Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
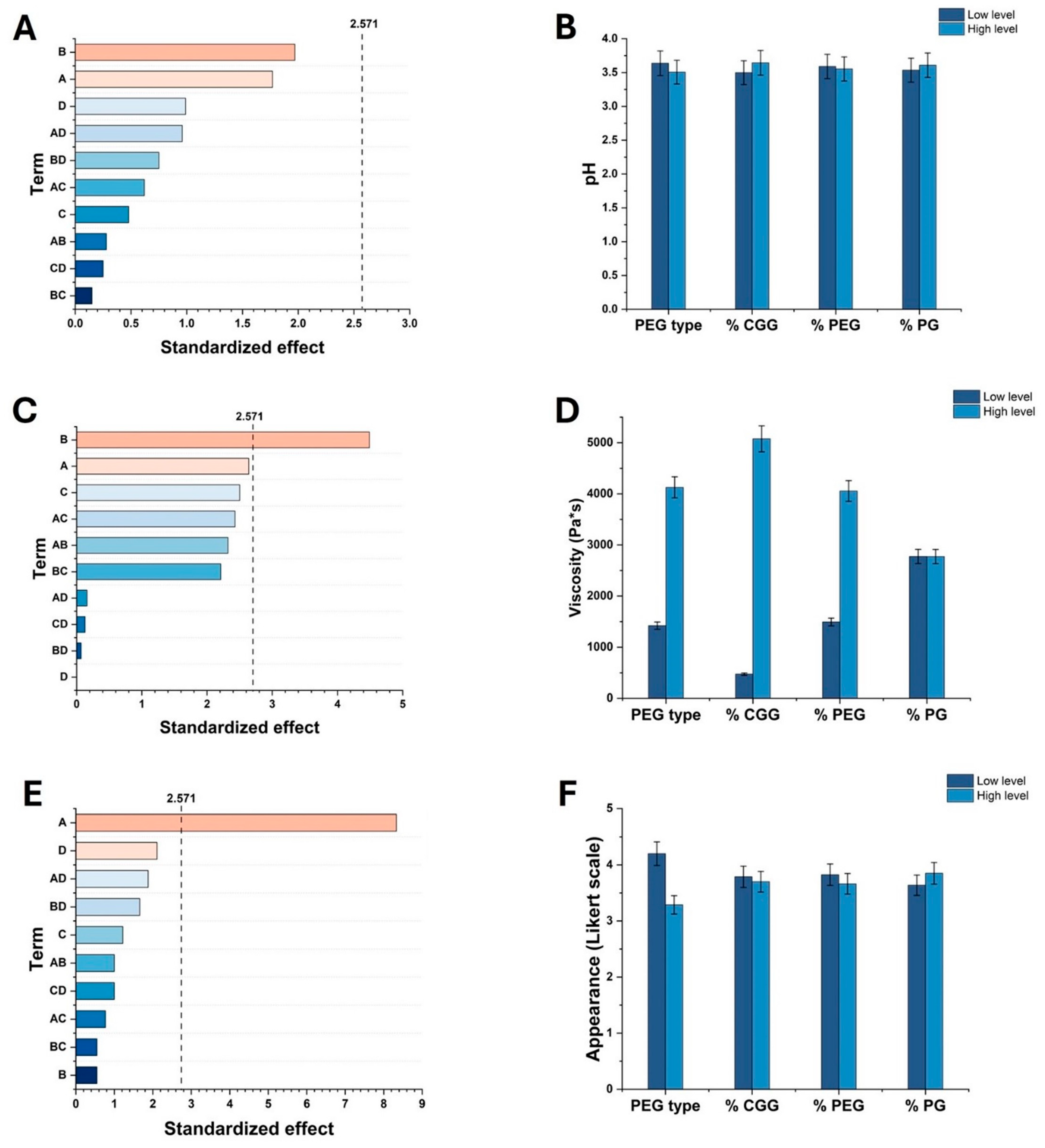
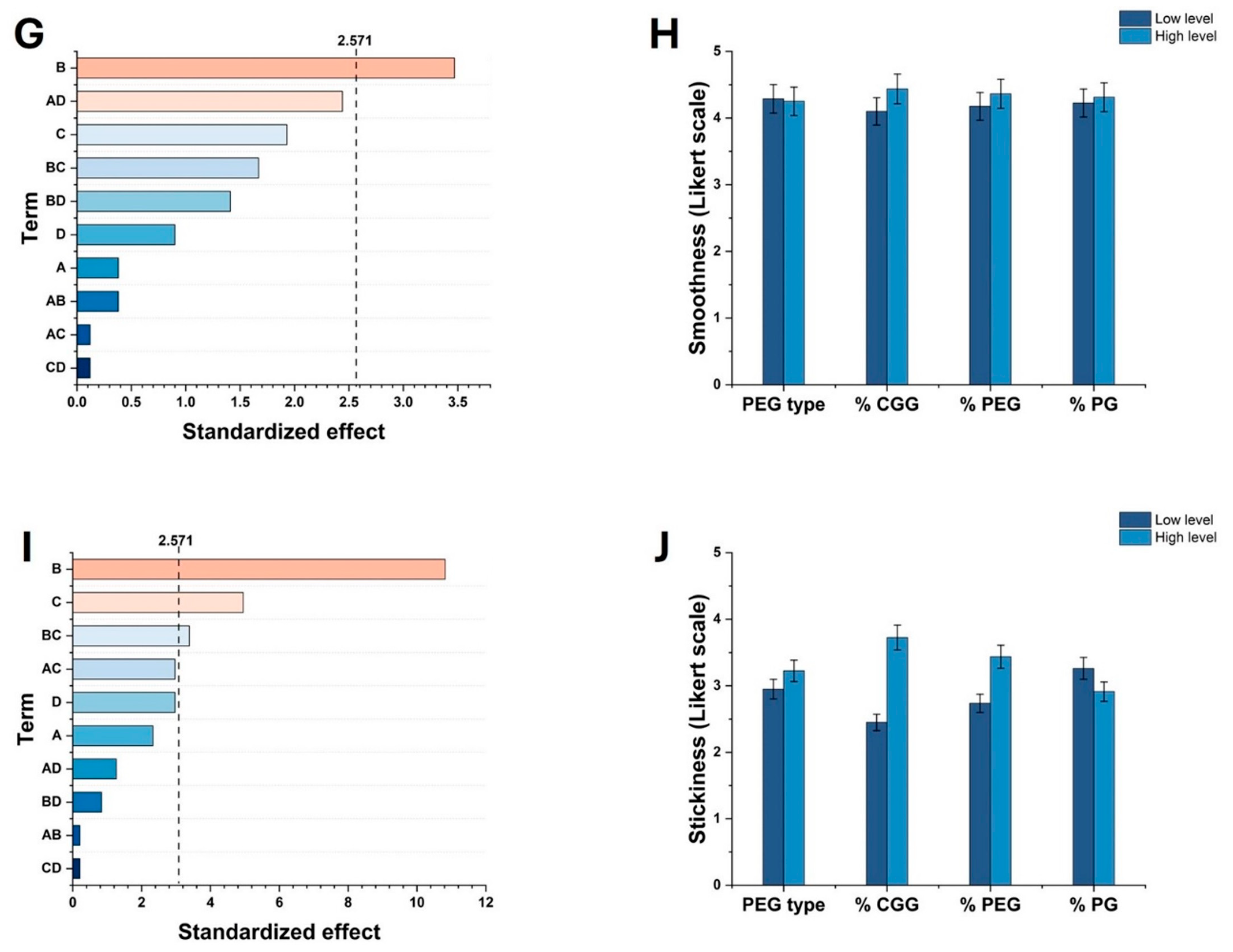
2.1. Effect of Factors on Key Properties of HGs
2.2. Optimization of the Formulation of HGs
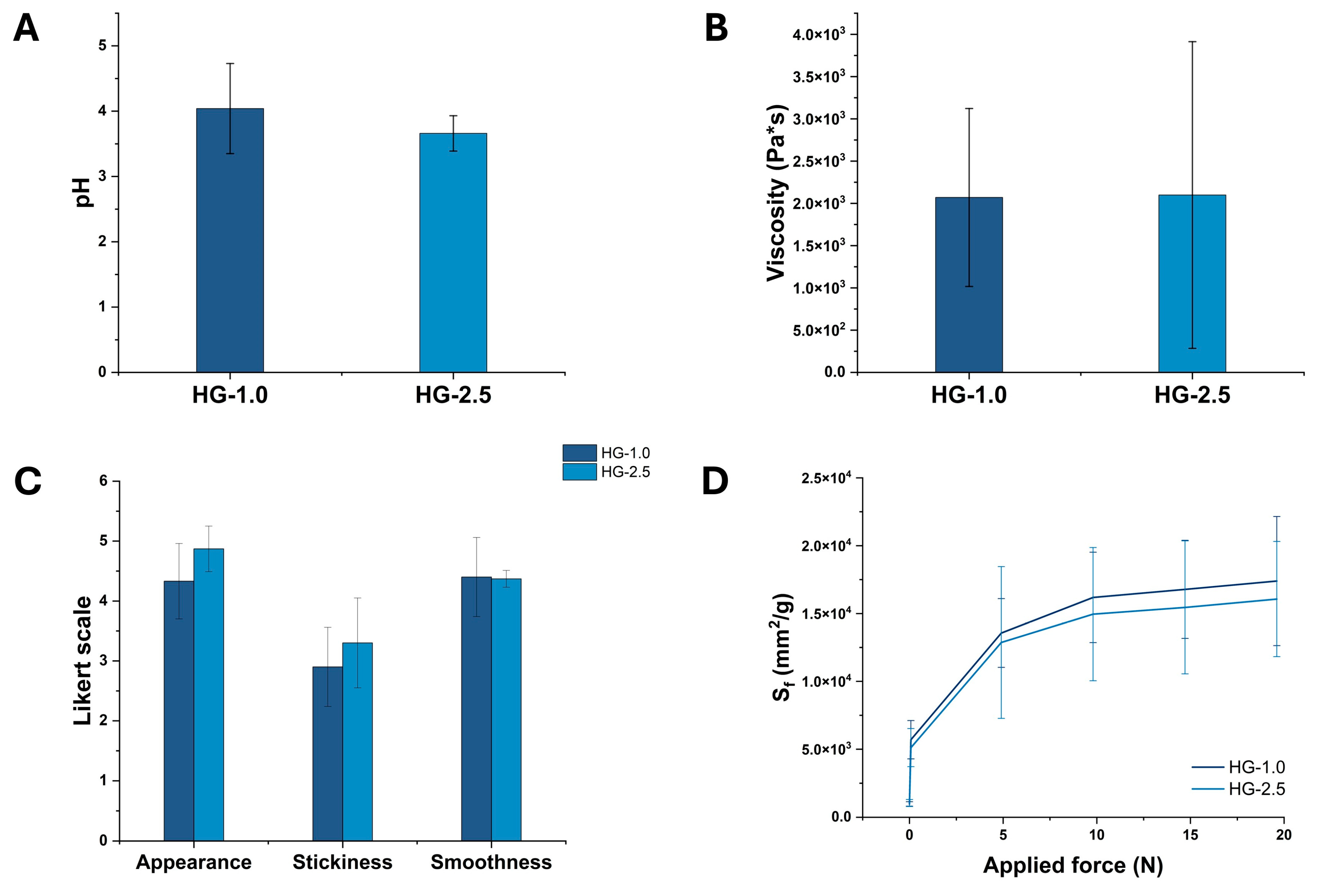
2.3. Evaluation of pH, Viscosity, Sensory, and Spreadability Analysis of Optimized HGs
2.4. Characterization of HGs
2.4.1. SEM Analysis
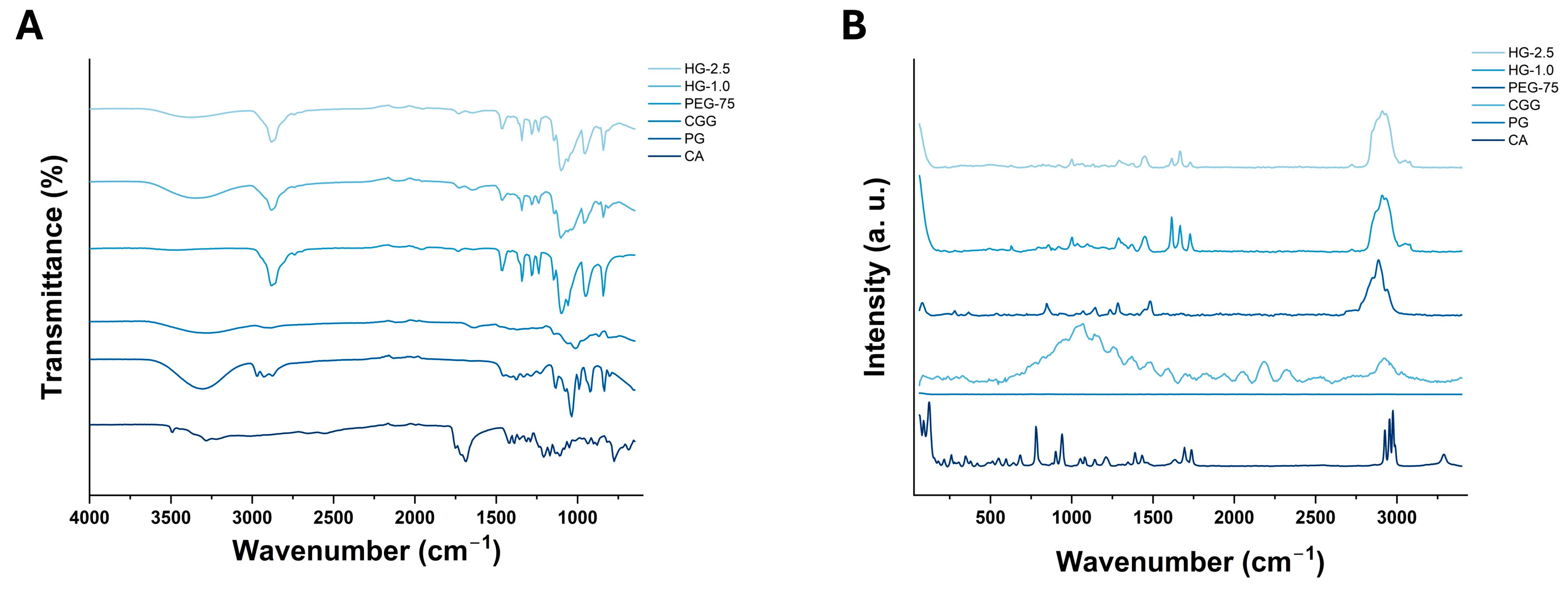
2.4.2. FTIR and Raman Spectroscopy Analysis
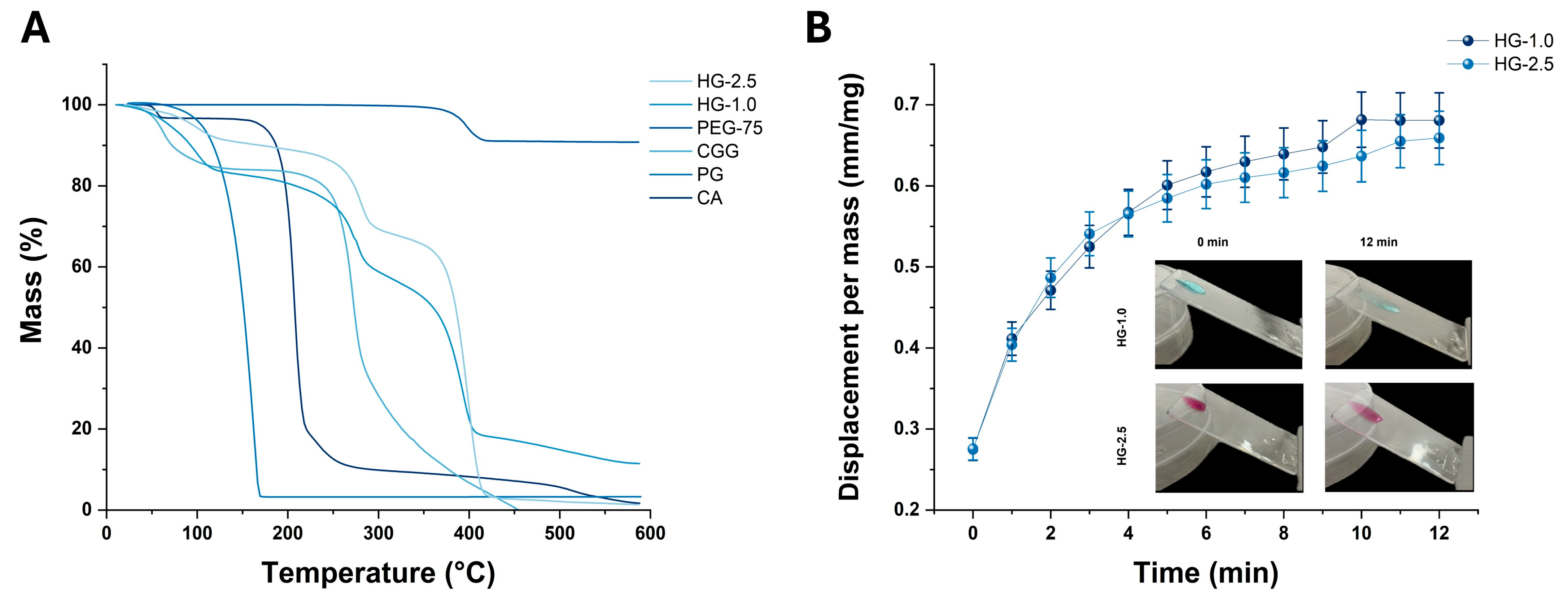
2.4.3. TGA and Mucoadhesive Analyses
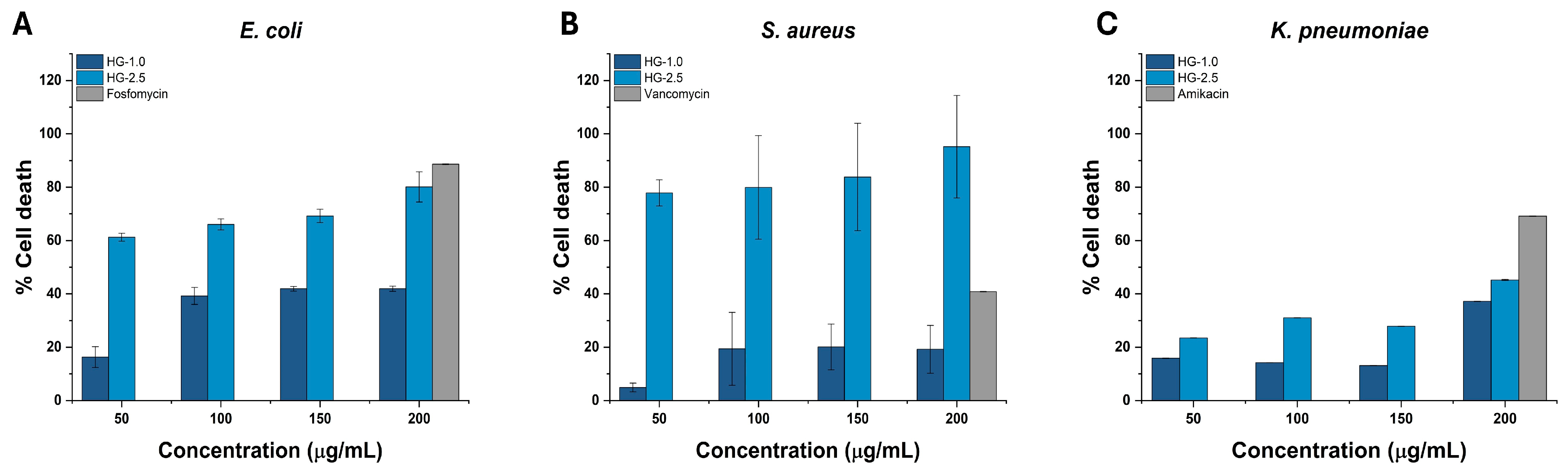
2.5. Antibacterial Activity
2.6. Toxicity Evaluation In Vivo
3. Conclusions
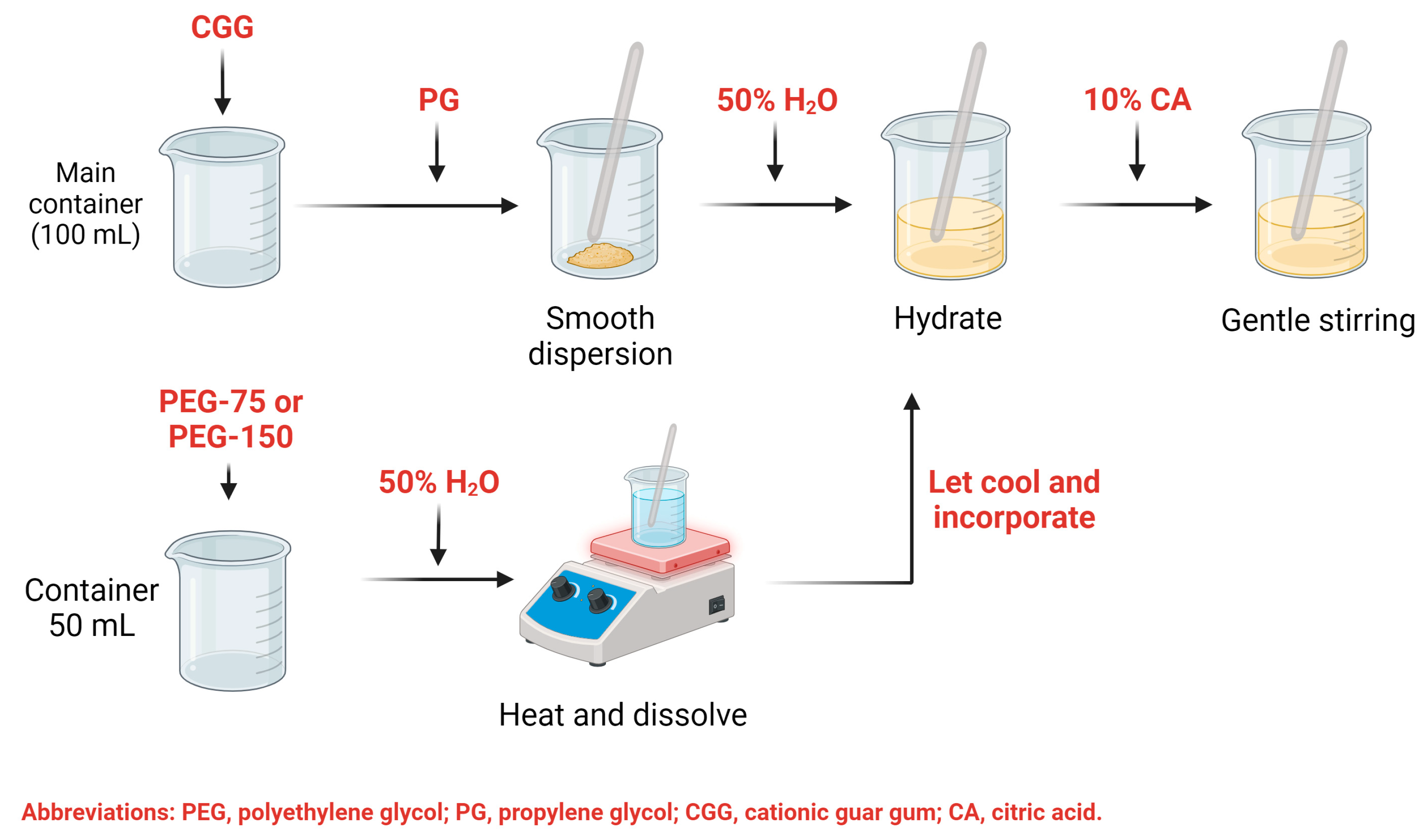
4. Materials and Methods
4.1. Materials
4.2. Fabrication of the Hydrogel through Design of Experiments
4.3. Viscosity, pH, and Sensory Analysis of HGs
4.4. Characterization of the Optimized Hydrogel
4.5. Spreadability
4.6. FTIR and Raman Spectroscopy Analyses
4.7. TGA Analysis
4.8. SEM Analysis
4.9. Mucoadhesive Properties Evaluation
4.10. Analysis of Antibacterial Activity
4.11. Evaluation of Toxicity in A. salina
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.Y.; Zhang, S.J.; Meng, H.F.; Xu, H.Q.; Guo, Z.X.; Yan, J.F.; Gao, J.L.; Niu, L.N.; Wang, S.L.; Jiao, K. DPSCs Regulate Epithelial-T Cell Interactions in Oral Submucous Fibrosis. Stem Cell Res. Ther. 2024, 15, 113. [Google Scholar] [CrossRef]
- Zhu, P.; Shao, R.; Xu, P.; Zhao, R.; Zhao, C.; Fei, J.; He, Y. Streptococcus Salivarius Ameliorates the Destructive Effect on the Epithelial Barrier by Inhibiting the Growth of Prevotella Melaninogenica via Metabolic Acid Production. Mol. Oral Microbiol. 2024; early view. [Google Scholar] [CrossRef]
- Azhamuthu, T.; Kathiresan, S.; Senkuttuvan, I.; Abulkalam Asath, N.A.; Ravichandran, P. Usnic Acid Attenuates 7,12-Dimethylbenz[a] Anthracene (DMBA) Induced Oral Carcinogenesis through Inhibiting Oxidative Stress, Inflammation, and Cell Proliferation in Male Golden Syrian Hamster Model. J. Biochem. Mol. Toxicol. 2024, 38, e23553. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Mansur, D.; Kuntari, W.L.; Syahnia, S.J.M.R.; Iskandar, B.; Arundina, I.; Liu, T.-W.; Lee, C.-K.; Ernawati, D.S. The Hydroxypropyl Methylcellulose-Sorbitol Thin Film Containing a Coconut Shell of Liquid Smoke for Treating Oral Ulcer. JCIS Open 2024, 15, 100119. [Google Scholar] [CrossRef]
- Roca, C.; Alkhateeb, A.A.; Deanhardt, B.K.; Macdonald, J.K.; Chi, D.L.; Wang, J.R.; Wolfgang, M.C. Saliva Sampling Method Influences Oral Microbiome Composition and Taxa Distribution Associated with Oral Diseases. PLoS ONE 2024, 19, e0301016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Umar, A.; Zhang, H.; Yang, L.; Huang, J.; Long, Y.; Yu, Z. Comparative Analysis of Microbial Composition and Functional Characteristics in Dental Plaque and Saliva of Oral Cancer Patients. BMC Oral Health 2024, 24, 411. [Google Scholar] [CrossRef]
