Sustainable Jam with Apple Pomace: Gelling, Rheology, and Composition Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Jam Production and Control Selection
2.2. Jam Production and Optimization
2.3. Jam Characterization
3. Conclusions
4. Materials and Methods
4.1. Preliminary Tests for Jam Production and Control Selection
4.2. Optimization of the Gelation Process
4.3. Rheology Behavior
4.4. Texture Profile
4.5. Chemical Characterization
4.5.1. Nutritional Composition
4.5.2. pH and Soluble Solids (°Brix)
4.5.3. Color
4.5.4. Total Phenolic Compounds (TPC)
4.5.5. Antioxidant Activity
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.; Tsai, M.L.; Nargotra, P.; Chen, C.W.; Kuo, C.H.; Sun, P.P.; Dong, C.D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste; European Union: Maastricht, The Netherlands, 2024; ISBN 9789280741391. [Google Scholar]
- Pathania, S.; Kaur, N. Utilization of Fruits and Vegetable By-Products for Isolation of Dietary Fibres and Its Potential Application as Functional Ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Pintado, M.; Lopes da Silva, J.A. Potential Nutritional and Functional Improvement of Extruded Breakfast Cereals Based on Incorporation of Fruit and Vegetable By-Products—A Review. Trends Food Sci. Technol. 2022, 125, 136–153. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef]
- Pascoalino, L.A.; Reis, F.S.; Prieto, M.A.; Barreira, J.C.M.; Ferreira, I.C.F.R.; Barros, L. Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds. Molecules 2021, 26, 2624. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical Properties of Pectin from Malus Domestica ‘Fălticeni’ Apple Pomace as Affected by Non-Conventional Extraction Techniques. Food Hydrocoll. 2020, 100, 105358. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging Concepts in the Nutraceutical and Functional Properties of Pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Yang, H.; Chou, L.Y.; Hua, C.C. Effects of Calcium and PH on Rheological Thermal Resistance of Composite Xanthan Gum and High-Methoxyl Apple Pectin Matrices Featuring Dysphagia-Friendly Consistency. Foods 2024, 13, 90. [Google Scholar] [CrossRef]
- Padma Ishwarya, S.; Sandhya, R.; Nisha, P. Advances and Prospects in the Food Applications of Pectin Hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef] [PubMed]
- Razak, R.A.; Karim, R.; Sulaiman, R.; Hussain, N. Effects of Different Types and Concentration of Hydrocolloids on Mango Filling. Int. Food Res. J. 2018, 25, 1109–1119. [Google Scholar]
- Sorour, M.A.; Abdou, E.S.; Bo Samri, S.M. Rheological Properties of Apricot Jam through Piping. J. TESCE Trans. Egypt. Soc. Chem. Eng. 2009, 35, 17–35. [Google Scholar]
- Cervera-Chiner, L.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Sugar Replacement by Non-Centrifugal Sugar on Physicochemical, Antioxidant and Sensory Properties of Strawberry and Kiwifruit Functional Jams. Heliyon 2021, 7, e05963. [Google Scholar] [CrossRef]
- Basu, S.; Shivhare, U.S. Rheological, Textural, Microstructural, and Sensory Properties of Sorbitol-Substituted Mango Jam. Food Bioprocess Technol. 2013, 6, 1401–1413. [Google Scholar] [CrossRef]
- Mohammadi-Moghaddam, T.; Firoozzare, A.; Daryadar, S.; Rahmani, Z. Black Plum Peel Jam: Physicochemical Properties, Sensory Attributes, and Antioxidant Capacity. Int. J. Food Prop. 2020, 23, 1737–1747. [Google Scholar] [CrossRef]
- Rong, L.; Shen, M.; Wen, H.; Xiao, W.; Li, J.; Xie, J. Effects of Xanthan, Guar and Mesona Chinensis Benth Gums on the Pasting, Rheological, Texture Properties and Microstructure of Pea Starch Gels. Food Hydrocoll. 2022, 125, 107391. [Google Scholar] [CrossRef]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of Formulation Variables on Rheology, Texture, Colour, and Acceptability of Apple Jelly: MODELLING and Optimization. LWT Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Brunchi, C.E.; Morariu, S.; Iftime, M.M.; Stoica, I. Xanthan Gum in Solution and Solid-like State: Effect of Temperature and Polymer Concentration. J. Mol. Liq. 2023, 387, 122600. [Google Scholar] [CrossRef]
