The Inhibitory Impact of a Co-Assembly Gel with Natural Carrier-Free Binary Small Molecules, as Used in Traditional Chinese Medicine, on the Viability of SW1990 Cells
Abstract
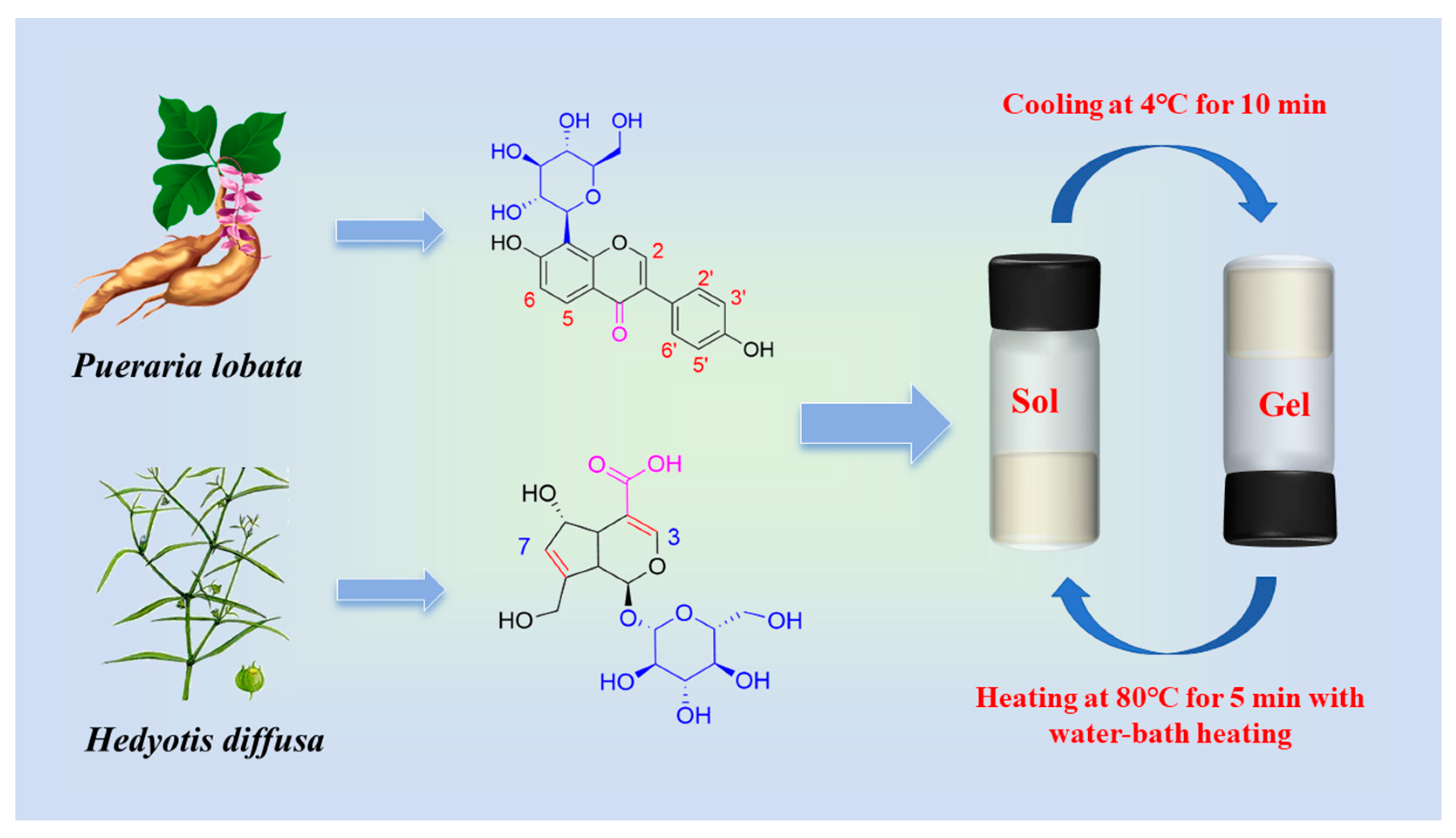
:1. Introduction
2. Results and Discussions
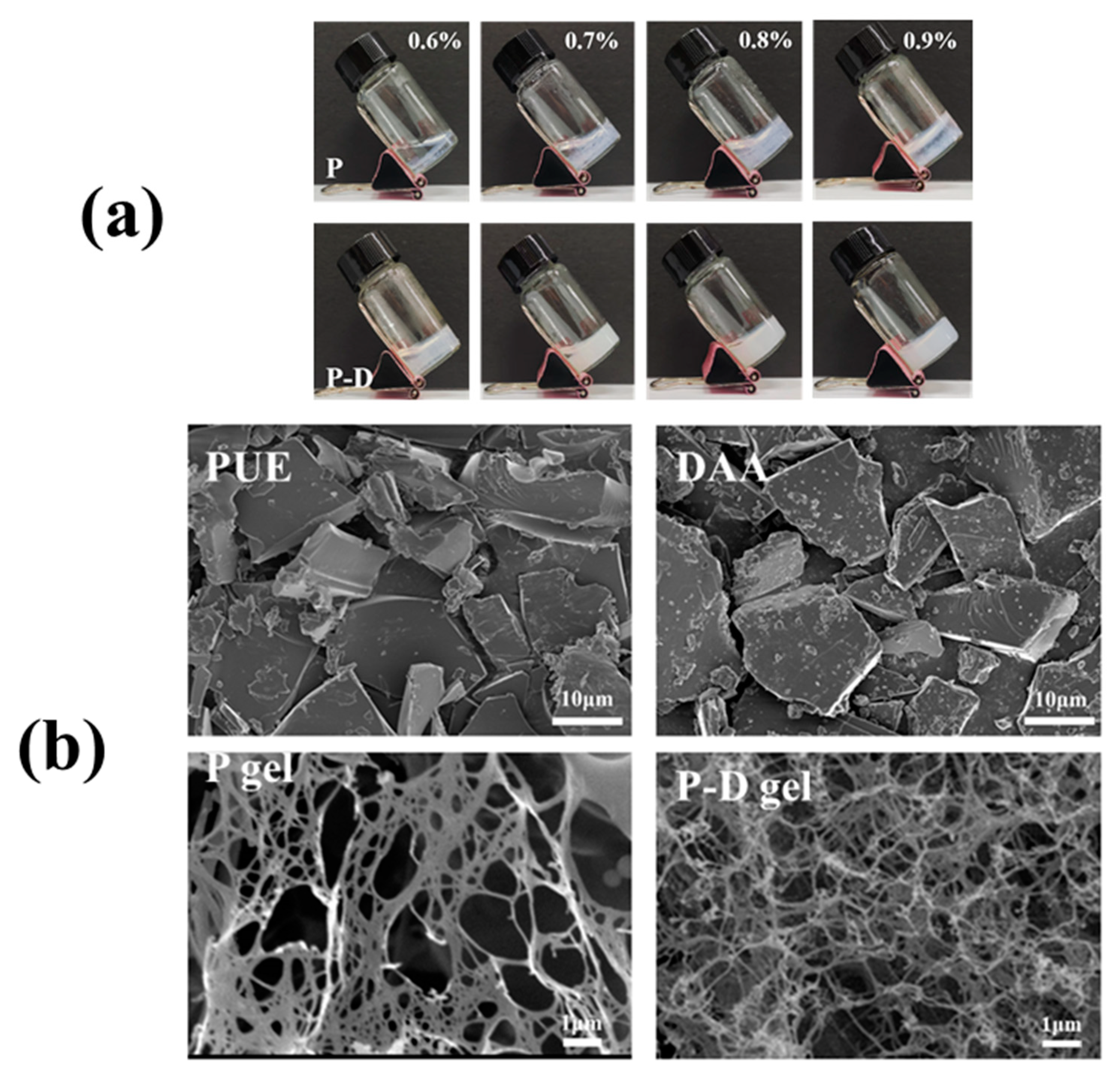
2.1. Gelation and Morphological Characterization of Hydrogels
2.2. Micro-Rheological and Rheological Analysis
2.3. Co-Assembled Mechanism of P − D Gel
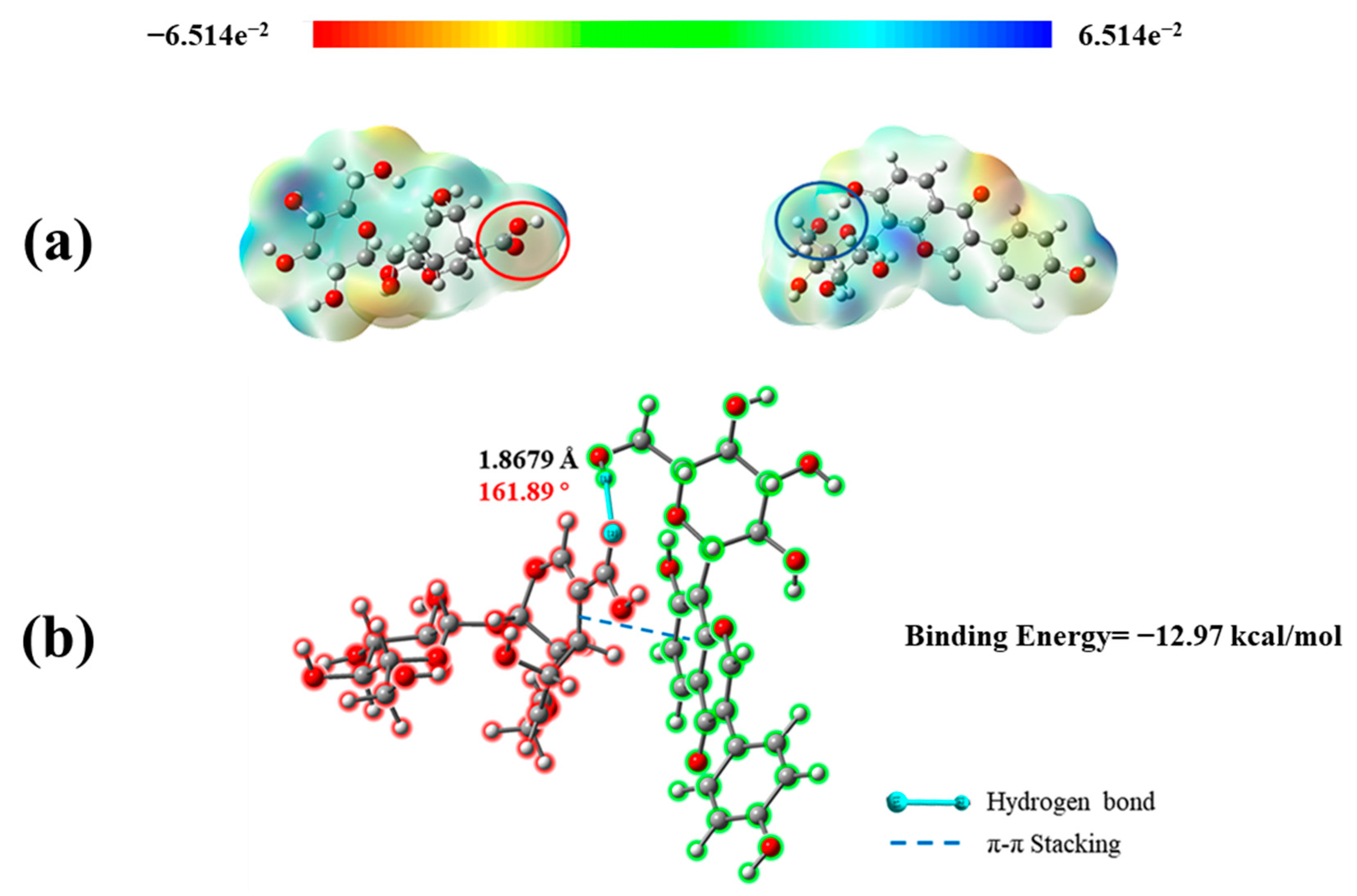
2.4. Quantum Chemical Calculation of P − D Gel
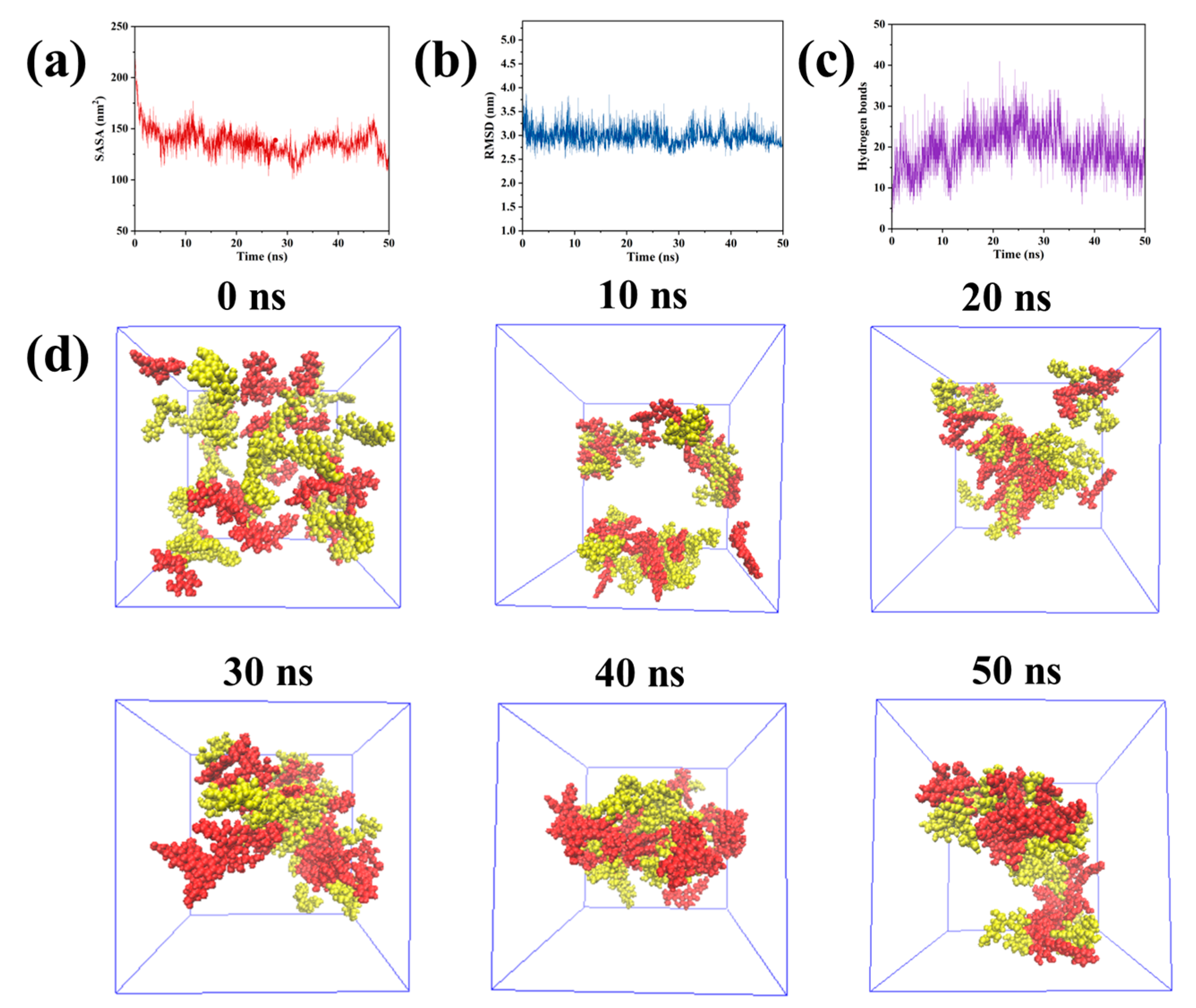
2.5. Molecular Dynamics Simulation
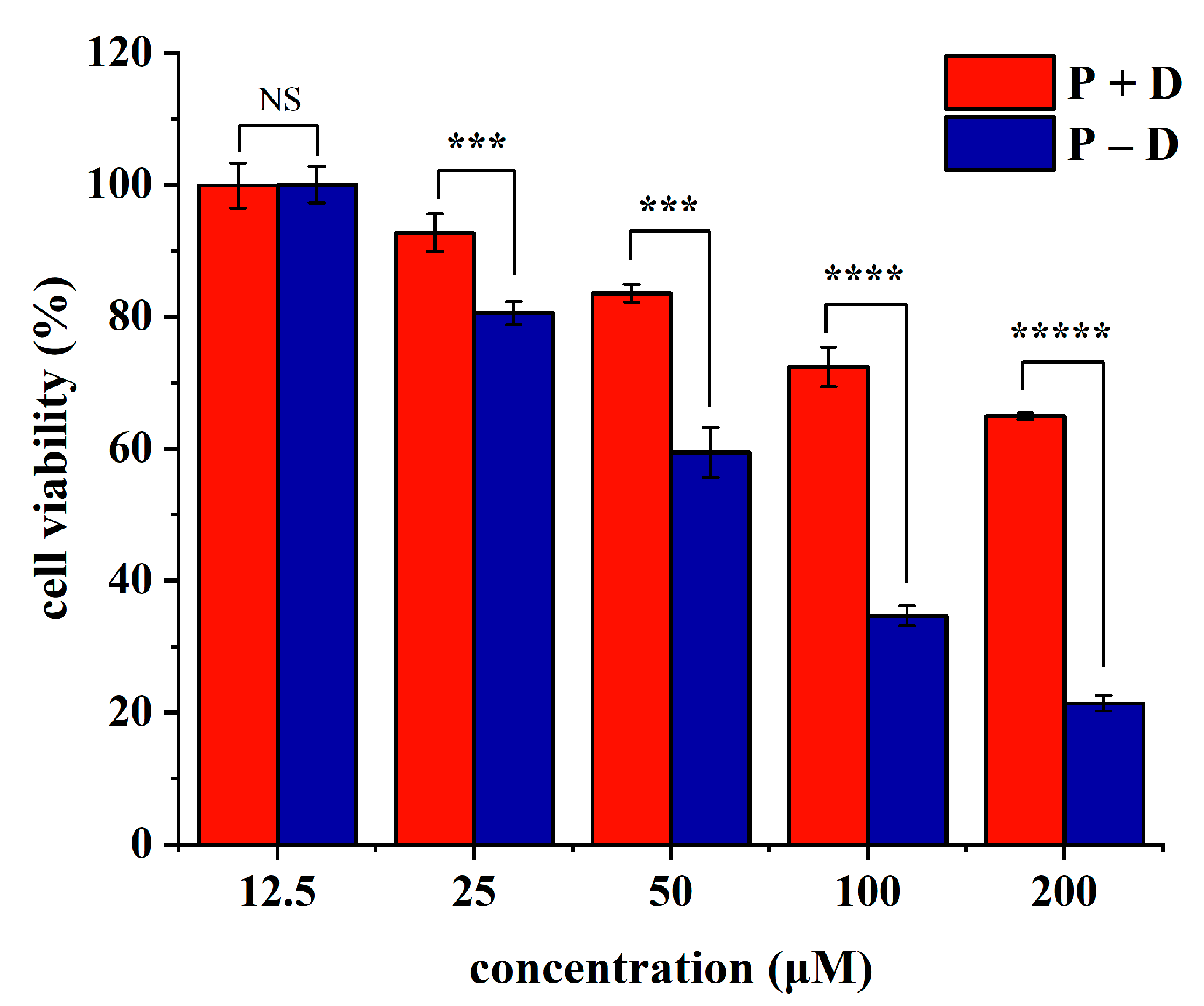
2.6. Study on Antitumor Activity In Vitro
3. Conclusions
4. Materials and Methods
4.1. Reagents, Cell Line, and Materials
4.2. Preparation of the P Gel and P − D Gel
4.3. Morphological Characterization
4.4. Microrheological Properties Test
4.5. Rheological Properties Test
4.6. Co-Assembled Mechanism Study
4.7. Quantum Chemical Calculation
4.8. Molecular Dynamics Simulation
4.9. Cell Test
4.9.1. Cell and Culture Conditions
4.9.2. Cytotoxicity Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.M.; Xie, J.J.; Peng, C.G.; Wang, B.H.; Wang, K.C.; Li, L.J. Enhancing Clinical Efficacy through the Gut Microbiota: A New Field of Traditional Chinese Medicine. Engineering 2019, 5, 40–49. [Google Scholar] [CrossRef]
- Qiao, L.; Han, M.S.; Gao, S.J.; Shao, X.X.; Wang, X.M.; Sun, L.L.; Fu, X.J.; Wei, Q.C. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J. Mater. Chem. B 2020, 8, 6333–6351. [Google Scholar] [CrossRef]
- Yin, B.; Fang, D.M.; Zhou, X.L.; Gao, F. Natural products as important tyrosine kinase inhibitors. Eur. J. Med. Chem. 2019, 182, 111664. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Huang, X.M.; Tian, X.H.; Yuan, Z.H.; Lu, J.H.; Nie, X.Q.; Wang, P.L.; Lei, H.M.; Wang, P.L. Natural Small-Molecule-Based Carrier-Free Self-Assembly Library Originated from Traditional Chinese Herbal Medicine. ACS Omega 2022, 7, 43510–43521. [Google Scholar] [CrossRef]