- Rulff, H.; Schmidt, R.F.; Wei, L.-F.; Fentker, K.; Kerkhoff, Y.; Mertins, P.; Mall, M.A.; Lauster, D.; Gradzielski, M. Comprehensive Characterization of the Viscoelastic Properties of Bovine Submaxillary Mucin (BSM) Hydrogels and the Effect of Additives. Biomacromolecules 2024, 25, 4014–4029. [Google Scholar] [CrossRef]
- Safferthal, M.; Bechtella, L.; Zappe, A.; Vos, G.M.; Pagel, K. Labeling of Mucin-Type O-Glycans for Quantification Using Liquid Chromatography and Fluorescence Detection. ACS Meas. Sci. Au 2024, 4, 223–230. [Google Scholar] [CrossRef]
- Thobor, B.M.; Tilstra, A.; Mueller, B.; Haas, A.; Hehemann, J.-H.; Wild, C. Mucus Carbohydrate Composition Correlates with Scleractinian Coral Phylogeny. Sci. Rep. 2024, 14, 14019. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.M.; Ottaiano, G.Y.; de Souza Guedes, L.; de Moraes, M.A.; Beppu, M.M. Analyzing the Interactions and Miscibility of Silk Fibroin/Mucin Blends. J. Appl. Polym. Sci. 2024, 141, e55343. [Google Scholar] [CrossRef]
- Bechtella, L.; Chunsheng, J.; Fentker, K.; Ertürk, G.R.; Safferthal, M.; Polewski, Ł.; Götze, M.; Graeber, S.Y.; Vos, G.M.; Struwe, W.B.; et al. Ion Mobility-Tandem Mass Spectrometry of Mucin-Type O-Glycans. Nat. Commun. 2024, 15, 2611. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, S.; Xu, M.; He, Q.; Xie, J.; Wu, J.; Huang, Y. Transepithelial Transport of Nanoparticles in Oral Drug Delivery: From the Perspective of Surface and Holistic Property Modulation. Acta Pharm. Sin. B, 2024; in press. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Haddad, F.F.; dos Santos, A.M.; Scarim, C.B.; Ferreira, L.M.B.; Meneguin, A.B.; Chorilli, M.; Gremião, M.P.D. Chitosan Surface Modification Modulates the Mucoadhesive, Permeation and Anti-Angiogenic Properties of Gellan Gum/Bevacizumab Nanoparticles. Int. J. Biol. Macromol. 2024, 263, 130272. [Google Scholar] [CrossRef]
- Jiang, Z.; Huo, S.; Qiao, L.; Lin, P.; Fu, L.; Wu, Y.; Li, W.; Bian, C.; Li, Y.; Li, N.; et al. Inhalable Mucin-Permeable Nanomicelles Deliver Antibiotics for Effective Treatment of Chronic Pneumonia. J. Mater. Chem. B 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Xue, S.; Sheng, Y.; Gao, B.; Dang, Y.; Zhang, Y.; Zhang, G. β-Cyclodextrin/Dialdehyde Glucan-Coated Keratin Nanoparticles for Oral Delivery of Insulin. Int. J. Biol. Macromol. 2024, 276, 133805. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xing, T.; Chen, S.; Feng, J. A Shape Memory Hydrogel with Excellent Mechanical Properties, Water Retention Capacity, and Tunable Fluorescence for Dual Encryption. Small 2024, 20, 2305928. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, P.; Mahajan, S.; Banerjee, S.K.; Seth, K.; Banerjee, S. Synthesis and Characterization of a pH/Temperature-Dual Responsive Hydrogel with Promising Biocompatibility Features for Stimuli-Responsive 5-FU Delivery. J. Mater. Chem. B 2024, 12, 5098–5110. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Ferreiro-Figueiras, D.; Noya-Padin, V.; Giraldez, M.J.; Yebra-Pimentel, E. Assessing Neophyte Response to Daily Disposable Silicone Hydrogel Contact Lenses: A Randomised Clinical Trial Investigation over One Month. Ophthalmic Physiol. Opt. 2024, 44, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Armengol, E.; Grassiri, B.; Piras, A.M.; Zambito, Y.; Fabiano, A.; Laffleur, F. Ocular Antibacterial Chitosan-Maleic Acid Hydrogels: In Vitro and in Vivo Studies for a Promising Approach with Enhanced Mucoadhesion. Int. J. Biol. Macromol. 2024, 254, 127939. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Yan, S.; Zhu, Z.; Wang, Y.; He, D.; Wang, K.; Zhou, D.; Jia, Q.; Wang, X.; Zhang, B.; et al. Ureido-Ionic Liquid Mediated Conductive Hydrogel: Superior Integrated Properties for Advanced Biosensing Applications. Adv. Sci. 2024, 2401869. [Google Scholar] [CrossRef]
- Han, Z.; Xiong, J.; Jin, X.; Dai, Q.; Han, M.; Wu, H.; Yang, J.; Tang, H.; He, L. Advances in Reparative Materials for Infectious Bone Defects and Their Applications in Maxillofacial Regions. J. Mater. Chem. B 2024, 12, 842–871. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Bai, H.; Niu, Y.; Wu, Y. Characterization and Application of in Situ Curcumin/ZNP Hydrogels for Periodontitis Treatment. BMC Oral Health 2024, 24, 395. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, C.; Shao, D.; Li, W.; Hu, X.; Tian, J.; Li, L.; Ding, S.; Zhou, C.; Lu, L. A Tough Janus Hydrogel Patch with Strong Wet Adhesion and Self-Debonding for Oral Ulcer Treatment. Chem. Mater. 2024, 36, 4976–4989. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, M.; Wang, P.; Wei, H.; Wei, X.; Wang, D.; Hao, Y.; Wang, Y.; Chen, H. Effects of a Semi-Interpenetrating Network Hydrogel Loaded with Oridonin and DNase-I on the Healing of Chemoradiotherapy-Induced Oral Mucositis. Biomater. Sci. 2024. [Google Scholar] [CrossRef]
- Kang, Y.; Xiong, Y.; Lu, B.; Wang, Y.; Zhang, D.; Feng, J.; Chen, L.; Zhang, Z. Application of In Situ Mucoadhesive Hydrogel with Anti-Inflammatory and Pro-Repairing Dual Properties for the Treatment of Chemotherapy-Induced Oral Mucositis. ACS Appl. Mater. Interfaces 2024, 16, 35949–35963. [Google Scholar] [CrossRef]
