Brown Adipose Stem Cell-Loaded Resilin Elastic Hydrogel Rebuilds Cardiac Function after Myocardial Infarction via Collagen I/III Reorganisation
Abstract
1. Introduction
2. Results and Discussion
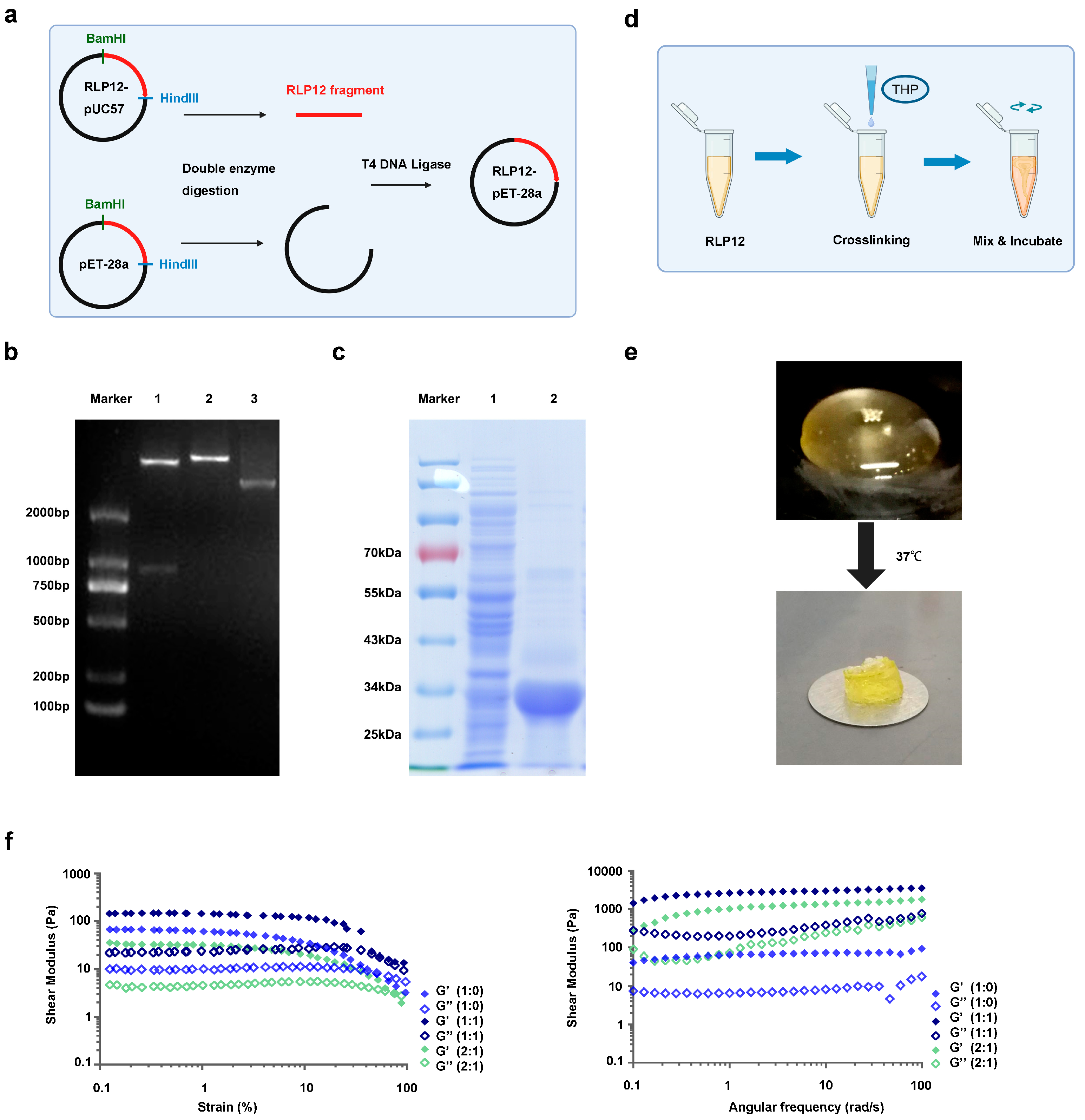
2.1. Synthesis of RLP12 Expression Vector and Protein Expression
2.2. Preparation and Mechanical Properties Identification of RLP12 Hydrogel
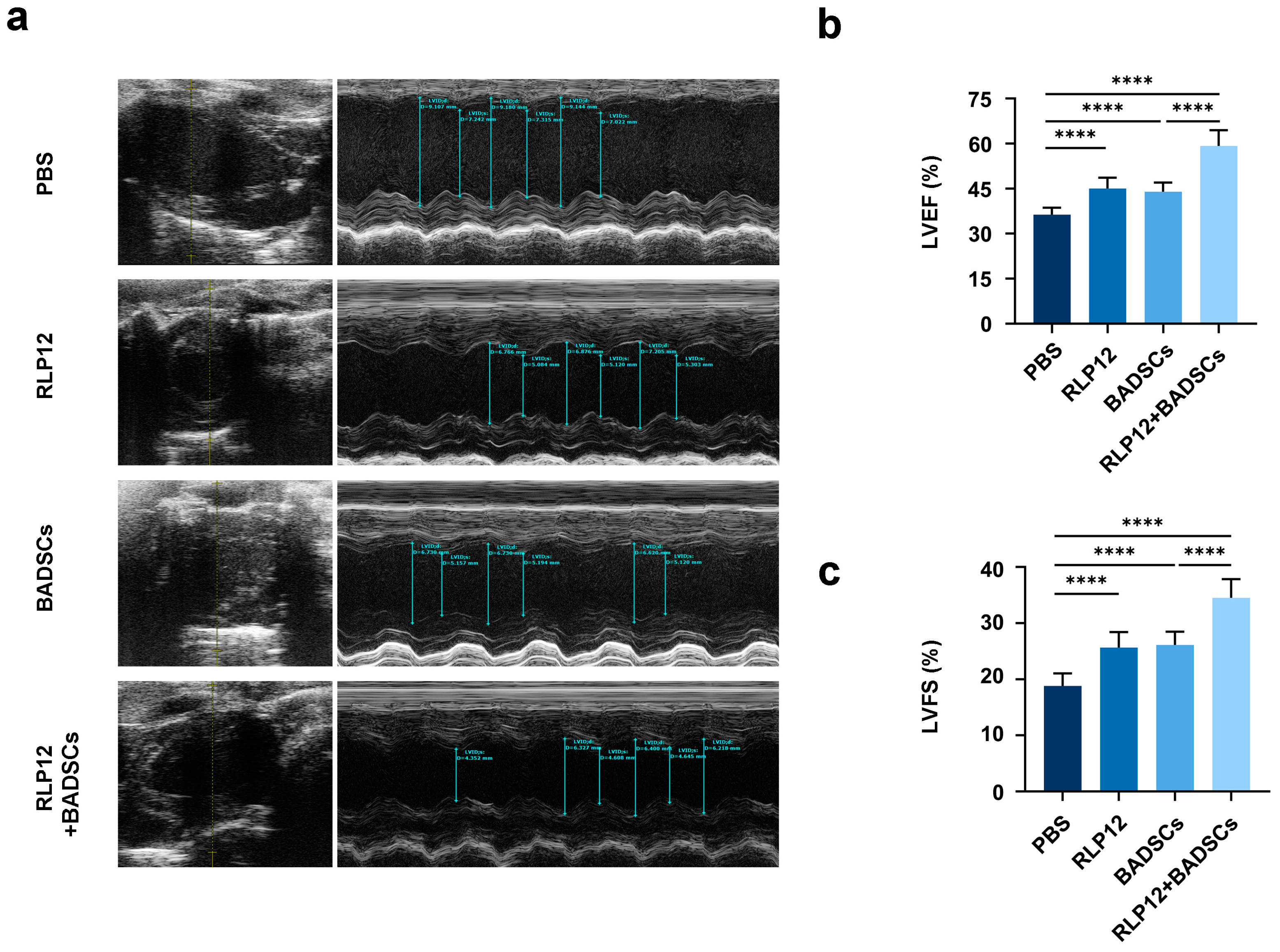