- Liu, S.L.; Su, J.Y. Chaigelancao decoction for the treatment of 46 cases of acute tonsillitis. J. Tradit. Chin. Med. 1983, 11, 19. [Google Scholar] [CrossRef]
- Qi, Y.F. Treatment of Tumor Diseases with Traditional Chinese Medicine Formulas, 1st ed.; People’s Medical Publishing House: Beijing, China, 2007; 242p. [Google Scholar]
- Hou, Y.; Chen, M.Y.; Ruan, H.N.; Sun, Z.C.; Wu, H.F.; Xu, X.D.; Yang, J.S.; Ma, G.X.; Zhou, X.L. A new supramolecular natural product gel based on self-assembled pomolic acid from traditional Chinese medicine. Colloid Interface Sci. 2022, 46, 100583. [Google Scholar] [CrossRef]
- Li, M.Y.; Wang, C.L.; Di, Z.H.; Li, H.; Zhang, J.F.; Xue, W.T.; Zhao, M.P.; Zhang, K.; Zhao, Y.L.; Li, L.L. Engineering Multifunctional DNA Hybrid Nanospheres through Coordination-Driven Self-Assembly. Angew. Chem. Int. Ed. 2019, 58, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Lu, J.H.; Yuan, Z.H.; Pi, W.M.; Huang, X.M.; Lin, X.Y.; Zhang, Y.Z.; Lei, H.M.; Wang, P.L. Natural Carrier-Free Binary Small Molecule Self-Assembled Hydrogel Synergize Antibacterial Effects and Promote Wound Healing by Inhibiting Virulence Factors and Alleviating the Inflammatory Response. Small 2022, 19, 2205528. [Google Scholar] [CrossRef]
- Fan, J.P.; Zhong, H.; Zhang, X.H.; Yuan, T.T.; Chen, H.P.; Peng, H.L. Preparation and Characterization of Oleanolic Acid-Based LowMolecular-Weight Supramolecular Hydrogels Induced by Heating. ACS Appl. Mater. Interfaces 2021, 13, 29130–29136. [Google Scholar] [CrossRef]
- Wang, X.C.; Huang, H.B.; Gong, W.; He, W.Y.; Li, X.; Xu, Y.; Gong, X.J.; Hu, J.N. Resveratrol Triggered the Quick Self-Assembly of Gallic Acid into Therapeutic Hydrogels for Healing of Bacterially Infected Wounds. Biomac. 2022, 23, 1680–1692. [Google Scholar] [CrossRef]
