pH-Sensitive Fluorescent Probe in Nanogel Particles as Theragnostic Agent for Imaging and Elimination of Latent Bacterial Cells Residing Inside Macrophages
Abstract
1. Introduction
2. Results and Discussion
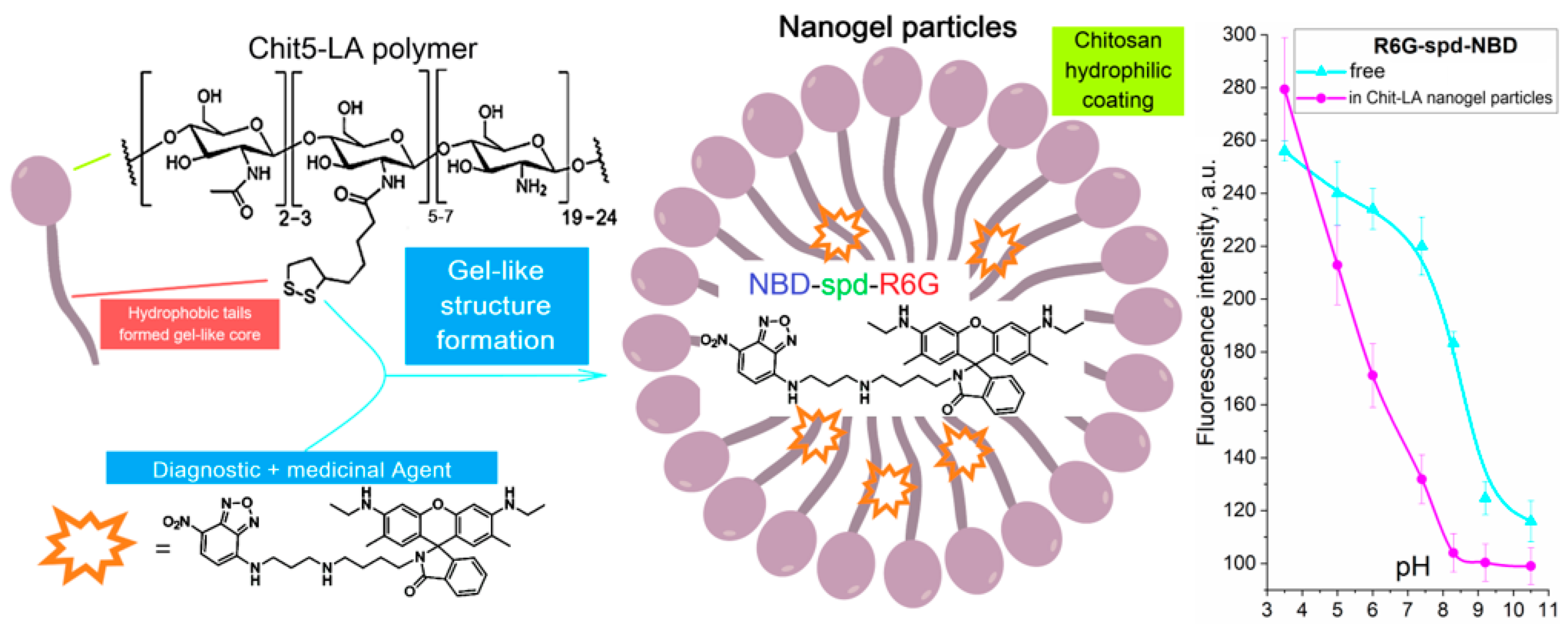
2.1. Work Design
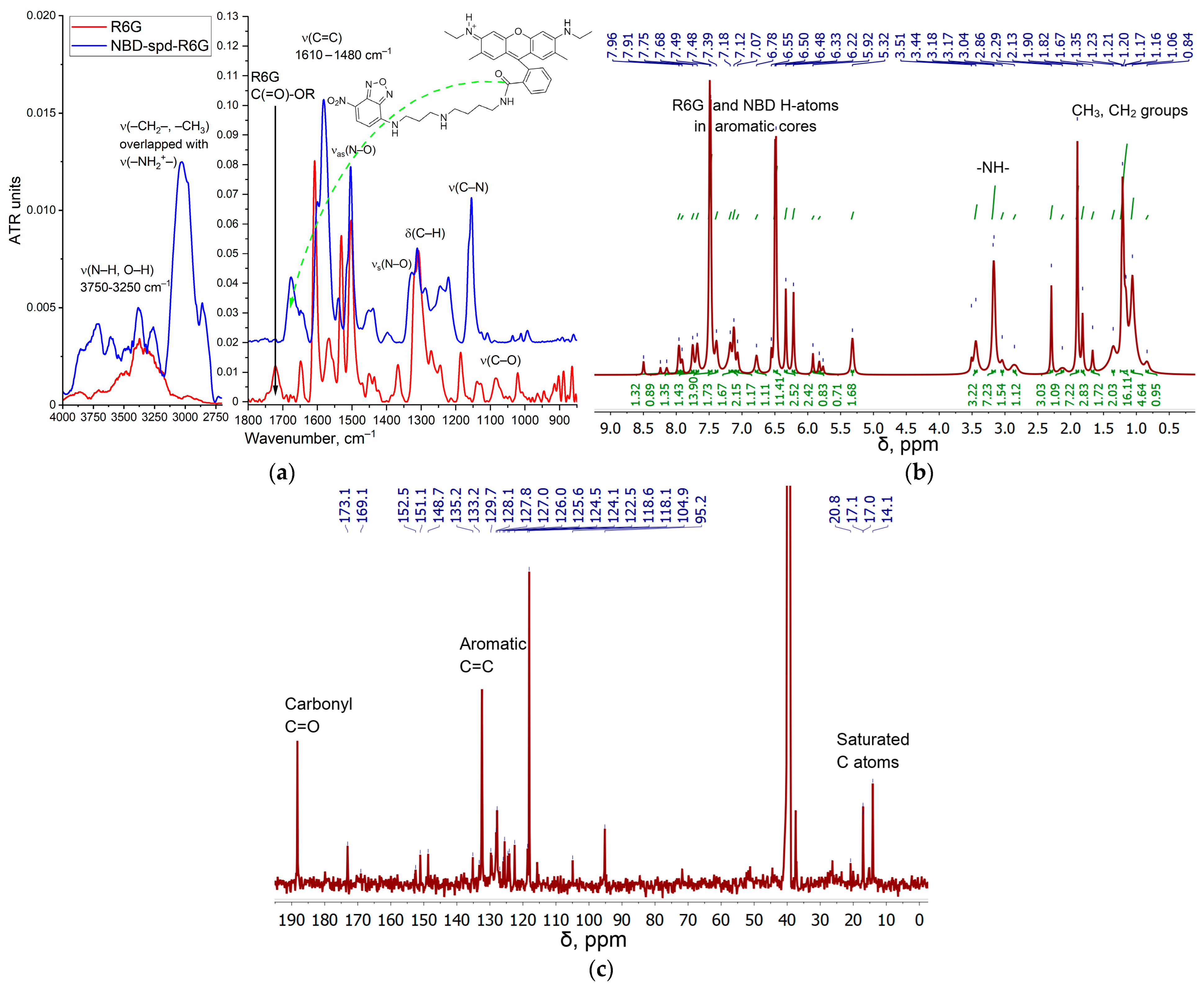
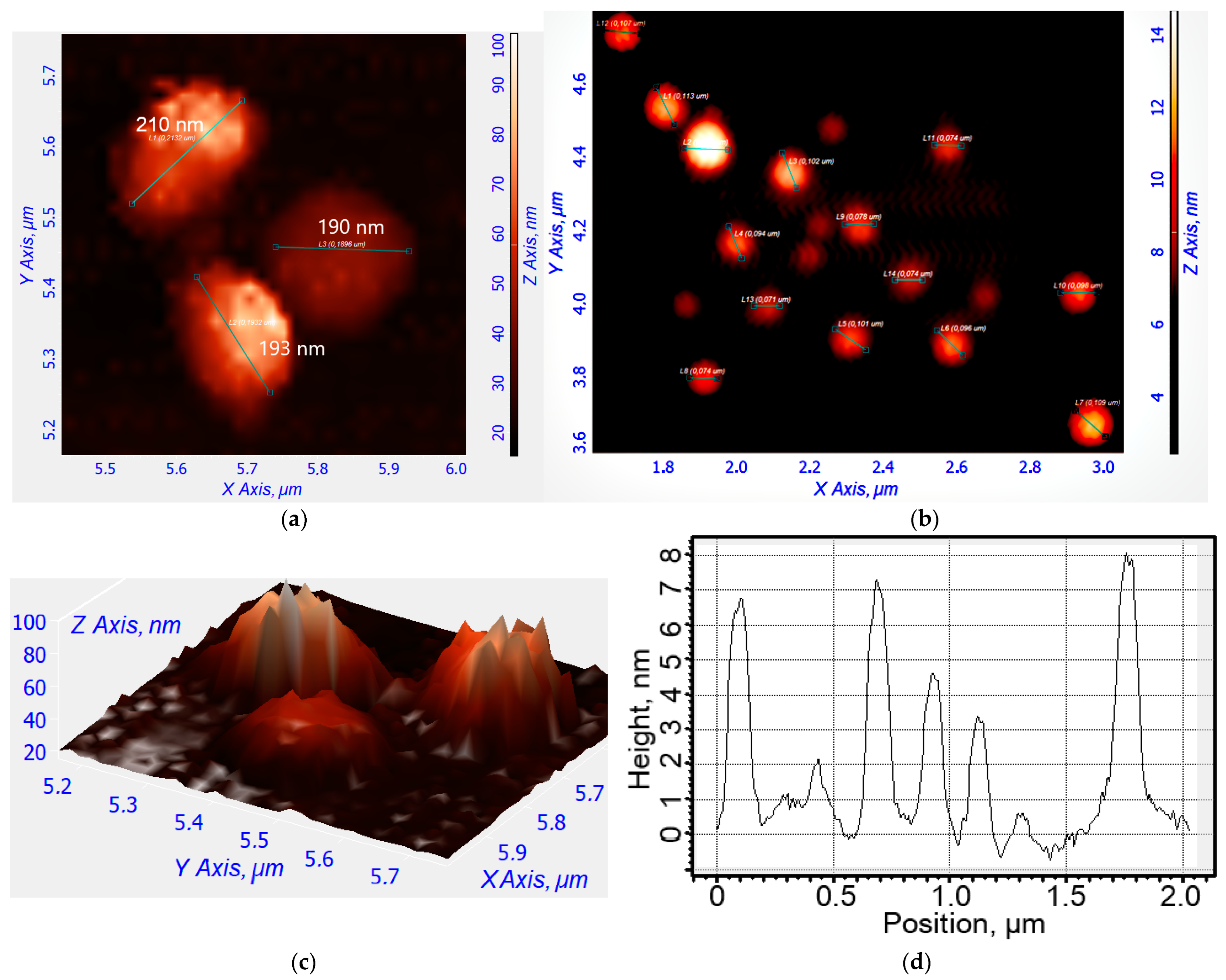
2.2. Synthesis, Characterization, and Preparation of R6G-spd-NBD and Its Nanogel Formulation
2.3. Fluorescent Properties of the Theranostic Agent R6G-spd-NBD in the Polysaccharide Nanogel Particles and Interaction with Bacterial Cells
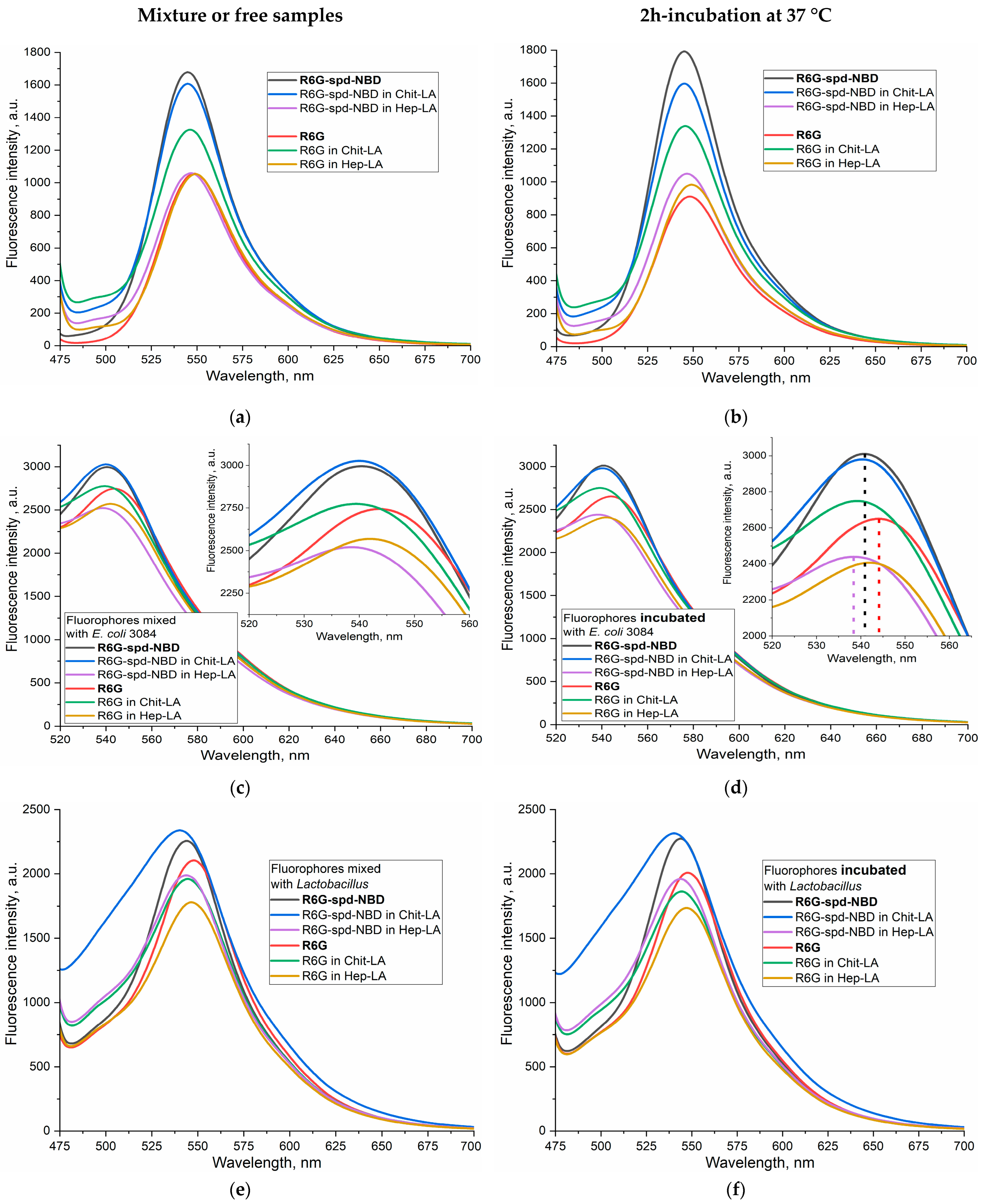
2.3.1. Fluorescence Spectra
2.3.2. The FRET Process in the Context of the Interaction between R6G-spd-NBD and Bacterial Cells
2.3.3. pH Sensitivity of R6G-spd-NBD in the Context of Its Interaction with Bacterial Cells
2.4. Antibacterial Effect of R6G-spd-NBD Nanogel Formulations and Correlation with Intracellular Permeability
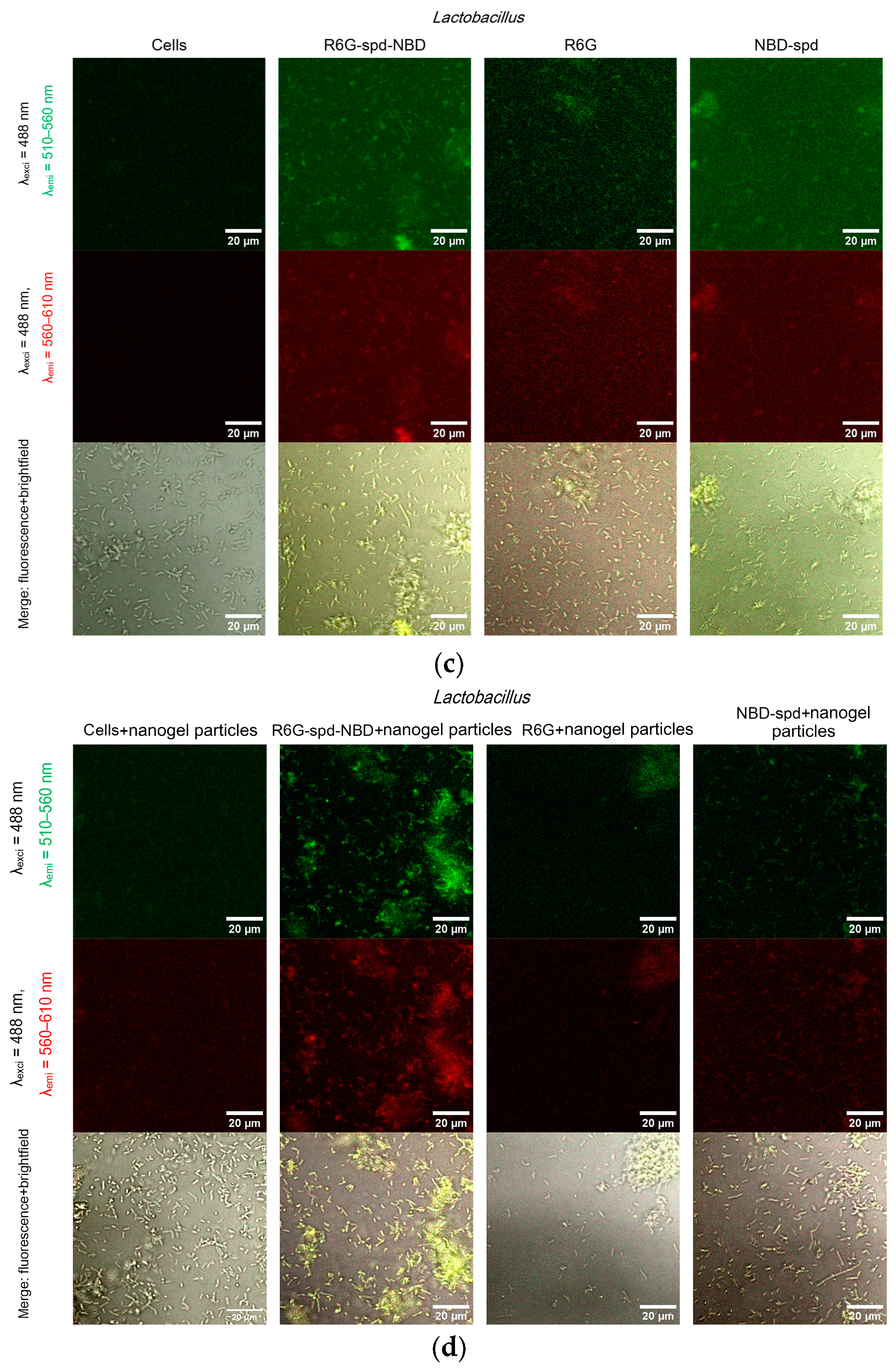
2.5. Confocal Laser Scanning Microscopy (CLSM) for Visualization of Bacterial Cell Staining
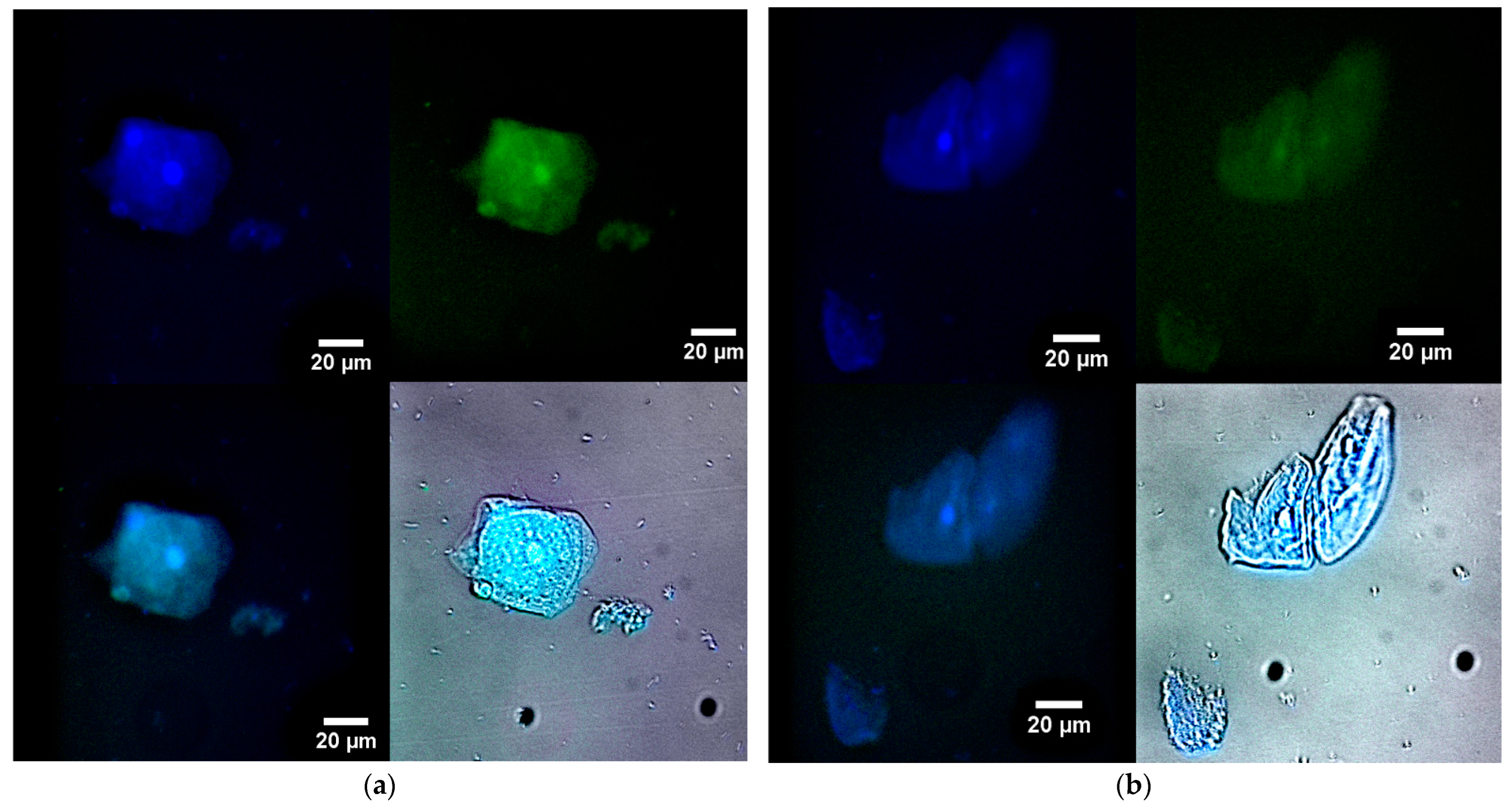
2.6. The Use of Fluorescence Microscopy for the Visualization of Model Latent Bacterial Infections Localized in Macrophages
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of R6G-spd-NBD Fluorophore
4.3. Synthesis of Chit5-LA and Hep-LA Amphiphilic Conjugates and Preparation of Polysaccharide-Based Nanogel Theranostic Formulations
4.3.1. Synthesis of Chit5-LA and Hep-LA Amphiphilic Conjugates
4.3.2. Preparation of Polysaccharide-Based Nanogel Theranostic Formulations
4.4. Characterization of Nanogel Particles
4.4.1. Fourier Infrared Spectroscopy
4.4.2. NMR Spectroscopy
4.4.3. Fluorescence Spectroscopy
4.4.4. Circular Dichroism Spectroscopy
4.4.5. Atomic Force Microscopy
4.4.6. Dynamic Light Scattering
4.5. Microbiology Experiments
4.5.1. Bacterial Cells Cultivation
4.5.2. Antibacterial Studies
4.5.3. Visualization of Petri Dishes with Bacteria Incubated with Fluorophores
4.6. Confocal Laser Scanning Microscopy (CLSM) and Fluorescence Microscopy
4.6.1. CLSM Visualization of Bacterial Cell Staining Using a Theranostic Preparation