2.3. Cardiac Function
2.4. MI Area Size and Fibrosis Level
2.5. Distribution Levels of Type I Collagen in the Infarct Area and Border Area
2.6. Distribution Levels of Type III Collagen in the Infarct Area and Border Area
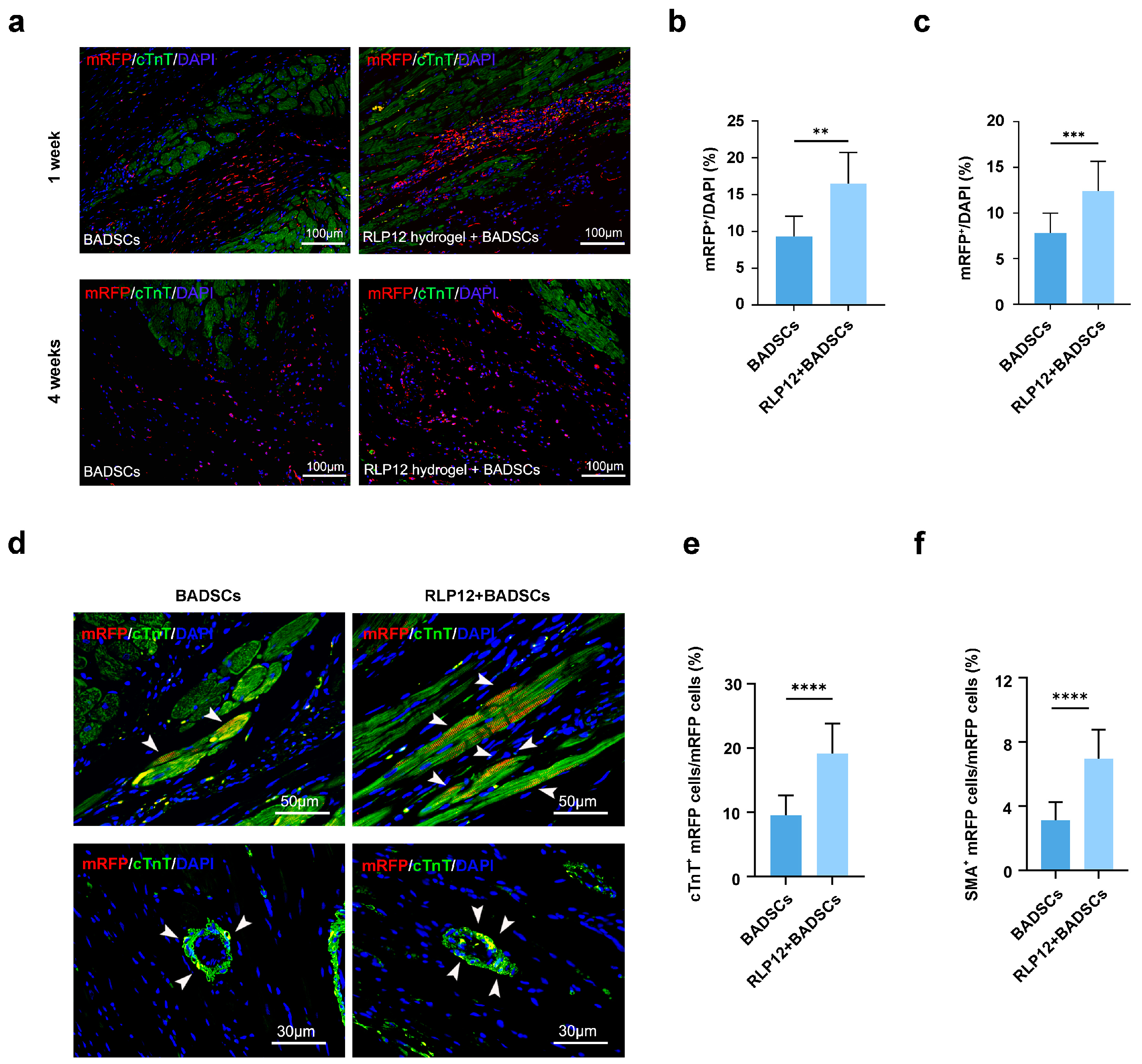
2.7. BADSC Survival Rate and In Vivo Differentiation after Transplantation
2.8. Microvessel Density (MVD)
3. Conclusion
4. Materials and Methods
4.1. Expression and Purification of RLP12 Protein
4.2. Preparation and Identification of the RLP12 Hydrogel
4.3. Examination of Mechanical and Rheometric Properties
4.4. Establishment of a Rat MI Model and Injection
4.5. Cardiac Function Testing
- EDD: End-Diastolic Diameter;
- ESD: End-Systolic Dimension;
- LVIDd: Left Ventricular Internal Diameter in Diastole;
- LVIDs: Left Ventricular Internal Diameter in Systole.