- Lu, J.H.; Yao, S.C.; Lin, X.Y.; Wang, Z.J.; Wu, L.Y.; Pi, W.M.; Yang, L.P.; Zhang, Y.Z.; Zhang, X.; Wang, Z.J.; et al. Nanoparticles Self-Assembled from Berberine and Chrysin for Generating Antimicrobial Films. ACS Appl. Nano Mater. 2023, 6, 23232–23244. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, H.C.; Xu, S.J.; Kong, J.W.; Gao, F.; Wang, Z.J.; Li, Y.; Dai, Z.Q.; Jiang, X.Q.; Ding, X.; et al. Novel Natural Carrier-Free Self-Assembled Nanoparticles for Treatment of Ulcerative Colitis by Balancing Immune Microenvironment and Intestinal Barrier. Adv. Healthc. Mater. 2023, 12, 2301826. [Google Scholar] [CrossRef]
- Tian, X.H.; Wang, P.L.; Li, T.; Huang, X.M.; Guo, W.B.; Yang, Y.Q.; Yan, M.M.; Zhang, H.; Cai, D.S.; Jia, X.H.; et al. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination. Acta Pharm. Sin. B 2020, 10, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Y.; Tang, G.; Gao, Y.H.; Chen, X.; Zhou, Z.Y.; Li, Y.; Li, X.; Wang, H.C.; Yu, X.Y.; Luo, L.X.; et al. Carrier-Free Small Molecular Self-Assembly Based on Berberine and Curcumin Incorporated in Submicron Particles for Improving Antimicrobial Activity. ACS Appl. Mater. Interfaces 2022, 14, 10055–10067. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Wang, P.L.; Li, T.; Tian, X.H.; Guo, W.B.; Xu, B.; Huang, G.R.; Cai, D.S.; Zhou, F.; Zhang, H.; et al. Self-Assemblies Based on Traditional Medicine Berberine and Cinnamic Acid for Adhesion-Induced Inhibition Multidrug-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2019, 12, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Wang, Z.W.; Guo, M.T.; Zhang, K.; Geng, L.; Mao, A.Q.; Yang, Y.J.; Yu, F. Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels. Food Funct. 2020, 11, 10137–10148. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.X.; Li, Z.B.; Xu, M.; Lu, Y.P.; Li, X.F. Puerarin-containing rhein-crosslinked tyramine-modified hyaluronic acid hydrogel for antibacterial and anti-inflammatory wound dressings. Int. J. Biol. Macromol. 2024, 271, 132527. [Google Scholar] [CrossRef]