- Nourmohammadi, A.; Ahmadi, E.; Heshmati, A. Optimization of Physicochemical, Textural, and Rheological Properties of Sour Cherry Jam Containing Stevioside by Using Response Surface Methodology. Food Sci. Nutr. 2021, 9, 2483–2496. [Google Scholar] [CrossRef]
- Rocha Parra, A.F.; Ribotta, P.D.; Ferrero, C. Apple Pomace in Gluten-Free Formulations: Effect on Rheology and Product Quality. Int. J. Food Sci. Technol. 2015, 50, 682–690. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Effects of Addition of Different Fibers on Rheological Characteristics of Cake Batter and Quality of Cakes. J. Food Sci. Technol. 2018, 55, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Kırbaş, Z.; Kumcuoglu, S.; Tavman, S. Effects of Apple, Orange and Carrot Pomace Powders on Gluten-Free Batter Rheology and Cake Properties. J. Food Sci. Technol. 2019, 56, 914–926. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, N.; Ktenioudaki, A.; Smyth, T.P.; McLoughlin, P.; Doran, L.; Auty, M.A.E.; Arendt, E.; Gallagher, E. Physicochemical Assessment of Two Fruit By-Products as Functional Ingredients: Apple and Orange Pomace. J. Food Eng. 2015, 153, 89–95. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Genovese, D.B. Pectin Gels Enriched with Dietary Fibre for the Development of Healthy Confectionery Jams. Food Technol. Biotechnol. 2018, 56, 441–453. [Google Scholar] [CrossRef]
- Dervisi, P.; Lamb, J.; Zabetakis, I. High Pressure Processing in Jam Manufacture: Effects on Textural and Colour Properties. Food Chem. 2001, 73, 85–91. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Ma, Y.; Bi, J. The Role of Amide Groups in the Mechanism of Acid-Induced Pectin Gelation: A Potential PH-Sensitive Hydrogel Based on Hydrogen Bond Interactions. Food Hydrocoll. 2023, 141, 108741. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, Y.; Yoo, B. Rheological and Pasting Properties of Naked Barley Flour as Modified by Guar, Xanthan, and Locust Bean Gums. Prev. Nutr. Food Sci. 2016, 21, 367–372. [Google Scholar] [CrossRef]
- Prakash, K.; Satyanarayana, V.M.; Nagiat, H.T.; Fathi, A.H.; Shanta, A.K.; Prameela, A.R. Formulation Development and Evaluation of Novel Oral Jellies of Carbamazepine Using Pectin, Guar Gum, and Gellan Gum. Asian J. Pharm. 2014, 8, 241–249. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, K.; Descallar, F.B.A.; Li, A.; Yang, X.; Yang, H. Gelation Behaviors of Some Special Plant-Sourced Pectins: A Review Inspired by Examples from Traditional Gel Foods in China. Trends Food Sci. Technol. 2022, 126, 26–40. [Google Scholar] [CrossRef]
- Badin, E.E.; Rossi, Y.E.; Montenegro, M.A.; Ibarz, A.; Ribotta, P.D.; Lespinard, A.R. Thermal Processing of Raspberry Pulp: Effect on the Color and Bioactive Compounds. Food Bioprod. Process. 2020, 124, 469–477. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R.; Da Silva, G.R.B. Effect of Processing on the Preservation of Bioactive Compounds in Traditional and Exotic Fruits: A Review. J. Food Bioact. 2022, 18, 18308. [Google Scholar] [CrossRef]
- Shinwari, K.J.; Rao, P.S. Development of a Reduced-Calorie High Pressure Processed Sapodilla (Manilkara zapota L.) Jam Based on Rheological, Textural, and Sensory Properties. J. Food Sci. 2020, 85, 2699–2710. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; da Silva, E.G.P.; dos Santos, W.N.L.; Quintella, C.M.; David, J.M.; de Andrade, J.B.; Breitkreitz, M.C.; Jardim, I.C.S.F.; Neto, B.B. Statistical Designs and Response Surface Techniques for the Optimization of Chromatographic Systems. J. Chromatogr. A 2007, 1158, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Benali, S.; Benamara, S.; Bigan, M.; Madani, K. Feasibility Study of Date (Phoenix dactylifera L.) Fruit Syrup-Based Natural Jelly Using Central Composite Design. J. Food Sci. Technol. 2015, 52, 4975–4984. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Optimization of Ultrasound Assisted Extraction of Polyphenols from Meghalayan Cherry Fruit (Prunus nepalensis) Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Approach. J. Food Meas. Charact. 2021, 15, 119–133. [Google Scholar] [CrossRef]