4.6. Histological Testing
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, A.S.; Dooley, E.E.; Master, H.; Spartano, N.L.; Brittain, E.L.; Gabriel, K.P. Physical Activity Over the Lifecourse and Cardiovascular Disease. Circ. Res. 2023, 132, 1725–1740. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Med. Assoc. 2021, 326, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Dong, J.; Li, W.; Li, Z.; Gao, R.; Liu, X.; Wang, J.; Su, Q.; Wen, B.; Ouyang, W.; et al. Extracellular Matrix/Glycopeptide Hybrid Hydrogel as an Immunomodulatory Niche for Endogenous Cardiac Repair after Myocardial Infarction. Adv. Sci. 2023, 10, e2301244. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, T.; Ichibori, Y.; Ohtani, T.; Mizote, I.; Kioka, H.; Tsukamoto, Y.; Nakamura, D.; Yokoi, K.; Ide, S.; Nakamoto, K.; et al. Pathophysiological evaluations of initial plaque development after heart transplantation via serial multimodality imaging and cytokine assessments. J. Heart Lung Transplant. 2022, 41, 877–885. [Google Scholar] [CrossRef]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left ventricular remodelling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef]
- Carvalho, A.B.; Kasai-Brunswick, T.H.; de Carvalho, A.C.C. Advanced cell and gene therapies in cardiology. eBioMedicine 2024, 103, 105125. [Google Scholar] [CrossRef]
- Bolli, R. Cell therapy for acute myocardial infarction: Requiescat in Pace. Eur. Heart J. 2020, 41, 3711–3714. [Google Scholar] [CrossRef]
- Thomas, D.; Cunningham, N.J.; Shenoy, S.; Wu, J.C. Human-induced pluripotent stem cells in cardiovascular research: Current approaches in cardiac differentiation, maturation strategies, and scalable production. Cardiovasc Res. 2022, 118, 20–36. [Google Scholar] [CrossRef]
- Qiao, L.; Kong, Y.; Shi, Y.; Sun, A.; Ji, R.; Huang, C.; Li, Y.; Yang, X. Synergistic effects of adipose-derived stem cells combined with decellularized myocardial matrix on the treatment of myocardial infarction in rats. Life Sci. 2019, 239, 116891. [Google Scholar] [CrossRef]
- Lu, K.Y.; Primus Dass, K.T.; Lin, S.Z.; Harn, H.J.; Liu, S.P. The application of stem cell therapy and brown adipose tissue transplantation in metabolic disorders. Cytotherapy 2020, 22, 521–528. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels 2023, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Qian, M.; Zhang, Y.; Liu, Q.; Midgley, A.C.; Liu, Y.; Che, Y.; Hou, J.; Zhao, Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9, e2105408. [Google Scholar] [CrossRef] [PubMed]
- Jalilinejad, N.; Rabiee, M.; Baheiraei, N.; Ghahremanzadeh, R.; Salarian, R.; Rabiee, N.; Akhavan, O.; Zarrintaj, P.; Hejna, A.; Saeb, M.R.; et al. Electrically conductive carbon-based (bio)-nanomaterials for cardiac tissue engineering. Bioeng. Transl. Med. 2022, 8, e10347. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Tian, L.; Li, Y.; Zhang, J.; Wei, Y.; Jin, Z.; Liu, Z.; Liu, H. Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair. Stem Cells Int. 2019, 2019, 6708435. [Google Scholar] [CrossRef] [PubMed]
- Shahshahani, S.; Shahgholi, M.; Karimipour, A. The thermal performance and mechanical stability of methacrylic acid porous hydrogels in an aqueous medium at different initial temperatures and hydrogel volume fraction using the molecular dynamics simulation. J. Mol. Liq. 2023, 382, 122001. [Google Scholar] [CrossRef]