- Borges, P.V.; Moret, K.H.; Raghavendra, N.M.; Maramaldo Costa, T.E.; Monteiro, A.P.; Carneiro, A.B.; Pacheco, P.; Temerozo, J.R.; Bou-Habib, D.C.; das Graças Henriques, M.; et al. Protective effect of gedunin on TLR-mediated inflammation by modulation of inflammasome activation and cytokine production: Evidence of a multitarget compound. Pharmacol. Res. 2017, 115, 65–77. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.Y.; Liu, M.R.; An, S.; Guan, K.Y.; Wang, M.L.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef]
- Wang, H.X.; Zeng, M.S.; Ye, Y.; Liu, J.Y.; Xu, P.P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2020, 35, 324–336. [Google Scholar] [CrossRef]
- Chen, X.F.; Wang, L.; Fan, S.S.; Song, S.Y.; Min, H.Y.; Wu, Y.Z.; He, X.; Liang, Q.; Wang, Y.; Yi, L.; et al. Puerarin acts on the skeletal muscle to improve insulin sensitivity in diabetic rats involving μ-opioid receptor. Eur. J. Pharmacol. 2018, 818, 115–123. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Effects of Puerarin on the Prevention and Treatment of Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef] [PubMed]
- Su, A.S.; Zhang, J.W.; Zou, J. The anxiolytic-like effects of puerarin on an animal model of PTSD. Biomed. Pharmacother. 2019, 115, 108978. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Yang, M.; Chen, S.; Fu, C.X.; Zhang, L.; Liu, S.X.; Kang, J.M.; Li, C.F. Multiple metabolite profiles uncover remarkable bioactive compounds and metabolic characteristics of noni fruit (Morinda citrifolia L.) at various stages of ripeness. Food Chem. 2024, 450, 139357. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Seong, G.S.; Kim, Y.D.; Choung, S.Y. Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells. Molecules 2021, 26, 3298. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Chen, M.; Su, C.X.; West, B.J. In VivoAntioxidant Activity of Deacetylasperulosidic Acid in Noni. J. Anal. Methods Chem. 2013, 2013, 804504. [Google Scholar] [CrossRef]