4.6.2. Fluorescence Microscopy for Visualization of Model Latent Bacterial Infections Localized in Macrophages
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in Nuclear Medicine Practice. OncoTargets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Liu, B. Multifunctional AIEgens for Future Theranostics. Small 2016, 12, 6528–6535. [Google Scholar] [CrossRef] [PubMed]
- Pusuluri, A.; Krishnan, V.; Wu, D.; Shields, C.W.; Wang, L.W.; Mitragotri, S. Role of Synergy and Immunostimulation in Design of Chemotherapy Combinations: An Analysis of Doxorubicin and Camptothecin. Bioeng. Transl. Med. 2019, 4, e10129. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wong, S.T.C. Cancer Theranostics: An Introduction. Cancer Theranostics 2014, 3–8. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Weber, W.A.; Barthel, H.; Bengel, F.; Eiber, M.; Herrmann, K.; Schäfers, M. What Is Theranostics? J. Nucl. Med. 2023, 64, 669–670. [Google Scholar] [CrossRef]
- Kalash, R.S.; Lakshmanan, V.K.; Cho, C.S.; Park, I.K. Theranostics. Biomater. Nanoarchitecton. 2016, 197–215. [Google Scholar] [CrossRef]
- Dalhoff, A. Global Fluoroquinolone Resistance Epidemiology and Implictions for Clinical Use. Interdiscip. Perspect. Infect. Dis. 2012, 1–37. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-Based Antimicrobial Therapies for the Treatment of Bacterial Infections. Nat. Rev. Mater. 2022, 7, 39–54. [Google Scholar] [CrossRef]
- Sharma, R.; O’Sullivan, K.M.; Holdsworth, S.R.; Bardin, P.G.; King, P.T. Visualizing Macrophage Extracellular Traps Using Confocal Microscopy. J. Vis. Exp. 2017, 2017, e56459. [Google Scholar] [CrossRef]
- Bhat, A.R.; Wani, F.A.; Behera, K.; Khan, A.B.; Patel, R. Formulation of Biocompatible Microemulsions for Encapsulation of Anti-TB Drug Rifampicin: A Physicochemical and Spectroscopic Study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128846. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Q.; Tan, Y.; Wang, R.; Wu, X.; Liu, J.; Liu, R.; Wang, S.; Dong, S. Nanoparticle-Based Lateral Flow Biosensor Integrated With Loop-Mediated Isothermal Amplification for Rapid and Visual Identification of Chlamydia Trachomatis for Point-of-Care Use. Front. Microbiol. 2022, 13, 914620. [Google Scholar] [CrossRef]