- Carrascal-Hernandez, D.C.; Mendez-Lopez, M.; Insuasty, D.; García-Freites, S.; Sanjuan, M.; Márquez, E. Molecular Recognition between Carbon Dioxide and Biodegradable Hydrogel Models: A Density Functional Theory (DFT) Investigation. Gels 2024, 10, 386. [Google Scholar] [CrossRef]
- Liu, Z.; Basem, A.; Mostafa, L.; Jasim, D.J.; Al-Rubaye, A.H.; Salahshour, S.; Hekmatifar, M.; Esmaeili, S. Investigating the Effect of pH on the Swelling Process, Mechanical and Thermal Attributes of Polyacrylamide Hydrogel Structure: A Molecular Dynamics Study. Case Stud. Therm. Eng. 2024, 55, 104148. [Google Scholar] [CrossRef]
- Miranda, P.; Castro, A.; Díaz, P.; Minini, L.; Ferraro, F.; Paulsen, E.; Faccio, R.; Pardo, H. Novel Thermosensitive and Mucoadhesive Nasal Hydrogel Containing 5-MeO-DMT Optimized Using Box-Behnken Experimental Design. Polymers 2024, 16, 2148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, X.; Li, J.; Zheng, H.; Zhang, Q. Nanoporous, Ultrastiff, and Transparent Plastic-like Polymer Hydrogels Enabled by Hydrogen Bonding-Induced Self-Assembly. ACS Appl. Mater. Interfaces 2024, 16, 42783–42793. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, K.; Szopa, D.; Pstrowska, K.; Wróbel, P.; Witek-Krowiak, A. Comparative Analysis of Crosslinking Methods and Their Impact on the Physicochemical Properties of SA/PVA Hydrogels. Materials 2024, 17, 1816. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Yang, Z.; Jiao, J.; Zhao, Z.; Liu, Y. “All-in-One” Self-Healing and Injectable Cationic Guar Gum Hydrogel Dressing Functionalized by Bioactive Complexes Composed of Natural Small Molecules. Int. J. Biol. Macromol. 2024, 275, 133517. [Google Scholar] [CrossRef]
- Chopra, L.; Sharma, A.; Chohan, J.S.; Upadhyay, V.V.; Singh, R.; Sharma, S.; Dwivedi, S.P.; Kumar, A.; Tag-Eldin, E.M. Synthesis and Characterizations of Super Adsorbent Hydrogel Based on Biopolymer, Guar Gum-Grafted-Poly (Hydroxyethyl Methacrylate) (Gg-g-Poly (HEMA)) for the Removal of Bismarck Brown Y Dye from Aqueous Solution. Int. J. Biol. Macromol. 2024, 256, 128518. [Google Scholar] [CrossRef]
- Bai, Y.; Lang, S.; Du, Y.; Hu, Q.; Li, X.; Liu, G. Metallic-Polyphenolic Nanoparticles Reinforced Cationic Guar Gum Hydrogel for Effectively Treating Burn Wound. Macromol. Biosci. 2024, 24, 2300396. [Google Scholar] [CrossRef]
- Hyldbakk, A.; Hansen, T.; Hak, S.; Borgos, S.E.F. Polyethylene Glycol (PEG) as a Broad Applicability Marker for LC–MS/MS-Based Biodistribution Analysis of Nanomedicines. J. Control Release 2024, 366, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.K.d.P.; Lima, A.K.M.; Miguel, T.B.A.R.; Filho, A.G.S.; Ferreira, O.P.; Pontes, M.d.S.; Grillo, R.; Miguel, E.d.C. Assessing Toxicity Mechanism of Silver Nanoparticles by Using Brine Shrimp (Artemia salina) as Model. Chemosphere 2024, 347, 140673. [Google Scholar] [CrossRef] [PubMed]
