Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Gel Polymer Electrolyte Modified with Multi-Walled Carbon Nanotubes and SiO2 Nanospheres to Increase Rechargeability of Zn–Air Batteries
Abstract
1. Introduction
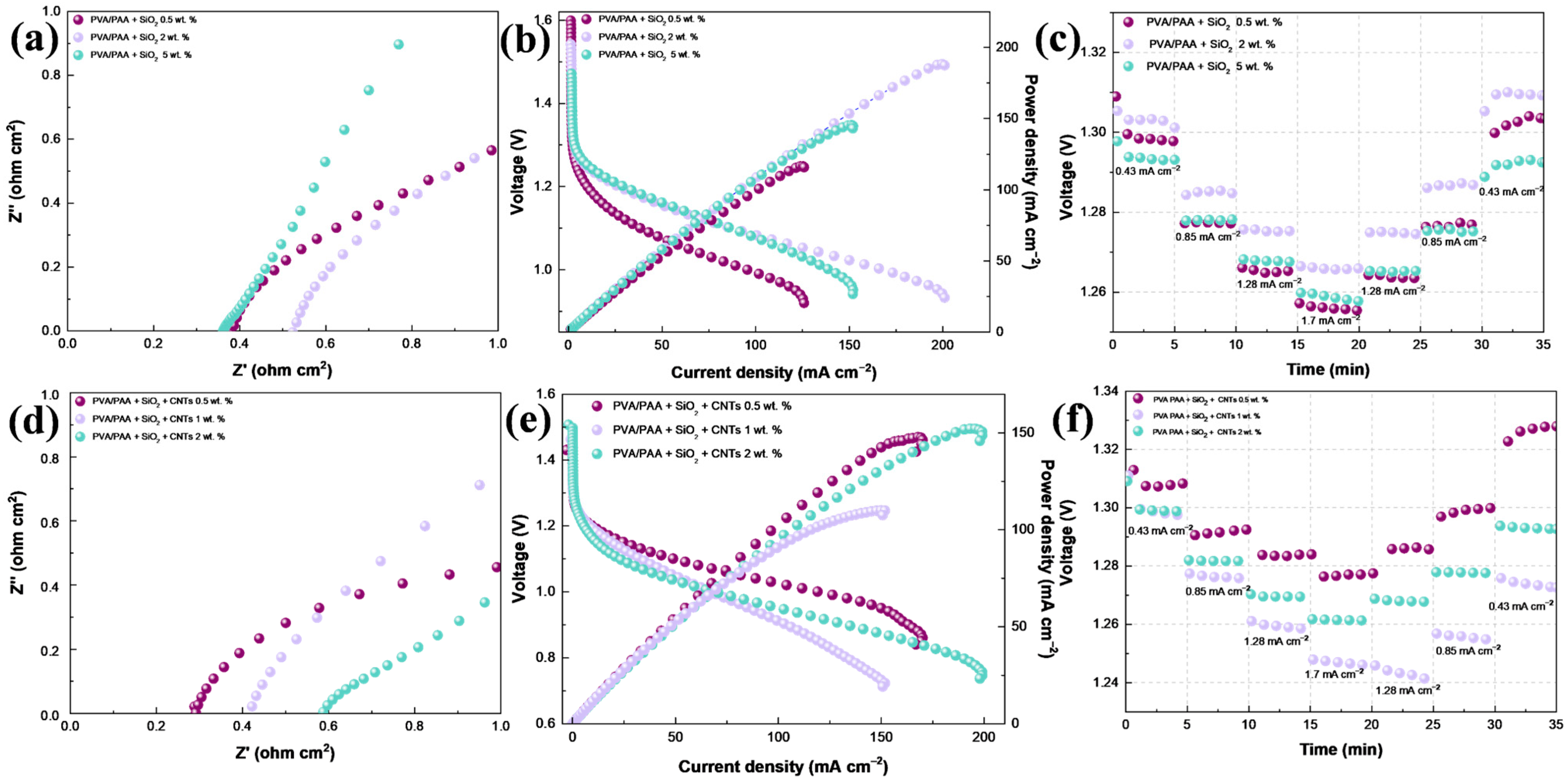