- Alshamrani, M. Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials. Polymers 2022, 14, 1221. [Google Scholar] [CrossRef]
- Qi, X.; Grafskaia, E.; Yu, Z.; Shen, N.; Fedina, E.; Masyutin, A.; Erokhina, M.; Lepoitevin, M.; Lazarev, V.; Zigangirova, N.; et al. Methylene Blue-Loaded NanoMOFs: Accumulation in Chlamydia Trachomatis Inclusions and Light/Dark Antibacterial Effects. ACS Infect. Dis. 2023, 9, 1558–1569. [Google Scholar] [CrossRef]
- Rai, M.; Jamil, B. Nanotheranostics: Applications and Limitations; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030297688. [Google Scholar]
- Bai, D.P.; Lin, X.Y.; Huang, Y.F.; Zhang, X.F. Theranostics Aspects of Various Nanoparticles in Veterinary Medicine. Int. J. Mol. Sci. 2018, 19, 3299. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Giottonini, K.Y.; Arellano-Reynoso, B.; Rodríguez-Córdova, R.J.; de la Vega-Olivas, J.; Díaz-Aparicio, E.; Lucero-Acuña, A. Enhancing Therapeutic Efficacy against Brucella Canis Infection in a Murine Model Using Rifampicin-Loaded PLGA Nanoparticles. ACS Omega 2023, 8, 49362–49371. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Sargazi, S.; Fatima, I.; Mobashar, A.; Rahdar, A.; Ajalli, N.; Kyzas, G.Z. Nanotechnology for Therapy of Zoonotic Diseases: A Comprehensive Overview. ChemistrySelect 2022, 7, e202201271. [Google Scholar] [CrossRef]
- Prasad, M.; Ghosh, M.; Kumar, R.; Brar, B.; Surjith, K.P.; Lambe, U.P.; Ranjan, K.; Banerjee, S.; Prasad, G.; Kumar Khurana, S.; et al. The Importance of Nanomedicine in Prophylactic and Theranostic Intervention of Bacterial Zoonoses and Reverse Zoonoses in the Era of Microbial Resistance. J. Nanosci. Nanotechnol. 2021, 21, 3404–3452. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, P.; Mishra, B.; Mudavath, S.L.; Patel, R.R.; Chaurasia, S.; Sundar, S.; Suvarna, V.; Monteiro, M. Mannose-Conjugated Curcumin-Chitosan Nanoparticles: Efficacy and Toxicity Assessments against Leishmania Donovani. Int. J. Biol. Macromol. 2018, 111, 109–120. [Google Scholar] [CrossRef]
- Nahar, M.; Dubey, V.; Mishra, D.; Mishra, P.K.; Dube, A.; Jain, N.K. In vitro evaluation of surface functionalized gelatin nanoparticles for macrophage targeting in the therapy of visceral leishmaniasis. J. Drug Target. 2010, 18, 93–105. [Google Scholar] [CrossRef]