- Šumić, Z.; Vakula, A.; Tepić, A.; Čakarević, J.; Vitas, J.; Pavlić, B. Modeling and Optimization of Red Currants Vacuum Drying Process by Response Surface Methodology (RSM). Food Chem. 2016, 203, 465–475. [Google Scholar] [CrossRef]
- Fernandes, A.; Simões, S.; Ferreira, I.M.P.L.V.O.; Alegria, M.J.; Mateus, N.; Raymundo, A.; de Freitas, V. Upcycling Rocha Do Oeste Pear Pomace as a Sustainable Food Ingredient: Composition, Rheological Behavior and Microstructure Alone and Combined with Yeast Protein Extract. Molecules 2023, 28, 179. [Google Scholar] [CrossRef]
- Falcão, A.P.; Chaves, E.S.; Falcão, L.D.; Gauche, C.; Barreto, P.L.M.; Bordignon-Luiz, M.T. Rheological Behavior and Color Stability of Anthocyanins from Merlot (Vitis vinifera L.) and Bordo (Vitis labrusca L.) Grapes in a Jam Model System. Food Sci. Technol. 2009, 29, 857–862. [Google Scholar] [CrossRef]
- Khouryieh, H.A.; Herald, T.J.; Aramouni, F.; Alavi, S. Influence of Mixing Temperature on Xanthan Conformation and Interaction of Xanthan-Guar Gum in Dilute Aqueous Solutions. Food Res. Int. 2006, 39, 964–973. [Google Scholar] [CrossRef]
- Barbieri, S.F.; de Oliveira Petkowicz, C.L.; de Godoy, R.C.B.; de Azeredo, H.C.M.; Franco, C.R.C.; Silveira, J.L.M. Pulp and Jam of Gabiroba (Campomanesia xanthocarpa Berg): Characterization and Rheological Properties. Food Chem. 2018, 263, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Weiss, J.; Hulbert, G.J.; Mount, J. Ultrasonic Processing Influences Rheological and Optical Properties of High-Methoxyl Pectin Dispersions. Food Hydrocolloils 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Yu, S.; Guo, X.; Ai, C.; Tang, X.; Chen, H.; Lin, J.; Zhang, X.; Meng, H. Effects of PH and Temperature on the Structure, Rheological and Gel-Forming Properties of Sugar Beet Pectins. Food Hydrocoll. 2021, 116, 106646. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Jin, H.; Kim, H.-S.; Kim, S.-K.; Shin, M.-K.; Kim, J.-H.; Lee, J.-W. Production of Heteropolysaccharide-7 by Beijerinckia indica from Agro-Industrial Byproducts. Enzym. Microb. 2002, 30, 822–827. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Cantero, L.; Salmerón, J.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.D.P.; Bustamante, M.Á.; Matias, S.; Navarro, V.; Simón, E.; Martínez, O. Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value. Appl. Sci. 2022, 12, 5934. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple Pomace as Food Fortification Ingredient: A Systematic Review and Meta-Analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Manzoor, M.; Anwar, F.; Saari, N.; Ashraf, M. Variations of Antioxidant Characteristics and Mineral Contents in Pulp and Peel of Different Apple (Malus domestica Borkh.) Cultivars from Pakistan. Molecules 2012, 17, 390–407. [Google Scholar] [CrossRef]
- Lomba-Viana, X.; Raymundo, A.; Prista, C.; Alegria, M.J.; Sousa, I. Clean Label “Rocha” Pear (Pyrus communis L.) Snack Containing Juice By-Products and Euglena Gracilis Microalgae. Front Nutr. 2022, 9, 825999. [Google Scholar] [CrossRef] [PubMed]
- Kruczek, M.; Gumul, D.; Kacániová, M.; Ivanišhová, E.; Marecek, J.; Gambus, H. Industrial Apple Pomace By-Products as a Potential Source of pro-Health Compounds in Functional Food. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 22–26. [Google Scholar] [CrossRef]
- Regulamento (CE) No. 1924:2006 do Parlamento Europeu e do Conselho Relativo às Alegaçȯes Nutricionais e de Saude Sobre Os Alimentos. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32006R1924 (accessed on 10 July 2024).