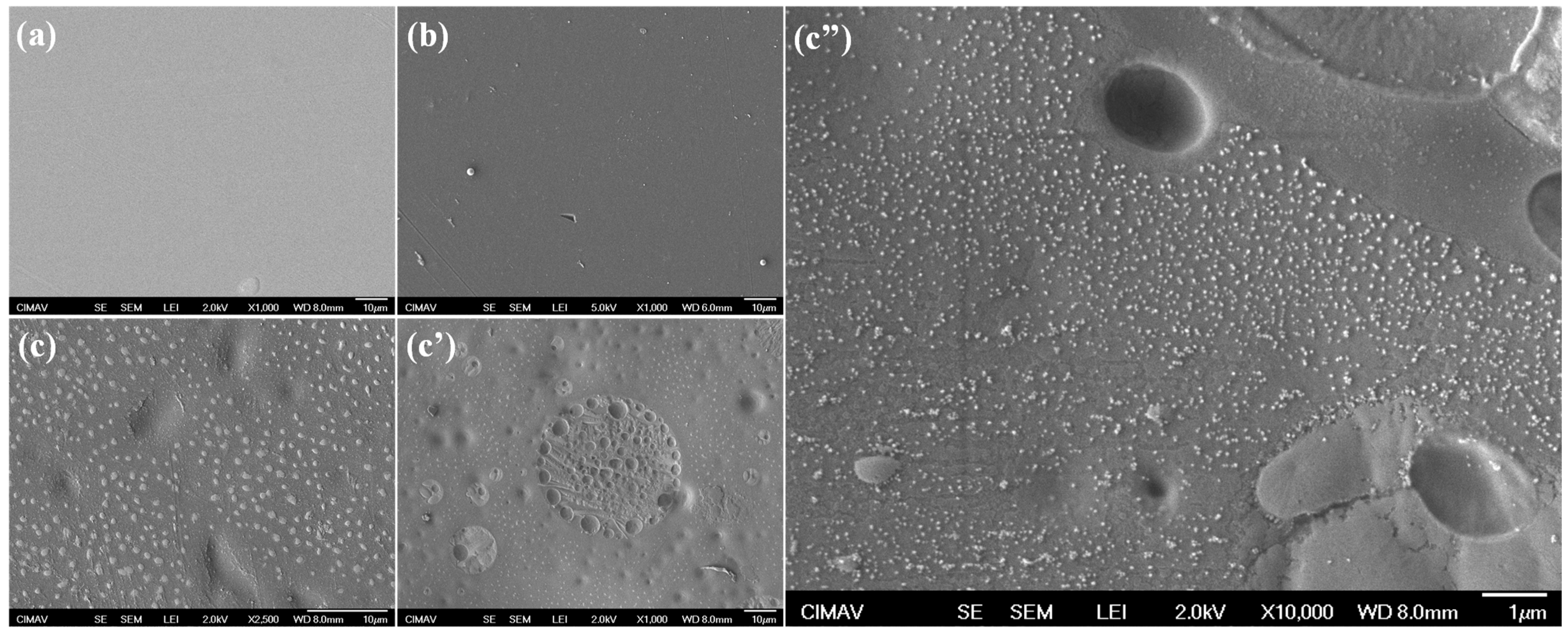
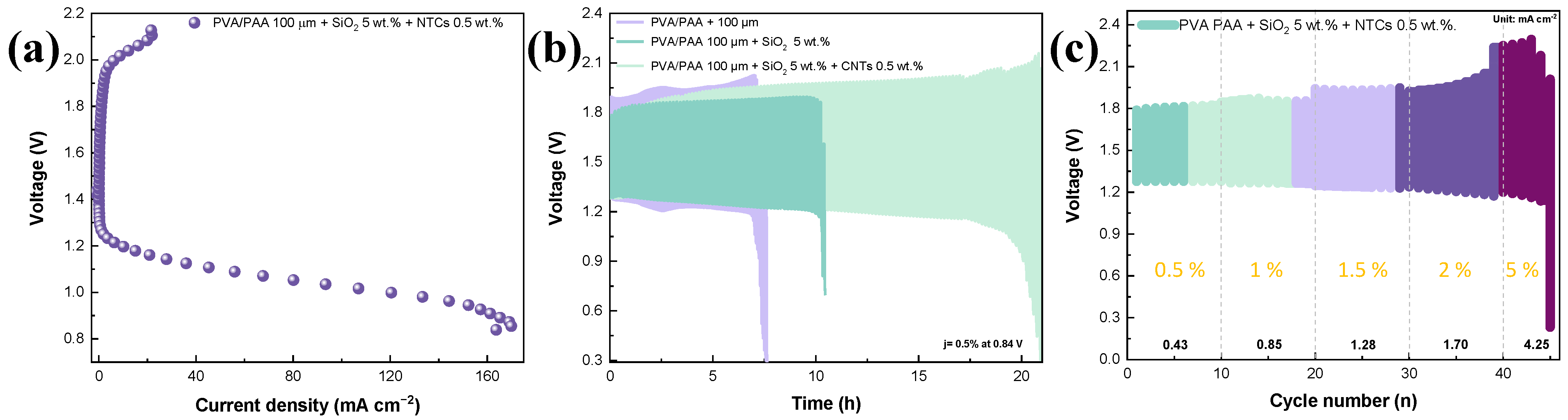