- Kunjachan, S.; Gupta, S.; Dwivedi, A.K.; Dube, A.; Chourasia, M.K. Chitosan-Based Macrophage-Mediated Drug Targeting for the Treatment of Experimental Visceral Leishmaniasis. J. Microencapsul. 2011, 28, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Hebrink, D.; Jenson, P.; Kottom, T.; Limper, A.H. Differential Macrophage Polarization from Pneumocystis in Immunocompetent and Immunosuppressed Hosts: Potential Adjunctive Therapy during Pneumonia. Infect. Immun. 2017, 85, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Cheruku, P.S.; Bazer, F.W.; Safe, S.H.; Zhou, B. Investigation of Macrophage Polarization Using Bone Marrow Derived Macrophages. J. Vis. Exp. 2013, 76, e50323. [Google Scholar] [CrossRef]
- Draijer, C.; Robbe, P.; Boorsma, C.E.; Hylkema, M.N.; Melgert, B.N. Characterization of Macrophage Phenotypes in Three Murine Models of House-Dust-Mite-Induced Asthma. Mediators Inflamm. 2013, 2013, 632049. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Lyamina, S.V.; Malysheva, E.V.; Yurenev, G.L.; Malyshev, I.Y. An Immune Response and an Alveolar Macrophage Phenotype in Asthma, Gastroesophageal Reflux Disease and Their Concurrence. Ter. Arkh. 2015, 87, 34–41. [Google Scholar] [CrossRef]
- Fraternale, A.; Brundu, S.; Magnani, M. Polarization and Repolarization of Macrophages. J. Clin. Cell. Immunol. 2015, 6, 100319. [Google Scholar] [CrossRef]
- Porta, C.; Riboldi, E.; Totaro, M.G.; Strauss, L.; Sica, A.; Mantovani, A. Macrophages in Cancer and Infectious Diseases: The ‘good’ and the ‘Bad’. Immunotherapy 2011, 3, 1185–1202. [Google Scholar] [CrossRef]
- Savchenko, I.V.; Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery. Biomimetics 2023, 8, 543. [Google Scholar] [CrossRef]
- Hong, J.Y.; Chung, Y.; Steenrod, J.; Chen, Q.; Lei, J.; Comstock, A.T.; Goldsmith, A.M.; Bentley, J.K.; Sajjan, U.S.; Hershenson, M.B. Macrophage Activation State Determines the Response to Rhinovirus Infection in a Mouse Model of Allergic Asthma. Respir. Res. 2014, 15, 63. [Google Scholar] [CrossRef]