- He, S.; Zhang, Z.; Luo, R.; Jiang, Q.; Yang, L.; Wang, Y. Advances in Injectable Hydrogel Strategies for Heart Failure Treatment. Adv. Healthc. Mater. 2023, 12, e2300029. [Google Scholar] [CrossRef]
- Alimirzaei, F.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Soleimani, M.; Pouri; Najafi-Gharavi, Z. pH-Sensitive Chitosan Hydrogel with Instant Gelation for Myocardial Re generation. J. Tissue Sci. Eng. 2017, 8, 3. [Google Scholar] [CrossRef]
- Mann, D.L.; Lee, R.J.; Coats, A.J.-S.; Neagoe, G.; Dragomir, D.; Pusineri, E.; Piredda, M.; Bettari, L.; Kirwan, B.A.; Dowling, R.; et al. One-year follow-up results from AUGMENT-HF: A multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur. J. Heart Fail. 2015, 18, 314–325. [Google Scholar] [CrossRef]
- Rao, S.V.; Zeymer, U.; Douglas, P.S.; Al-Khalidi, H.; White, J.A.; Liu, J.; Levy, H.; Guetta, V.; Gibson, C.M.; Tanguay, J.F.; et al. Bioabsorbable Intracoronary Matrix for Prevention of Ventricular Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 715–723. [Google Scholar] [CrossRef]
- Seif-Naraghi, S.B.; Singelyn, J.M.; Salvatore, M.A.; Osborn, K.G.; Wang, J.J.; Sampat, U.; Kwan, O.L.; Strachan, G.M.; Wong, J.; Schup-Magoffin, P.J.; et al. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Sci. Transl. Med. 2013, 5, 173ra25. [Google Scholar] [CrossRef]
- Maruyama, K.; Imanaka-Yoshida, K. The Pathogenesis of Cardiac Fibrosis: A Review of Recent Progress. Int. J. Mol. Sci. 2022, 23, 2617. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Niu, Y.; Liang, K.; Du, Y. Mechanical communication in fibrosis progression. Trends Cell Biol. 2022, 32, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Vettori, L.; Tran, H.A.; Mahmodi, H.; Filipe, E.C.; Wyllie, K.; Liu Chung Ming, C.; Cox, T.R.; Tipper, J.; Kabakova, I.V.; Rnjak-Kovacina, J.; et al. Silk fibroin increases the elasticity of alginate-gelatin hydrogels and regulates cardiac cell contractile function in cardiac bioinks. Biofabrication 2024, 16, 035025. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rodell, C.B.; Lee, M.E.; Dusaj, N.N.; Gorman, J.H., 3rd; Burdick, J.A.; Gorman, R.C.; Wenk, J.F. Computational sensitivity investigation of hydrogel injection characteristics for myocardial support. J. Biomech. 2017, 64, 231–235. [Google Scholar] [CrossRef]
- Montgomery, M.; Ahadian, S.; Huyer, L.D.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef]
- Tallawi, M.; Rai, R.; Boccaccini, A.R.; Aifantis, K.E. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng. Part B Rev. 2015, 21, 157–165. [Google Scholar] [CrossRef]
- Su, R.S.-C.; Kim, Y.; Liu, J.C. Resilin: Protein-based elastomeric biomaterials. Acta Biomater. 2014, 10, 1601–1611. [Google Scholar] [CrossRef]
- van Eldijk, M.B.; McGann, C.L.; Kiick, K.L.; van Hest, J.C. Elastomeric polypeptides. Top. Curr. Chem. 2012, 310, 71–116. [Google Scholar]
- Li, L.; Kiick, K.L. Transient dynamic mechanical properties of resilin-based elastomeric hydrogels. Front. Chem. 2014, 2, 21. [Google Scholar] [CrossRef]
- Gosline, J.M. The elastic properties of rubber-like proteins and highly extensible tissues. Symp. Soc. Exp. Biol. 1980, 34, 332–357. [Google Scholar]