- Regulamento (UE) No. 1169/2011 do Parlamento Europeu e do Conselho Relativo à Prestação de Informação Aos Consumidores Sobre Os Géneros Alimentícios. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:PT:PDF (accessed on 10 July 2024).
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determinación de La Capacidad Antioxidante, Contenido de Fenoles Totales y Composición Mineral de Diferentes Tejidos de Frutos de Cinco Variedades de Manzana Cultivadas En Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]
- Sudha, M.L.; Dharmesh, S.M.; Pynam, H.; Bhimangouder, S.V.; Eipson, S.W.; Somasundaram, R.; Nanjarajurs, S.M. Antioxidant and Cyto/DNA Protective Properties of Apple Pomace Enriched Bakery Products. J. Food Sci. Technol. 2016, 53, 1909–1918. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Application of the Pulsed Electric Field to Release Bound Phenolics in Sorghum Flour and Apple Pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- Bindon, K.; Qi, S.; Kassara, S.; Nicolotti, L.; Jouin, A.; Beer, M. Apple Pomace Compositional Data Highlighting the Proportional Contribution of Polymeric Procyanidins. Molecules 2023, 28, 5494. [Google Scholar] [CrossRef]
- Castellar, M.R.; Obón, J.M.; Fernández-López, J.A. The Isolation and Properties of a Concentrated Red-Purple Betacyanin Food Colourant from Opuntia Stricta Fruits. J. Sci. Food Agric. 2006, 86, 122–128. [Google Scholar] [CrossRef]
- Ismail, B.P. Ash Content Determination. In Food Analysis; Nielsen, S.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 117–119. [Google Scholar]