- Esakkimuthu, S.; Nagulkumar, S.; Darvin, S.S.; Buvanesvaragurunathan, K.; Sathya, T.N.; Navaneethakrishnan, K.R.; Kumaravel, T.S.; Murugan, S.S.; Shirota, O.; Balakrishna, K.; et al. Antihyperlipidemic effect of iridoid glycoside deacetylasperulosidic acid isolated from the seeds of Spermacoce hispida L.—A traditional antiobesity herb. J. Ethnopharmacol. 2019, 245, 112170. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.Q.; Huang, J.; Chen, X.W.; Xiong, H.Y.; Kang, Y.; Wu, J. Synthesis, characterization, and formulation of poly-puerarin as a biodegradable and biosafe drug delivery platform for anti-cancer therapy. Biomater. Sci. 2019, 7, 2152–2164. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.T.; Bao, Z.W.; Zhu, D.S.; Chen, G.Q.; Li, W.R.; Xiao, Y.X.; Wang, Z.Z.; Zhang, Y.X.; Liu, H.F.; et al. Pericardial Delivery of SDF-1α Puerarin Hydrogel Promotes Heart Repair and Electrical Coupling. Adv. Mater. 2023, 36, 2302686. [Google Scholar] [CrossRef]
- Zheng, L.J.; Xu, H.J.; Hu, H.; Ruan, J.X.; Shi, C.H.; Cao, J.Q.; Zhang, X.G. Preparation, characterization and antioxidant activity of inclusion complex loaded with puerarin and corn peptide. Food Biosci. 2022, 49, 101886. [Google Scholar] [CrossRef]
- Ouyang, L.P.; Chen, B.H.; Liu, X.D.; Wang, D.H.; Li, Y.; Liao, Y.; Yeung, K.W.K.; Liu, X.Y. Puerarin@Chitosan composite for infected bone repair through mimicking the bio-functions of antimicrobial peptides. Bioact. Mater. 2023, 21, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, W.; Zhang, S.Y.; Liu, Y.; Teng, J.M.; Ma, Y.X.; Huang, R.J.; Wei, H.; Chen, H.L.; Zhang, J.T.; et al. Dual Self-Assembly of Puerarin and Silk Fibroin into Supramolecular Nanofibrillar Hydrogel for Infected Wound Treatment. Adv. Healthc. Mater. 