- Staples, K.J.; Hinks, T.S.C.; Ward, J.A.; Gunn, V.; Smith, C.; Djukanović, R. Phenotypic Characterization of Lung Macrophages in Asthmatic Patients: Overexpression of CCL17. J. Allergy Clin. Immunol. 2012, 130, 1404–1412.e7. [Google Scholar] [CrossRef]
- Ruan, G.X.; Chen, Y.Z.; Yao, X.L.; Du, A.; Tang, G.P.; Shen, Y.Q.; Tabata, Y.; Gao, J.Q. Macrophage Mannose Receptor-Specific Gene Delivery Vehicle for Macrophage Engineering. Acta Biomater. 2014, 10, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, P.; Ruscitti, P.; Vadasz, Z.; Toubi, E.; Giacomelli, R. Macrophages with Regulatory Functions, a Possible New Therapeutic Perspective in Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 102369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Akkoyunlu, M.; Rabin, R.L. Macrophages—Common Culprit in Obesity and Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Jefferies, C.; Cryan, S.-A. Targeted Liposomal Drug Delivery to Monocytes and Macrophages. J. Drug Deliv. 2011, 2011, 727241. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 2023, 8, 14290–14320. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current State of Breast Cancer Diagnosis, Treatment, and Theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-Targeted Nanomedicines for Cancer Theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Ezhov, A.A.; Dobryakova, N.V.; Kudryashova, E.V. Disulfide Cross-Linked Polymeric Redox-Responsive Nanocarrier Based on Heparin, Chitosan and Lipoic Acid Improved Drug Accumulation, Increased Cytotoxicity and Selectivity to Leukemia Cells by Tumor Targeting via “Aikido” Principle. Gels 2024, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceutics 2023, 15, 1287. [Google Scholar] [CrossRef]
- Huynh, N.T.; Roger, E.; Lautram, N.; Benoît, J.P.; Passirani, C. The Rise and Rise of Stealth Nanocarriers for Cancer Therapy: Passive versus Active Targeting. Nanomedicine 2010, 5, 1415–1433. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A.; Kudryashova, E.V. Smart PH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Belogurova, N.G.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Plant Alkylbenzenes and Terpenoids in the Form of Cyclodextrin Inclusion Complexes as Antibacterial Agents and Levofloxacin Synergists. Pharmaceuticals 2022, 15, 861. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Dobryakova, N.V.; Ezhov, A.A.; Kudryashova, E.V. Achievement of the Selectivity of Cytotoxic Agents against Cancer Cells by Creation of Combined Formulation with Terpenoid Adjuvants as Prospects to Overcome Multidrug Resistance. Int. J. Mol. Sci. 2023, 24, 8023. [Google Scholar] [CrossRef] [PubMed]