- Pandey, J.; Bastola, T.; Tripathi, J.; Tripathi, M.; Rokaya, R.K.; Dhakal, B.; Rabin, D.C.; Bhandari, R.; Poudel, A. Estimation of Total Quercetin and Rutin Content in Malus Domestica of Nepalese Origin by HPLC Method and Determination of Their Antioxidative Activity. J. Food Qual. 2020, 2020, 8853426. [Google Scholar] [CrossRef]
- Matheus, J.; Alegria, M.J.; Nunes, M.C.; Raymundo, A. Algae-Boosted Chickpea Hummus: Improving Nutrition and Texture with Seaweeds and Microalgae. Foods 2024, 13, 2178. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Messaoud, C.; Smaali, I. Bioprospecting of Microalgae for a Potential Use as Enzyme Inhibitors, Anti-Ageing and Prebiotic Agents. Biocatal. Agric. Biotechnol. 2023, 51, 102759. [Google Scholar] [CrossRef]

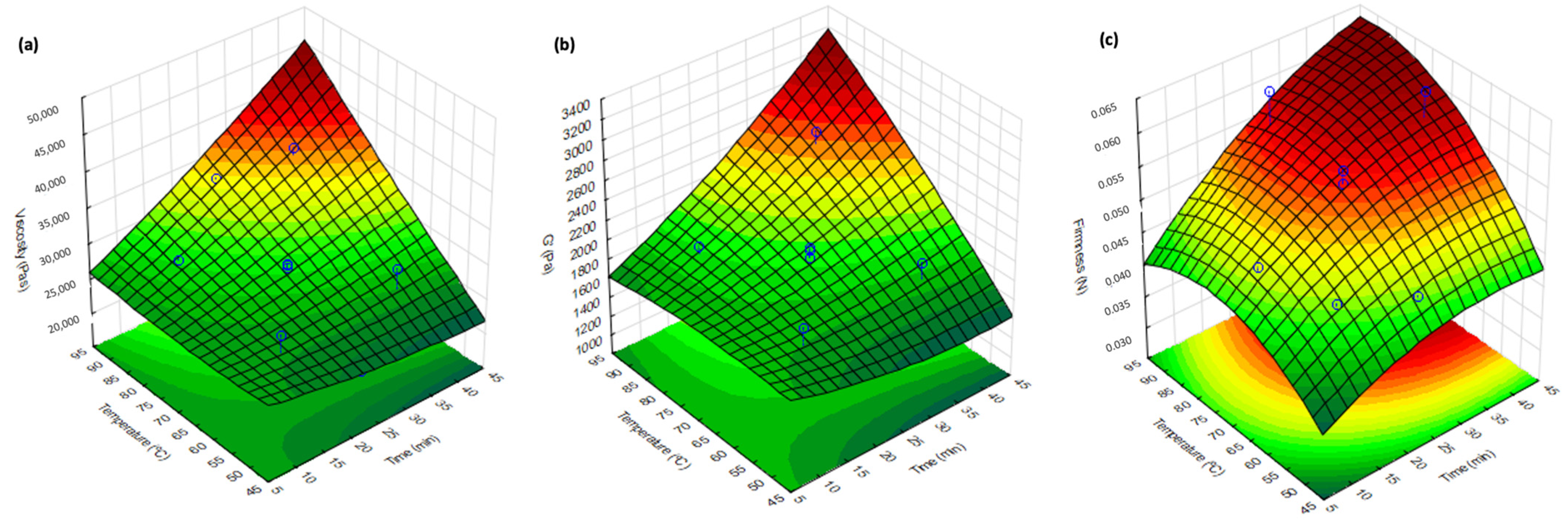
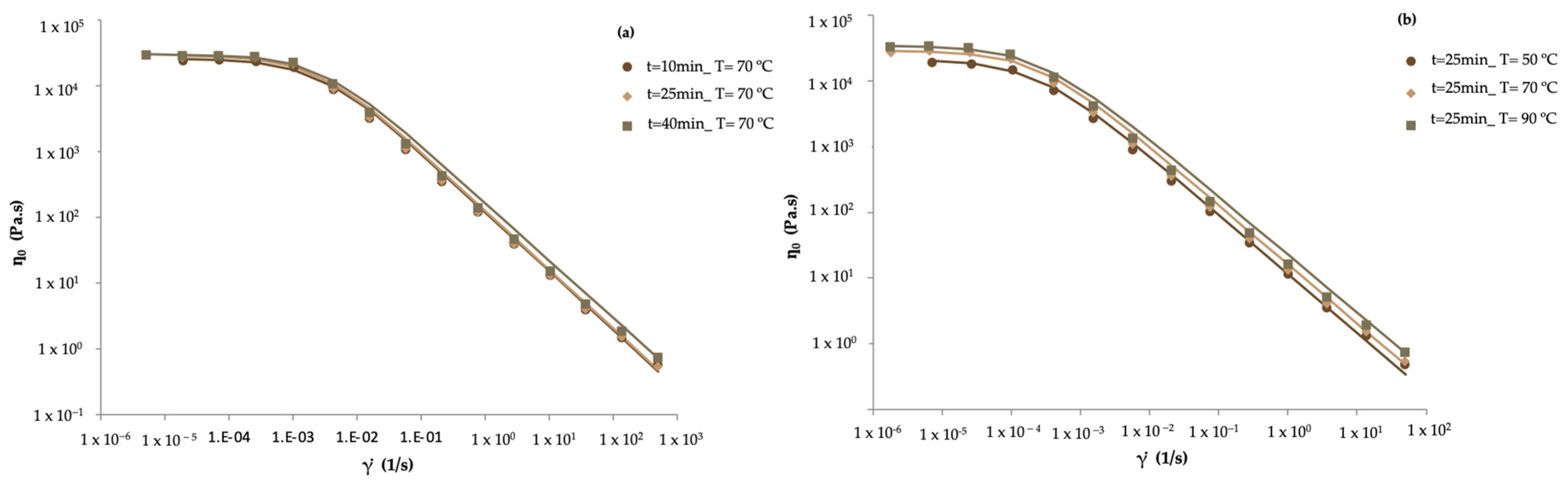
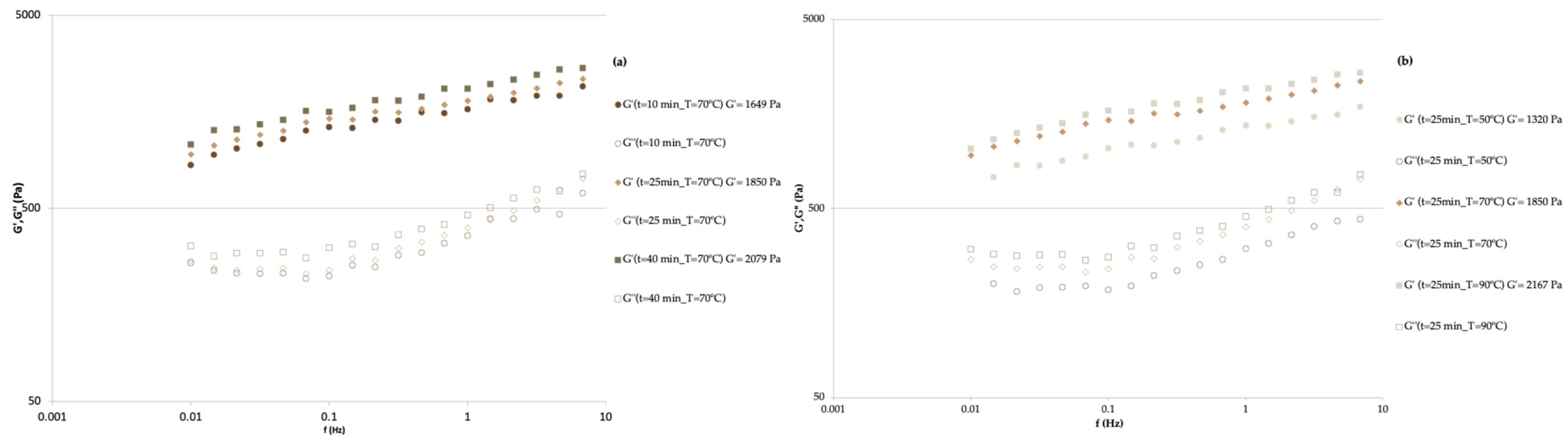

| Formulation | Firmness (N) | G′1Hz (Pa) | ƞ0 (Pa·s) | m | k (Pa·s) | R2 |
|---|---|---|---|---|---|---|
| 0.25% P | 0.044 ± 0.001 bc | 1190 ± 84.1 c | 3.49 × 104 ± 2.42 × 103 cde | 1.02 ± 0.04 ab | 545.46 ± 17.77 bcde | 0.998 ± 0.004 |
| 0.5% P | 0.047 ± 0.002 bc | 1419 ± 25 bc | 3.77 × 104 ± 4.41 × 102 cd | 0.96 ± 0.07 ab | 735.71 ± 107 b | 0.999 ± 0.001 |
| 0.75% P | 0.049 ± 0.001 abc | 1575 ± 133.5 ab | 4.24 × 104 ± 3.62 × 103 c | 1.04 ± 0.04 ab | 664.33 ± 52.37 bcd | 0.999 ± 0.001 |
| 0.25% XG | 0.041 ± 0.001 c | 1504 ± 133.4 ab | 3.13 × 104 ± 3.15 × 103 de | 1.22 ± 0.06 a | 564.29 ± 47.84 bcde | 0.998 ± 0.002 |
| 0.5% XG | 0.042 ± 0.001 bc | 1693 ± 9.2 a | 3.54 × 104 ± 7.71 × 103 cde | 0.94 ± 0.09 ab | 700.75 ±59.85 bc | 0.999 ± 0.001 |