- Gosline, J.; Bailey, A.J.; Macmillan, J.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ardell, D.H.; Andersen, S.O. Tentative identification of a resilin gene in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2001, 31, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Charati, M.B.; KiickK, L. Elastomeric polypeptide-based biomaterials. Polym. Chem. 2010, 1, 1160–1170. [Google Scholar] [CrossRef]
- Qin, G.; Rivkin, A.; Lapidot, S.; Hu, X.; Preis, I.; Arinus, S.B.; Dgany, O.; Shoseyov, O.; Kaplan, D.L. Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials 2011, 32, 9231–9243. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mahara, A.; Tong, Z.; Levenson, E.A.; McGann, C.L.; Jia, X.; Yamaoka, T.; Kiick, K.L. Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications. Adv. Healthc. Mater. 2015, 5, 266–275. [Google Scholar] [CrossRef]
- Li, L.; Stiadle, J.M.; Levendoski, E.E.; Lau, H.K.; Thibeault, S.L.; Kiick, K.L. Biocompatibility of injectable resilin-based hydrogels. J. Biomed. Mater. Res. Part A 2018, 106, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.L.; Levenson, E.A.; Kiick, K.L. Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering. Macromol. Chem. Phys. 2012, 214, 203–213. [Google Scholar] [CrossRef]
- Li, L.; Teller, S.; Clifton, R.J.; Jia, X.; Kiick, K.L. Tunable Mechanical Stability and Deformation Response of a Resilin-Based Elastomer. Biomacromolecules 2011, 12, 2302–2310. [Google Scholar] [CrossRef]
- Renner, J.N.; Cherry, K.M.; Su, R.S.; Liu, J.C. Characterization of resilin-based materials for tissue engineering applications. Biomacromolecules 2012, 13, 3678–3685. [Google Scholar] [CrossRef]
- Lv, S.; Dudek, D.M.; Cao, Y.; Balamurali, M.M.; Gosline, J.; Li, H. Designed biomaterials to mimic the mechanical properties of muscles. Nature 2010, 465, 69–73. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Boroujeni, N.N.; Jahangiri, S.; Rabiee, N.; Rabiee, M.; Makvandi, P.; Akhavan, O.; Varma, R.S. Prevascularized Micro-/Nano-Sized Spheroid/Bead Aggregates for Vascular Tissue Engineering. Nano-Micro Lett. 2021, 13, 182. [Google Scholar] [CrossRef]
- Charati, M.B.; Ifkovits, J.L.; Burdick, J.A.; Linhardt, J.G.; Kiick, K.L. Hydrophilic elastomeric biomaterials based on resilin-like polypeptides. Soft Matter. 2009, 5, 3412–3416. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Nettles, D.L.; Setton, L.A.; Chilkoti, A. Rapid Cross-Linking of Elastin-like Polypeptides with (Hydroxymethyl)phosphines in Aqueous Solution. Biomacromolecules 2007, 8, 1463–1470. [Google Scholar] [CrossRef]
- Su, R.S.-C.; Gill, E.E.; Kim, Y.; Liu, J.C. Characterization of resilin-like proteins with tunable mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 91, 68–75. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Chen, M. Research Status of the Measurement for Young’s Modulus of Myocardial Cells Using Atomic Force Microscopy. Chin. J. Biomed. Eng. 2014, 33, 93–97. [Google Scholar]
- Soni, S.S.; D’Elia, A.M.; Rodell, C.B. Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches. Drug Deliv. Transl. Res. 2023, 13, 1983–2014. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019, 65, 70–99. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef]
- Hernandez, M.J.; Christman, K.L. Designing Acellular Injectable Biomaterial Therapeutics for Treating Myocardial Infarction and Peripheral Artery Disease. JACC Basic Transl. Sci. 2017, 2, 212–226. [Google Scholar] [CrossRef]