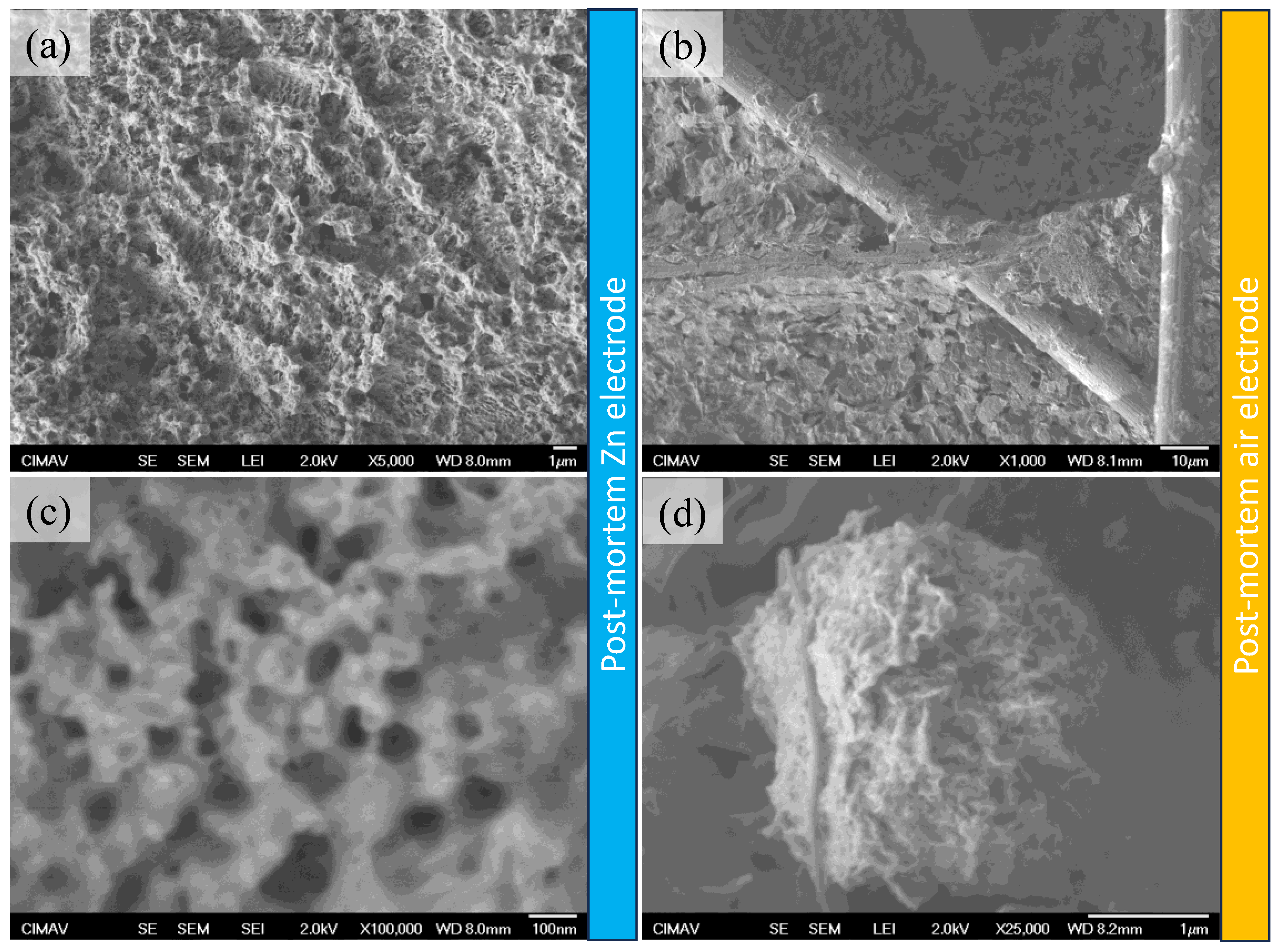
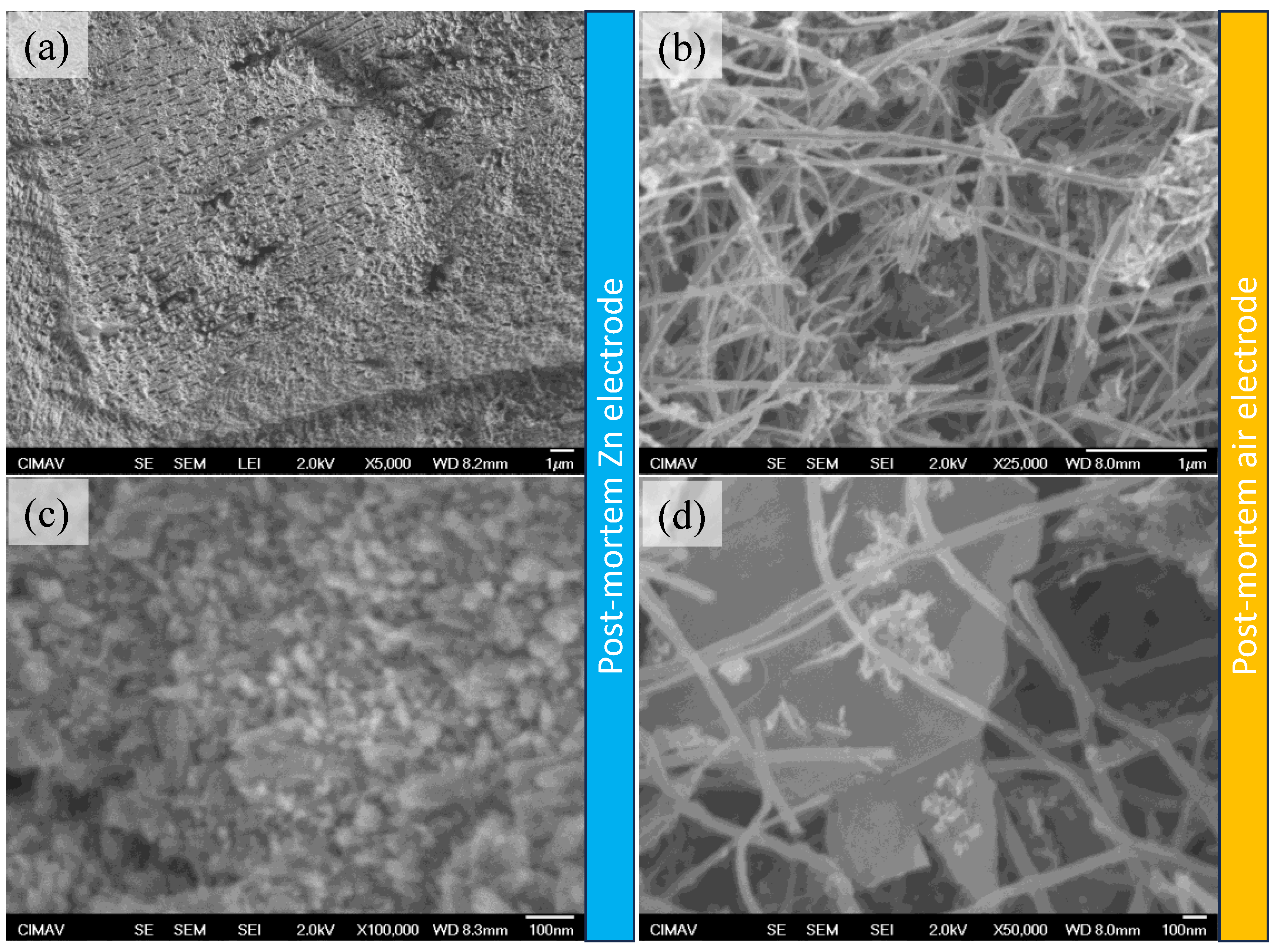
2. Results and Discussion