- Casiano-Muñiz, I.M.; Ortiz-Román, M.I.; Lorenzana-Vázquez, G.; Román-Velázquez, F.R. Synthesis, Characterization, and Ecotoxicology Assessment of Zinc Oxide Nanoparticles by In Vivo Models. Nanomaterials 2024, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, L.; Jenifer, M.A.; Mukherjee, A. Influence of Algal-Extracellular Polymeric Substances (EPS) on the Pristine and Combined Toxicity of TiO2 NPs and PSNPs in Artemia Salina: Eco-Corona Enhances the Toxic Effects. Ecotoxicol. Environ. Saf. 2024, 282, 116760. [Google Scholar] [CrossRef]
- Araya, X.; Okumu, M.; Durán, G.; Gómez, A.; Gutiérrez, J.M.; León, G. Assessment of the Artemia salina Toxicity Assay as a Substitute of the Mouse Lethality Assay in the Determination of Venom-Induced Toxicity and Preclinical Efficacy of Antivenom. Toxicon X 2024, 22, 100195. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, M.; Liang, G.; Li, S.; Zhao, C.; Jing, K.; Feng, S. Prevalence and Clinical Markers of Herpes Simplex Virus Infection in Oral Lesions of Bullous Pemphigoid. Front. Immunol. 2024, 15, 1387503. [Google Scholar] [CrossRef]
- Ouchi, C.; Hasebe, A.; Sakata, K.; Sato, J.; Yamazaki, Y.; Ohga, N.; Kitagawa, Y. Genotypes and Virulence-Related Activities of Candida albicans Derived from Oral Cavity of Patients in Hokkaido. Arch. Oral Biol. 2024, 157, 105827. [Google Scholar] [CrossRef]
- Xing, Y.; Shi, H.; Wang, C.; Yang, Y. Clinical Features and Risk Factors for Sjogren’s Syndrome Patients Suffering from Oral Candidiasis in Shanxi, China. BMC Oral Health 2024, 24, 812. [Google Scholar] [CrossRef]
- Reeve, M.P.; Vehviläinen, M.; Luo, S.; Ritari, J.; Karjalainen, J.; Gracia-Tabuenca, J.; Mehtonen, J.; Padmanabhuni, S.S.; Kolosov, N.; Artomov, M.; et al. Oral and Non-Oral Lichen Planus Show Genetic Heterogeneity and Differential Risk for Autoimmune Disease and Oral Cancer. Am. J. Hum. Genet. 2024, 111, 1047–1060. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamashita, Y.; Fujio, M.; Hanai, Y.; Koizumi, H.; Ogawa-Momohara, M.; Takeichi, T.; Muro, Y.; Akiyama, M. Combined Oral Lichen Planus and Oral Lichenoid Contact Hypersensitivity Reaction in a Patient with Hashimoto’s Thyroiditis. Oral Oncol. Rep. 2024, 10, 100356. [Google Scholar] [CrossRef]
- Salehi, M.; Saeedi, M.; Negarandeh, R.; Savabi, A.; Lotfizadeh, A.; Hosseinnataj, A.; Molania, T. Evaluation of Caffeic Acid Mucoadhesive Tablets on Minor Recurrent Aphthous Stomatitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. BMC Oral Health 2024, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Lešić, S.; Ivanišević, Z.; Špiljak, B.; Tomas, M.; Šoštarić, M.; Včev, A. The Impact of Vitamin Deficiencies on Oral Manifestations in Children. Dent. J. 2024, 12, 109. [Google Scholar] [CrossRef]