- Matsumura, Y.; Zhu, Y.; Jiang, H.; D’Amore, A.; Luketich, S.K.; Charwat, V.; Yoshizumi, T.; Sato, H.; Yang, B.; Uchibori, T.; et al. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289. [Google Scholar] [CrossRef]
- Nerger, B.A.; Sinha, S.; Lee, N.N.; Cheriyan, M.; Bertsch, P.; Johnson, C.P.; Mahadevan, L.; Bonventre, J.V.; Mooney, D.J. 3D Hydrogel Encapsulation Regulates Nephrogenesis in Kidney Organoids. Adv. Mater. 2024, 36, 2308325. [Google Scholar] [CrossRef]
- King, R.E.; Lau, H.K.; Zhang, H.; Sidhu, I.; Christensen, M.B.; Fowler, E.W.; Li, L.; Jia, X.; Kiick, K.L.; Thibeault, S.L. Biocompatibility and Viscoelastic Properties of Injectable Resilin-Like Polypeptide and Hyaluronan Hybrid Hydrogels in Rabbit Vocal Folds. Regen. Eng. Transl. Med. 2019, 5, 373–386. [Google Scholar] [CrossRef]
- Carver, W.; Nagpal, M.; Nachtigal, M.; Borg, T.K.; Terracio, L. Collagen Expression in MechanicallyStimulated Cardiac Fibroblasts. Circ. Res. 1991, 69, 116–122. [Google Scholar] [CrossRef]
- Roman, B.; Kumar, S.A.; Allen, S.C.; Delgado, M.; Moncayo, S.; Reyes, A.M.; Suggs, L.J.; Chintalapalle, R.; Li, C.; Joddar, B. A Model for Studying the Biomechanical Effects of Varying Ratios of Collagen Types I and III on Cardiomyocytes. Cardiovasc. Eng. Technol. 2021, 12, 311–324. [Google Scholar] [CrossRef]
- Ivan, M.; Ruthard, J. Characterisation of left ventricular collagen in the rat. Cardiovasc. Res. 1983, 17, 15–21. [Google Scholar]
- Wen, Y.; Li, X.; Li, Z.; Wang, M.L.; Chen, P.P.; Liu, Y.; Zhang, X.Z.; Jiang, X.J. Intra-myocardial Delivery of a Novel Thermosensitive Hydrogel Inhibits Post-infarct Heart Failure After Degradation in Rat. J. Cardiovasc. Transl. Res. 2020, 13, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Sen, S. Alternation of Cardiac Collagen Phenotypes in Hypertensive Hypertrophy: Role of Blood Pressure. J. Mol. Cell. Cardiol. 1993, 25, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Xin, P.; Li, S.; Tao, J.; Li, Y.; Li, J.; Liu, M.; Li, J.; Zhu, W.; Redington, A.N. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ. Res. 2011, 108, 1220–1225. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Liu, H.; Gao, R.; Zhang, K.; Gong, Y.; Cui, Y.; Ke, S.; Wang, J.; Wang, H. Brown Adipose Stem Cell-Loaded Resilin Elastic Hydrogel Rebuilds Cardiac Function after Myocardial Infarction via Collagen I/III Reorganisation. Gels 2024, 10, 568. https://doi.org/10.3390/gels10090568
Zhao L, Liu H, Gao R, Zhang K, Gong Y, Cui Y, Ke S, Wang J, Wang H. Brown Adipose Stem Cell-Loaded Resilin Elastic Hydrogel Rebuilds Cardiac Function after Myocardial Infarction via Collagen I/III Reorganisation. Gels. 2024; 10(9):568. https://doi.org/10.3390/gels10090568
Chicago/Turabian StyleZhao, Le, Huaying Liu, Rui Gao, Kaihui Zhang, Yuxuan Gong, Yaya Cui, Shen Ke, Jing Wang, and Haibin Wang. 2024. "Brown Adipose Stem Cell-Loaded Resilin Elastic Hydrogel Rebuilds Cardiac Function after Myocardial Infarction via Collagen I/III Reorganisation" Gels 10, no. 9: 568. https://doi.org/10.3390/gels10090568
APA StyleZhao, L., Liu, H., Gao, R., Zhang, K., Gong, Y., Cui, Y., Ke, S., Wang, J., & Wang, H. (2024). Brown Adipose Stem Cell-Loaded Resilin Elastic Hydrogel Rebuilds Cardiac Function after Myocardial Infarction via Collagen I/III Reorganisation. Gels, 10(9), 568. https://doi.org/10.3390/gels10090568