| 0.75% XG | 0.049 ± 0.001 ab | 1703 ± 33.6 a | 3.50 × 104 ± 1.50 × 103 cde | 0.94 ± 0.01 ab | 502.79 ± 46.43 cde | 0.999 ± 0.001 |
| 0.5% XG + 0.25% P | 0.053 ± 0.002 a | 1376 ± 6.5 c | 2.84 × 104 ± 1.83 × 103 b | 1.01 ± 0.07 ab | 475.70 ± 37.55 de | 0.998 ± 0.002 |
| 0.5% P + 0.25% XG | 0.043 ± 0.001 bc | 984 ± 15.1 abc | 2.15 × 104 ± 1.90 × 103 e | 1.13 ± 0.13 a | 435.30 ± 56.72 e | 0.995 ± 0.004 |
| Target | 0.044 ± 0.006 bc | 419 ± 6.2 d | 5.55 × 104 ± 7.45 × 102 a | 0.82 ± 0.01 b | 1272.3 ± 156.78 a | 0.999 ± 0.003 |
| Coded Value | Decoded Value | |||
|---|---|---|---|---|
(Time) | (Temperature) | Time (min) | Temperature (°C) | |
| Factorial points | −1 | −1 | 14.4 | 55.8 |
| 1 | −1 | 35.6 | 55.8 | |
| −1 | 1 | 14.4 | 84.2 | |
| 1 | 1 | 35.6 | 84.2 | |
| Star point | −1.414 | 0 | 10 | 70 |
| 1.414 | 0 | 40 | 70 | |
| 0 | −1.414 | 25 | 50 | |
| 0 | 1.414 | 25 | 90 | |
| Central Points | 0 | 0 | 25 | 70 |
| 0 | 0 | 25 | 70 | |
| 0 | 0 | 25 | 70 | |
| 0 | 0 | 25 | 70 | |
| Formulation | ƞ0 (Pa·s) | G′1Hz (Pa) | Firmness (N) | ||||
|---|---|---|---|---|---|---|---|
| Time | Temperature | Experimental | Predicted | Experimental | Predicted | Experimental | Predicted |
| 14.4 | 55.8 | 2.7 × 104 ± 5.7 × 102 | 2.3 × 104 | 1880 ± 104 | 1697 | 0.046 ± 0.002 | 0.047 |
| 35.6 | 55.8 | 2.9 × 104 ± 1.4 × 103 | 2.7 × 104 | 1959 ± 63 | 1748 | 0.049 ± 0.002 | 0.055 |
| 14.4 | 84.2 | 2.8 × 104 ± 5.6 × 102 | 2.8 × 104 | 1957 ± 71 | 1882 | 0.047 ± 0.001 | 0.054 |
| 35.6 | 84.2 | 3.6 × 104 ± 4.5 × 102 | 3.4 × 104 | 2596 ± 128 | 2494 | 0.062 ± 0.001 | 0.062 |