2024, 13, 2400071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Liu, J.; Zheng, Y.X.; Zhang, B.H.; Hu, Y.; Wu, Y.F.; Li, Y.M.; Liu, L.; Zhu, H.X.; Liu, Q.; et al. Copper Ion-Inspired Dual Controllable Drug Release Hydrogels for Wound Management: Driven by Hydrogen Bonds. Small 2024, 19, 2401152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.D.; Ma, D.Z.; Wang, J.; Zhang, J.W.; Zhang, S. Multi-responsive hydrogel based on lotus root starch. Int. J. Biol. Macromol. 2016, 89, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, X.; Liang, S.; Chen, L.; Dong, L.; Rahaman, A.; He, S.; Shen, Y.; Su, D. Polysaccharides improved the viscoelasticity, microstructure, and physical stability of ovalbumin-ferulic acid complex stabilized emulsion. Int. J. Biol. Macromol. 2022, 211, 150–158. [Google Scholar] [CrossRef]
- Aslam, A.; Umer Ashraf, M.; Barkat, K.; Mahmood, A.; Muhammad Sarfraz, R.; Malatani, R.T.; Gad, H.A. Green synthesis of quince/pectin cross-linked superporous hydrogel sponges for pH-regulated sustained domperidone delivery. Int. J. Pharm. 2023, 644, 123305. [Google Scholar] [CrossRef]
- Li, Q.L.; Hou, Y.; Xing, Y.J.; Wang, Y.M.; Sun, Z.H.; Sun, Z.C.; Xu, X.D.; Yang, L.F.; Huo, X.W.; Ma, G.X. Directed co-assembly of binary natural small molecules into carrier-free sprayable gel with synergistic multifunctional activity for perishable fruits preservation. Chem. Eng. J. 2024, 491, 152104. [Google Scholar] [CrossRef]
- Yang, X.; Ma, C.; Chen, Z.M.; Liu, J.; Liu, F.Y.; Xie, R.B.; Zhao, H.T.; Deng, G.; Chen, A.T.; Gong, N.B.; et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019, 12, 2468–2476. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Xu, S.Q.; Du, Y.N.; Zhang, Z.J.; Yan, J.N.; Sun, J.J.; Zhang, L.C.; Wang, C.; Lai, B.; Wu, H.T. Gel properties and interactions of hydrogels constructed with low acyl gellan gum and puerarin. Carbohydr. Polym. 