- Peyron, P.; Vaubourgeix, J.; Poquet, Y.; Levillain, F.; Botanch, C.; Bardou, F.; Daffé, M.; Emile, J.F.; Marchou, B.; Cardona, P.J.; et al. Foamy Macrophages from Tuberculous Patients’ Granulomas Constitute a Nutrient-Rich Reservoir for M. Tuberculosis Persistence. PLoS Pathog. 2008, 4, e1000204. [Google Scholar] [CrossRef]
- Dua, K.; Rapalli, V.K.; Shukla, S.D.; Singhvi, G.; Shastri, M.D.; Chellappan, D.K.; Satija, S.; Mehta, M.; Gulati, M.; Pinto, T.D.J.A.; et al. Multi-Drug Resistant Mycobacterium Tuberculosis & Oxidative Stress Complexity: Emerging Need for Novel Drug Delivery Approaches. Biomed. Pharmacother. 2018, 107, 1218–1229. [Google Scholar] [CrossRef]
- Laval, T.; Chaumont, L.; Demangel, C. Not Too Fat to Fight: The Emerging Role of Macrophage Fatty Acid Metabolism in Immunity to Mycobacterium Tuberculosis. Immunol. Rev. 2021, 301, 84–97. [Google Scholar] [CrossRef]
- Tulu, D. Bovine Brucellosis: Epidemiology, Public Health Implications, and Status of Brucellosis in Ethiopia. Vet. Med. Res. Rep. 2022, 13, 21–30. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Angeletti, S.; Ianni, A.; Petitti, T.; Gherardi, G. Microbiological Laboratory Diagnosis of Human Brucellosis: An Overview. Pathogens 2021, 10, 1623. [Google Scholar] [CrossRef]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine Brucellosis—A Comprehensive Review. Vet. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef]
- Boon, T.H.; Williams, E. Diagnosis of Brucellosis. Lancet 1970, 296, 51. [Google Scholar] [CrossRef]
- Corbel, M. Brucellosis: An Overview. Emerg. Infect. Dis. 1997, 3, 213–221. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Kudryashova, E.V. PH-Sensitive Fluorescent Marker Based on Rhodamine 6G Conjugate with Its FRET/PeT Pair in “Smart” Polymeric Micelles for Selective Imaging of Cancer Cells. Pharmaceutics 2024, 16, 1007. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Kudryashova, E.V. Smart Polymeric Micelles System, Designed to Carry a Combined Cargo of L-Asparaginase and Doxorubicin, Shows Vast Improve- Ment in Cytostatic Efficacy. Polymers 2024, 4, 2132. [Google Scholar] [CrossRef]