| 10 | 70 | 2.4 × 104 ± 1.4 × 102 | 2.6 × 104 | 1649 ± 63 | 1771 | 0.048 ± 0.001 | 0.049 |
| 40 | 70 | 2.9 × 104 ± 4.3 × 102 | 3.1 × 104 | 2080 ± 24 | 2240 | 0.055 ± 0.002 | 0.060 |
| 25 | 50 | 2.1 × 104 ± 7.6 × 102 | 2.4 × 104 | 1357 ± 63 | 1577 | 0.045 ± 0.002 | 0.049 |
| 25 | 90 | 3.4 × 104 ± 6.5 × 102 | 3.3 × 104 | 2167 ± 41 | 2233 | 0.061 ± 0.001 | 0.060 |
| 25 | 70 | 2.9 × 104 ± 9.7 × 102 | 2.9 × 104 | 2024 ± 45 | 1948 | 0.054 ± 0.001 | 0.055 |
| 25 | 70 | 2.8 × 104 ± 1.6 × 103 | 2.9 × 104 | 1919 ± 32 | 1948 | 0.054 ± 0.002 | 0.055 |
| 25 | 70 | 2.9 × 104 ± 3.6 × 103 | 2.9 × 104 | 2000 ± 132 | 1948 | 0.056 ± 0.001 | 0.055 |
| 25 | 70 | 2.8 × 104 ± 1.3 × 103 | 2.9 × 104 | 1850 ± 13 | 1948 | 0.044 ± 0.001 | 0.055 |
| Factor | ƞ0 (Pa·s) | G′1Hz (Pa) | Firmness (N) |
|---|---|---|---|
| Intercept | 2.84 × 104 | 1948.67 | 0.054 |
| 2.09 × 103 * | 165.76 * | 0.004 * | |
| 3.33 × 103 * | 232.96 * | 0.004 * | |
| 2 | 2.08 × 102 ns | 29.29 ns | −0.000 ns |
| 2 | 1.84 × 102 ns | −21.39 ns | −0.002 ns |
| 1.76 × 102 ns | 140.08 ns | 0.001 ns | |
| R2 | 0.80 | 0.78 | 0.76 |
| R2 Adjusted | 0.63 | 0.60 | 0.56 |
| Lack-of-fit (p-value) | 0.001 ns | 0.07 * | 0.03 ** |
| Present Study | Wang et al. [45] | Jin et al. [46] | Bhushan et al. [47] | |
|---|---|---|---|---|
| Moisture (g/100 g DW) | 15.29 ± 0.06 | 4.4 | 5.8 | 3.90–10.80 |
| Ash (g/100 g DW) | 2.73 ± 0.02 | 1.8 | 1.5 | 0.5–6.10 |
| Protein (g/100 g DW) | 4.00 ± 0.02 | 3.8 | 4.7 | 2.94–5.67 |
| Lipids (g/100 g DW) | 0.29 ± 0.02 | 3.8 | 4.2 | 1.20–3.90 |