- Zhu, J.; He, Y.; Feng, H.; Wang, Y.; Ge, Z. B12 Deficiency-Related Glossitis Is Highly Associated with High Gastrin-17 and Low Pepsinogen I. J. Oral Pathol. Med. 2024, 53, 142–149. [Google Scholar] [CrossRef]
- Richbourg, N.; Wechsler, M.E.; Rodriguez-Cruz, J.J.; Peppas, N.A. Model-Based Modular Hydrogel Design. Nat. Rev. Bioeng. 2024, 2, 575–587. [Google Scholar] [CrossRef]
- Wancura, M.; Nkansah, A.; Robinson, A.; Toubbeh, S.; Talanker, M.; Jones, S.; Cosgriff-Hernandez, E. PEG-Based Hydrogel Coatings: Design Tools for Biomedical Applications. Ann. Biomed. Eng. 2024, 52, 1804–1815. [Google Scholar] [CrossRef]
- Verger, A.; Kichou, H.; Huang, N.; Perse, X.; Ardeza, I.-M.; Pradel, C.; Goncalves Martins Da Conceicao, R.; Atanasova, B.; Legrand, F.-X.; Despres, A.; et al. Effects of Hydrophilic Natural Deep Eutectic Solvents on the Rheological, Textural, and Sensory Properties of Carboxymethylcellulose-Based Cosmetic Hydrogels. ACS Sustain. Chem. Eng. 2024, 12, 7187–7199. [Google Scholar] [CrossRef]
- Wang, P.; Li, R.; Ma, J.; Zhang, W.; Shen, H.; Ren, Y.; Zhang, X.; Li, S.; Chi, B. Facilitating Safe and Sustained Submucosal Lift through an Endoscopically Injectable Shear-Thinning Carboxymethyl Starch Sodium Hydrogel. Carbohydr. Polym. 2024, 336, 122128. [Google Scholar] [CrossRef]
- Gonella, S.; Domingues, M.F.; Miguel, F.; Moura, C.S.; Rodrigues, C.A.V.; Ferreira, F.C.; Silva, J.C. Fabrication and Characterization of Porous PEGDA Hydrogels for Articular Cartilage Regeneration. Gels 2024, 10, 422. [Google Scholar] [CrossRef]
- Permana, A.D.; Asri, R.M.; Amir, M.N.; Himawan, A.; Arjuna, A.; Juniarti, N.; Utami, R.N.; Mardikasari, S.A. Development of Thermoresponsive Hydrogels with Mucoadhesion Properties Loaded with Metronidazole Gel-Flakes for Improved Bacterial Vaginosis Treatment. Pharmaceutics 2023, 15, 1529. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of Mucoadhesion of Poly(Acrylic Acid) Hydrogels. Pharm. Res. 1987, 04, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, K.; Iyer, K.A.; Diaz, L.L.; Bird, R.; Maind, A.; Zhou, Q.A. Navigating Antibacterial Frontiers: A Panoramic Exploration of Antibacterial Landscapes, Resistance Mechanisms, and Emerging Therapeutic Strategies. ACS Infect. Dis. 2024, 10, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Traczewski, M.M.; Ambler, J.E.; Schuch, R. Determination of MIC Quality Control Parameters for Exebacase, a Novel Lysin with Antistaphylococcal Activity. J. Clin. Microbiol. 2021, 59, e03117-20. [Google Scholar] [CrossRef]