- Huster, D.; Müller, P.; Arnold, K.; Herrmann, A. Dynamics of Membrane Penetration of the Fluorescent 7-Nitrobenz-2-Oxa-1,3-Diazol-4-Yl (NBD) Group Attached to an Acyl Chain of Phosphatidylcholine. Biophys. J. 2001, 80, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.J.; Chen, L.N.; Zhang, X.; Liu, J.T.; Chen, M.Y.; Wu, Q.R.; Miao, J.Y.; Zhao, B.X. A NBD-Based Simple but Effective Fluorescent PH Probe for Imaging of Lysosomes in Living Cells. Anal. Chim. Acta 2016, 920, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kraithong, S.; Sangsuwan, R.; Worawannotai, N.; Sirirak, J.; Charoenpanich, A.; Thamyongkit, P.; Wanichachewa, N. Triple Detection Modes for Hg2+ Sensing Based on a NBD-Fluorescent and Colorimetric Sensor and Its Potential in Cell Imaging. New J. Chem. 2018, 42, 12412–12420. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Li, J.; Wu, S.; Qiu, X.; Xi, Z.; Yi, L. Investigation of Thiolysis of NBD Amines for the Development of H2S Probes and Evaluating the Stability of NBD Dyes. Org. Biomol. Chem. 2016, 14, 11117–11124. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E.V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13, 446. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic System Based on Reconstituted Macrophage Membranes for Analyzing and Selection of Higher-Affinity Ligands Specific to Mannose Receptor to Develop the Macrophage-Focused Medicines. Biomedicines 2023, 11, 2769. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A. Intermolecular FRET Pairs as An Approach to Visualize Specific Enzyme Activity in Model Biomembranes and Living Cells. Biophysica 2024, 4, 340–356. [Google Scholar] [CrossRef]


| Designation * | Polymer Modification Degree per Glycoside Unit, % | The Average Molecular Mass of One Structural Unit, kDa | Hydrodynamic Diameter, nm | ζ-Potential, mV | Aggregative Stability |
|---|---|---|---|---|---|
| Chit5-LA | 25 ± 3 | 6.4 ± 0.5 | 210 ± 40 | +8 ± 1 | >36 h |
| Hep-LA | 10 ± 2 | 14 ± 2 | 310 ± 50 | –12 ± 1.5 | >48 h |
