Peptide-Cross-Linked Hyaluronic Acid Nanogels: Synthesis and Targeted Intracellular Protein Delivery to CD44 Positive Cancer Cells. Biomacromolecules 2018, 19, 555–562. [Google Scholar] [CrossRef]
- Zhong, L. Enzyme Responsive Delivery of Engineered Antibody Fragments. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2024. [Google Scholar]
- Lutolf, M.P.; Gilbert, P.N.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Baynton, K.J.; Bewtra, J.K.; Biswas, N.; Taylor, K.E. Inactivation of horseradish peroxidase by phenol and hydrogen peroxide: A kinetic investigation. Biochim. Biophys. Acta 1994, 1206, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Acosta, M.; del Rio, J.A.; Varon, R.; Garcia-Canovas, F. A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide. Biochim. Biophys. Acta 1990, 1041, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Sugimura, T.; Horie, K.; Mita, I. Effect of Intramolecular Crosslinks on Microgel and Macrogel Formation by Photocrosslinking Reaction in Solution. Polym. J. 1990, 22, 63–69. [Google Scholar] [CrossRef]
- Fu, Y.; Zoetebier, B.; Both, S.; Dijkstra, P.J.; Karperien, M. Engineering of Optimized Hydrogel Formulations for Cartilage Repair. Polymers 2021, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Kamperman, T. Microgel Technology to Advance Modular Tissue Engineering. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2018. [Google Scholar]
- Henke, S. Microgel Technology for Improved Beta Cell Transplantation. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2016. [Google Scholar]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef]
- Kamperman, T.; Henke, S.; Crispim, J.F.; Willemen, N.G.A.; Dijkstra, P.J.; Lee, W.; Offerhaus, H.L.; Neubauer, M.; Smink, A.M.; de Vos, P.; et al. Tethering Cells via Enzymatic Oxidative Crosslinking Enables Mechanotransduction in Non-Cell-Adhesive Materials. Adv. Mater. 2021, 33, 2102660. [Google Scholar] [CrossRef]
- Both, S.K.; van der Muijsenberg, A.J.C.; van Blitterswijk, C.A.; de Boer, J.; de Bruijn, J.D. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 2007, 13, 3–9. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Banigo, A.T.; Zoetebier, B.; Karperien, M. Bioactive Hydrogels Based on Tyramine and Maleimide Functionalized Dextran for Tissue Engineering Applications. Gels 2024, 10, 566. https://doi.org/10.3390/gels10090566
Zhong L, Banigo AT, Zoetebier B, Karperien M. Bioactive Hydrogels Based on Tyramine and Maleimide Functionalized Dextran for Tissue Engineering Applications. Gels. 2024; 10(9):566. https://doi.org/10.3390/gels10090566
Chicago/Turabian StyleZhong, Lin, Alma Tamunonengiofori Banigo, Bram Zoetebier, and Marcel Karperien. 2024. "Bioactive Hydrogels Based on Tyramine and Maleimide Functionalized Dextran for Tissue Engineering Applications" Gels 10, no. 9: 566. https://doi.org/10.3390/gels10090566