2024, 326, 121594. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, H.C.; Yang, Z.M.; Ma, J.; Su, Y.H.; Li, W.F.; Lei, Z.Q. Methyl Cinnamate-Derived Fluorescent Rigid Organogels Based on Cooperative π−π Stacking and C=O···π Interactions Instead of H-Bonding and Alkyl Chains. Langmuir 2015, 31, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. WIREs Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Lei, Y.H.; Du, M.Q.; Zhang, G.; Chen, L.; Fu, Y.L.; Zhong, Y.Q.; Zhang, E.X. Bioinformatics and Network Pharmacology-Based Approaches to Explore the Potential Mechanism of the Antidepressant Effect of Cyperi Rhizoma through Soothing the Liver. Evid-Based. Compl. Alt. 2021, 2021, 8614963. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Requena, J.V.; Saldías, C.; Inostroza-Rivera, R.; Díaz Díaz, D. Understanding hydrogelation processes through molecular dynamics. J. Mater. Chem. B 2019, 7, 1652–1673. [Google Scholar] [CrossRef] [PubMed]
- Van Lommel, R.; Zhao, J.Y.; De Borggraeve, W.; De Proft, F.; Alonso, M. Molecular dynamics based descriptors for predicting supramolecular gelation. Chem. Sci. 2020, 11, 4226–4238. [Google Scholar] [CrossRef]
- Puyathorn, N.; Tamdee, P.; Sirirak, J.; Okonogi, S.; Phaechamud, T.; Chantadee, T. Computational Insight of Phase Transformation and Drug Release Behaviour of Doxycycline-Loaded Ibuprofen-Based In-Situ Forming Gel. Pharmaceutics 2023, 15, 2315. [Google Scholar] [CrossRef]
- Ma, Y.K.; Wu, Q.; Li, X.; Gu, X.Q.; Xu, J.H.; Yang, J.Z. Pancreatic cancer: From bench to bedside. Ann. Transl. Med. 2016, 4, 458. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Xiao, Y.Y.; Guo, H.C.; Guo, Y.Y.; Huang, Y.Z.; Shan, Y.F.; Bai, Y.H.; Lin, X.Y. The isoflavone puerarin exerts anti-tumor activity in pancreatic ductal adenocarcinoma by suppressing mTOR-mediated glucose metabolism. Aging 2021, 13, 25089–25105. [Google Scholar] [CrossRef]