- Carrion, C.C.; Nasrollahzadeh, M.; Sajjadi, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Lignin, Lipid, Protein, Hyaluronic Acid, Starch, Cellulose, Gum, Pectin, Alginate and Chitosan-Based Nanomaterials for Cancer Nanotherapy: Challenges and Opportunities. Int. J. Biol. Macromol. 2021, 178, 193–228. [Google Scholar] [CrossRef]
- Liang, L.; Su, Q.; Ma, Y.; Zhao, S.; Zhang, H.; Gao, X. Research Progress on the Polysaccharide Extraction and Antibacterial Activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Qiao, Y.; Shen, Y.; Jiang, H.; Li, D.; Li, B. Structural Characterization, Antioxidant and Antibacterial Activity of Three Pectin Polysaccharides from Blueberry. Int. J. Biol. Macromol. 2024, 262, 129707. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Zhang, H.; Wang, J.; Fu, Q.; Qiao, H.; Wang, Q. Insight into Antibacterial Mechanism of Polysaccharides: A Review. LWT 2021, 150, 111929. [Google Scholar] [CrossRef]
- Russell, S.K.; Harrison, J.K.; Olson, B.S.; Lee, H.J.; O’Brien, V.P.; Xing, X.; Livny, J.; Yu, L.; Roberson, E.D.O.; Bomjan, R.; et al. Uropathogenic Escherichia Coli Infection-Induced Epithelial Trained Immunity Impacts Urinary Tract Disease Outcome. Nat. Microbiol. 2023, 8, 875–888. [Google Scholar] [CrossRef]
- Yang, R.; Chen, J.; Qu, X.; Liu, H.; Wang, X.; Tan, C.; Chen, H.; Wang, X. Interleukin-22 Contributes to Blood–Brain Barrier Disruption via STAT3/VEGFA Activation in Escherichia Coli Meningitis. ACS Infect. Dis. 2024, 10, 988–999. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, L.; Zhuge, Z.; Li, W.; Zheng, Y.; Mai, J.; Lin, Z.; Lin, J. Risk Factors Associated with Multi-Drug Resistance in Neonatal Sepsis Caused by Escherichia coli. Infect. Drug Resist. 2023, 16, 2097–2106. [Google Scholar] [CrossRef]
- Powers, J.E.; Mureithi, M.; Mboya, J.; Campolo, J.; Swarthout, J.M.; Pajka, J.; Null, C.; Pickering, A.J. Effects of High Temperature and Heavy Precipitation on Drinking Water Quality and Child Hand Contamination Levels in Rural Kenya. Environ. Sci. Technol. 2023, 57, 6975–6988. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, S.; Zhang, L.; Wu, H.; Wu, H. Characteristics of Antibiotic Resistance Mechanisms and Genes of Klebsiella Pneumoniae. Open Med. 2023, 18, 20230707. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Dong, H.; Wang, M.; Qin, S.; Chen, S.; Li, R. Emergence of Tet(X4)-Positive Hypervirulent Klebsiella Pneumoniae of Food Origin in China. LWT 2023, 173, 114280. [Google Scholar] [CrossRef]
- Piewngam, P.; Otto, M. Staphylococcus aureus Colonisation and Strategies for Decolonisation. Lancet Microbe 2024, 5, e606–e618. [Google Scholar] [CrossRef]