| Carbohydrates (g/100 g DW) * | 77.69 * | 45.1 | 83.8 | 48.0–62.0 |
| Total Sugar (g glucose/100 g DW) | 58.33 ± 0.59 |
| Minerals | Recommended Values in mg/100 g Source of/Rich in | Present Study (mg/100 g Fresh Matter) |
|---|---|---|
| Sodium | - | 11.51 ± 0.16 |
| Potassium | 300/600 | 103.28 ± 1.39 |
| Calcium | 120/240 | 18.32 ± 0.30 |
| Magnesium | 57/114 | 5.38 ± 0.11 |
| Phosphorus | 105/210 | 34.94 ± 0.60 |
| Sulfur | - | 3.99 ± 0.01 |
| Iron | 2.1/4.2 | 0.47 ± 0.13 |
| Copper | 0.15/0.3 | 0.06 ± 0.005 |
| Zinc | 1.5/3 | 0.03 ± 0.002 |
| Manganese | 0.3/0.6 | 0.09 ± 0.001 |
| Boron | - | 0.28 ± 0.004 |
| Present Study | Other Studies | References | |
|---|---|---|---|
| pH * | 3.67 ± 0.01 | 3.9 | [57] |
| °Brix * | 15.73 ± 0.06 | 13.3–15.8 | [56] |
| TPC (mgGAE/100 g DW) | 308.04 ± 0.01 | 385.4–402.7 | [58] |
| DPPH (TEAC µmol Trolox/100 g DW) | 513.0 ± 0.07 | 780.6–799.3 | [58] |
| FRAP (TEAC µmol Trolox/100 g DW) | 1110.0 ± 0.01 | 3336–8525 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, Â.; Alegria, M.J.; Raymundo, A. Sustainable Jam with Apple Pomace: Gelling, Rheology, and Composition Analysis. Gels 2024, 10, 580. https://doi.org/10.3390/gels10090580
Viegas Â, Alegria MJ, Raymundo A. Sustainable Jam with Apple Pomace: Gelling, Rheology, and Composition Analysis. Gels. 2024; 10(9):580. https://doi.org/10.3390/gels10090580
Chicago/Turabian StyleViegas, Ândria, Maria João Alegria, and Anabela Raymundo. 2024. "Sustainable Jam with Apple Pomace: Gelling, Rheology, and Composition Analysis" Gels 10, no. 9: 580. https://doi.org/10.3390/gels10090580