- Yuan, C.; Morales-Oyarvide, V.; Khalaf, N.; Perez, K.; Tabung, F.K.; Ho, G.Y.F.; Kooperberg, C.; Shadyab, A.H.; Qi, L.; Kraft, P.; et al. Prediagnostic Inflammation and Pancreatic Cancer Survival. J. Natl. Cancer Inst. 2021, 113, 1186–1193. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zheng, Y.L.; Shi, H.F.; Xie, H.Z.; Yang, Y.; Zhu, F.Y.; Ke, L.J.; Chen, H.J.; Gao, Y. Convenient Tuning of the Elasticity of Self-Assembled Nano-Sized Triterpenoids to Regulate Their Biological Activities. ACS Appl. Mater. Interfaces 2021, 13, 44065–44078. [Google Scholar] [CrossRef]
- Deng, J.J.; Xu, W.D.; Lei, S.Y.; Li, Q.H.; Li, K.Q.; Lyu, J.X.; Wang, J.L.; Wang, Z. Activated Natural Killer Cells-Dependent Dendritic Cells Recruitment and Maturation by Responsive Nanogels for Targeting Pancreatic Cancer Immunotherapy. Small 2022, 18, 2203114. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Liu, S.; Huang, Q.; Wu, H.; Zheng, Q.; Xu, X.; Li, B.; Ma, G.; Zhou, X.; Liu, S.; et al. The Inhibitory Impact of a Co-Assembly Gel with Natural Carrier-Free Binary Small Molecules, as Used in Traditional Chinese Medicine, on the Viability of SW1990 Cells. Gels 2024, 10, 569. https://doi.org/10.3390/gels10090569
Nie X, Liu S, Huang Q, Wu H, Zheng Q, Xu X, Li B, Ma G, Zhou X, Liu S, et al. The Inhibitory Impact of a Co-Assembly Gel with Natural Carrier-Free Binary Small Molecules, as Used in Traditional Chinese Medicine, on the Viability of SW1990 Cells. Gels. 2024; 10(9):569. https://doi.org/10.3390/gels10090569
Chicago/Turabian StyleNie, Xueqiang, Sifan Liu, Qiongxue Huang, Haifeng Wu, Qingxia Zheng, Xudong Xu, Bowen Li, Guoxu Ma, Xiaolei Zhou, Shuchen Liu, and et al. 2024. "The Inhibitory Impact of a Co-Assembly Gel with Natural Carrier-Free Binary Small Molecules, as Used in Traditional Chinese Medicine, on the Viability of SW1990 Cells" Gels 10, no. 9: 569. https://doi.org/10.3390/gels10090569