- Serrão, C.; Marques-Santos, L.F. The Genus Artemia, the Nanoplastics, the Microplastics, and Their Toxic Effects: A Review. Environ. Sci. Pollut. Res. 2023, 30, 83025–83050. [Google Scholar] [CrossRef]
- Magalhães, L.S.S.M.; Andrade, D.B.; Bezerra, R.D.S.; Morais, A.I.S.; Oliveira, F.C.; Rizzo, M.S.; Silva-Filho, E.C.; Lobo, A.O. Nanocomposite Hydrogel Produced from PEGDA and Laponite for Bone Regeneration. J. Funct. Biomater. 2022, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.B.; Soares, L.L.S.; Cardoso, F.L.A.; Lima, I.S.; Silva, J.G.V.; Carvalho, M.A.M.; Fonseca, M.G.; Brito, G.d.C.; Santos, F.E.P.; Osajima, J.A.; et al. Hydrogel Based on Nanoclay and Gelatin Methacrylate Polymeric Matrix as a Potential Osteogenic Application. J. Funct. Biomater. 2023, 14, 74. [Google Scholar] [CrossRef]
- Sahar, F.; Riaz, A.; Malik, N.S.; Gohar, N.; Rasheed, A.; Tulain, U.R.; Erum, A.; Barkat, K.; Badshah, S.F.; Shah, S.I. Design, Characterization and Evaluation of Gelatin/Carboxymethyl Cellulose Hydrogels for Effective Delivery of Ciprofloxacin. Polym. Bull. 2023, 80, 12271–12299. [Google Scholar] [CrossRef]


| Factors | Levels | Response Variables | |
|---|---|---|---|
| Low | High | Viscosity, pH, sensory analysis (smoothness, appearance, stickiness) | |
| PEG concentration | 1% | 2.5% | |
| CGG concentration | 0.5% | 1% | |
| PG concentration | 2% | 5% | |
| PEG type | PEG-75 Lanolin | PEG-150 Distearate | |
| Factor (F) | Optimized Level by Minitab® XVII Software | |
|---|---|---|
| HG-1.0 | HG-2.5 | |
| [PEG] (% w/v) | 1 | 2.5 |
| [CGG] (% w/v) | 1 | 1 |
| [PG] (% w/v) | 5 | 5 |
| PEG type | PEG-75 Lanolin | PEG-75 Lanolin |
| Sample | E. coli | S. aureus | K. pneumoniae |
|---|---|---|---|
| HG-1.0 | 471.81 | 972.74 | 681.45 |
| HG-2.0 | 299.66 | 787.91 | 593.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Rendón, A.G.; Mejía-Méndez, J.L.; López-Mena, E.R.; Bernal-Chávez, S.A. Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery. Gels 2024, 10, 574. https://doi.org/10.3390/gels10090574
Pardo-Rendón AG, Mejía-Méndez JL, López-Mena ER, Bernal-Chávez SA. Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery. Gels. 2024; 10(9):574. https://doi.org/10.3390/gels10090574
Chicago/Turabian StylePardo-Rendón, Ana G., Jorge L. Mejía-Méndez, Edgar R. López-Mena, and Sergio A. Bernal-Chávez. 2024. "Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery" Gels 10, no. 9: 574. https://doi.org/10.3390/gels10090574
APA StylePardo-Rendón, A. G., Mejía-Méndez, J. L., López-Mena, E. R., & Bernal-Chávez, S. A. (2024). Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery. Gels, 10(9), 574. https://doi.org/10.3390/gels10090574