| Sample * | Cell Viability, % | Cin/Cout | ||
|---|---|---|---|---|
| E. coli | Lactobacilli | E. coli | Lactobacilli | |
| R6G | 84 ± 5 | 87 ± 2 | 3.2 ± 0.3 | 2.2 ± 0.1 |
| R6G in Chit-LA in nanogel | 67 ± 4 | 85 ± 2 | 2.3 ± 0.1 | 1.4 ± 0.1 |
| R6G in Hep-LA in nanogel | 88 ± 7 | 81 ± 4 | 2.5 ± 0.2 | 1.7 ± 0.2 |
| R6G-spd-NBD | 70 ± 3 | 92 ± 1 | 2.1 ± 0.2 | 1.4 ± 0.1 |
| R6G-spd-NBD in Chit-LA in nanogel | 45 ± 2 | 89 ± 4 | 3.6 ± 0.4 | 1.7 ± 0.3 |
| R6G-spd-NBD in Hep-LA in nanogel | 62 ± 6 | 93 ± 3 | 2.8 ± 0.2 | 1.3 ± 0.1 |
| Chit-LA in nanogel | 97 ± 1 | >99 | Polymers mostly adsorbed onto cell surface | |
| Hep-LA in nanogel | >99 | >99 | ||
| Probe | Blue Channel | Green Channel | ||||||
|---|---|---|---|---|---|---|---|---|
| Background | Nucleus | Lysosomes | Cytoplasm | Background | Nucleus | Lysosomes | Cytoplasm | |
| R6G-spd-NBD | 14 | 253 | 250 and 134 | 130 | 2 | 164 | 105 and 108 | 91 |
| R6G | 22 | 198 | 130 | 105 | 16 | 84 | 62 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlotnikov, I.D.; Ezhov, A.A.; Belogurova, N.G.; Kudryashova, E.V. pH-Sensitive Fluorescent Probe in Nanogel Particles as Theragnostic Agent for Imaging and Elimination of Latent Bacterial Cells Residing Inside Macrophages. Gels 2024, 10, 567. https://doi.org/10.3390/gels10090567
Zlotnikov ID, Ezhov AA, Belogurova NG, Kudryashova EV. pH-Sensitive Fluorescent Probe in Nanogel Particles as Theragnostic Agent for Imaging and Elimination of Latent Bacterial Cells Residing Inside Macrophages. Gels. 2024; 10(9):567. https://doi.org/10.3390/gels10090567
Chicago/Turabian StyleZlotnikov, Igor D., Alexander A. Ezhov, Natalya G. Belogurova, and Elena V. Kudryashova. 2024. "pH-Sensitive Fluorescent Probe in Nanogel Particles as Theragnostic Agent for Imaging and Elimination of Latent Bacterial Cells Residing Inside Macrophages" Gels 10, no. 9: 567. https://doi.org/10.3390/gels10090567
APA StyleZlotnikov, I. D., Ezhov, A. A., Belogurova, N. G., & Kudryashova, E. V. (2024). pH-Sensitive Fluorescent Probe in Nanogel Particles as Theragnostic Agent for Imaging and Elimination of Latent Bacterial Cells Residing Inside Macrophages. Gels, 10(9), 567. https://doi.org/10.3390/gels10090567