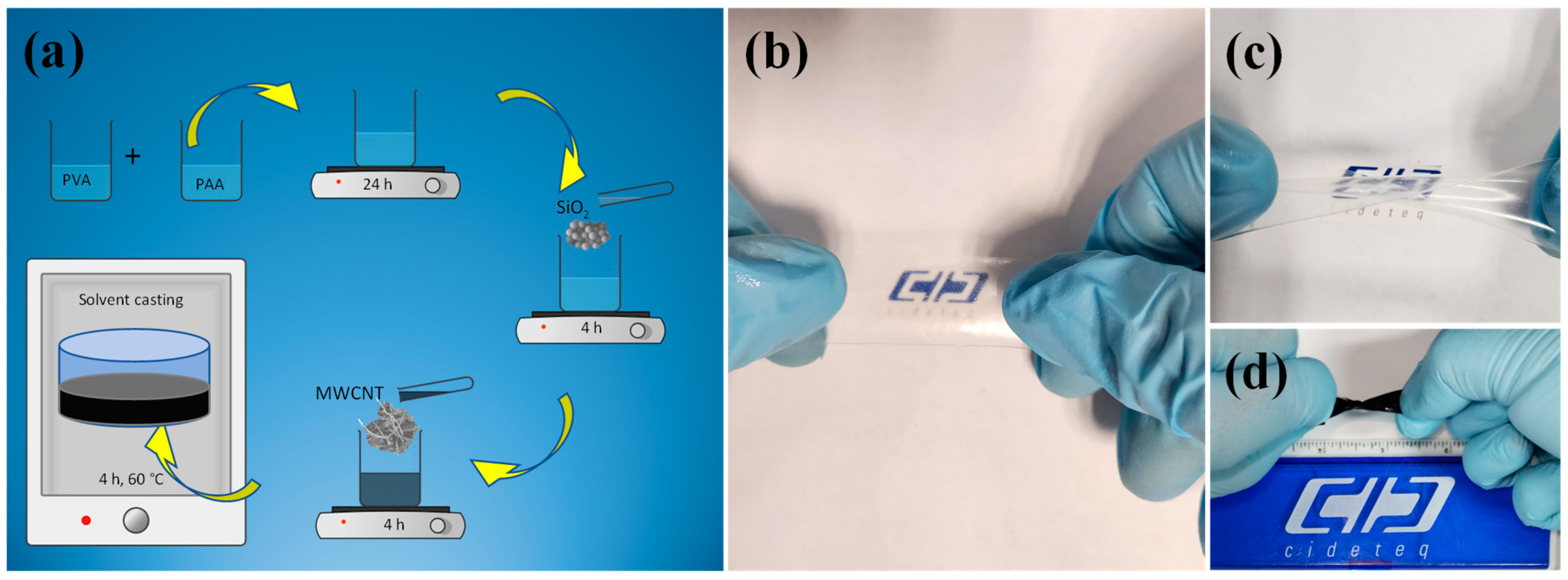
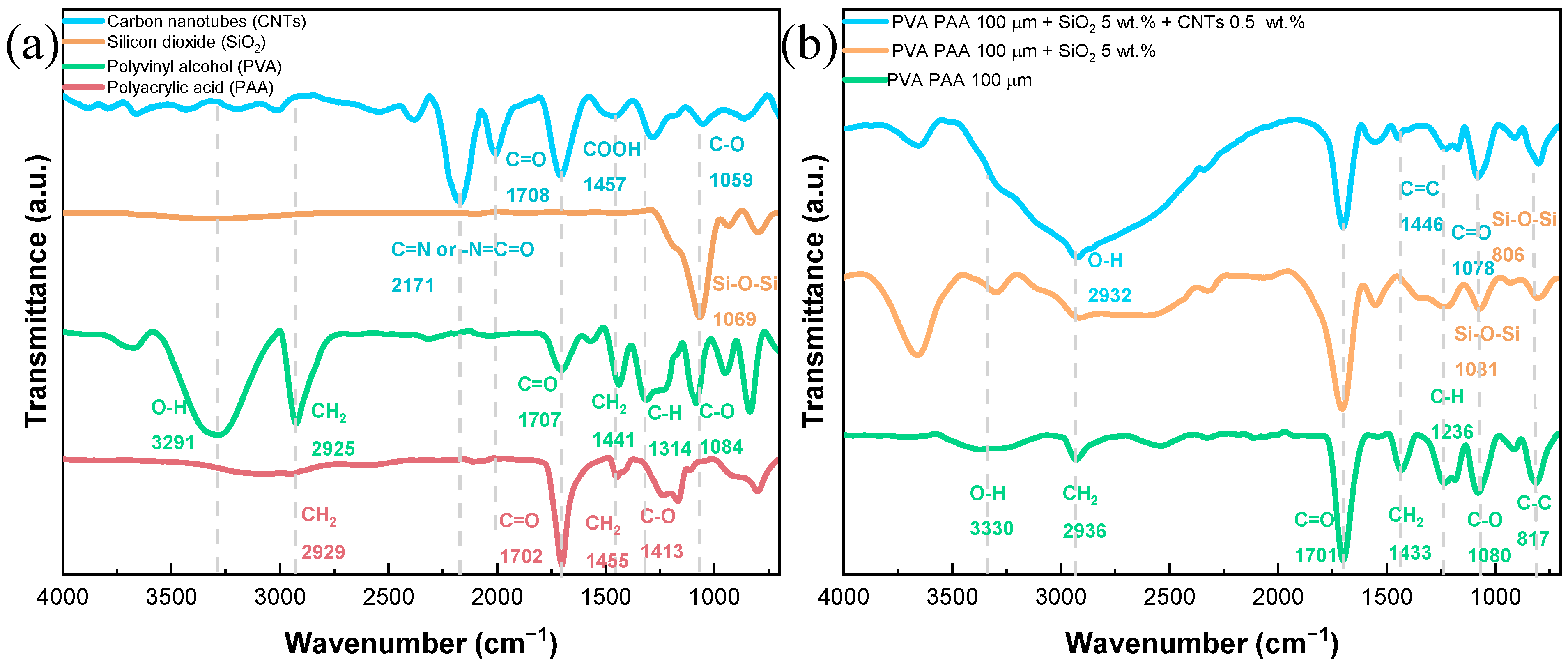
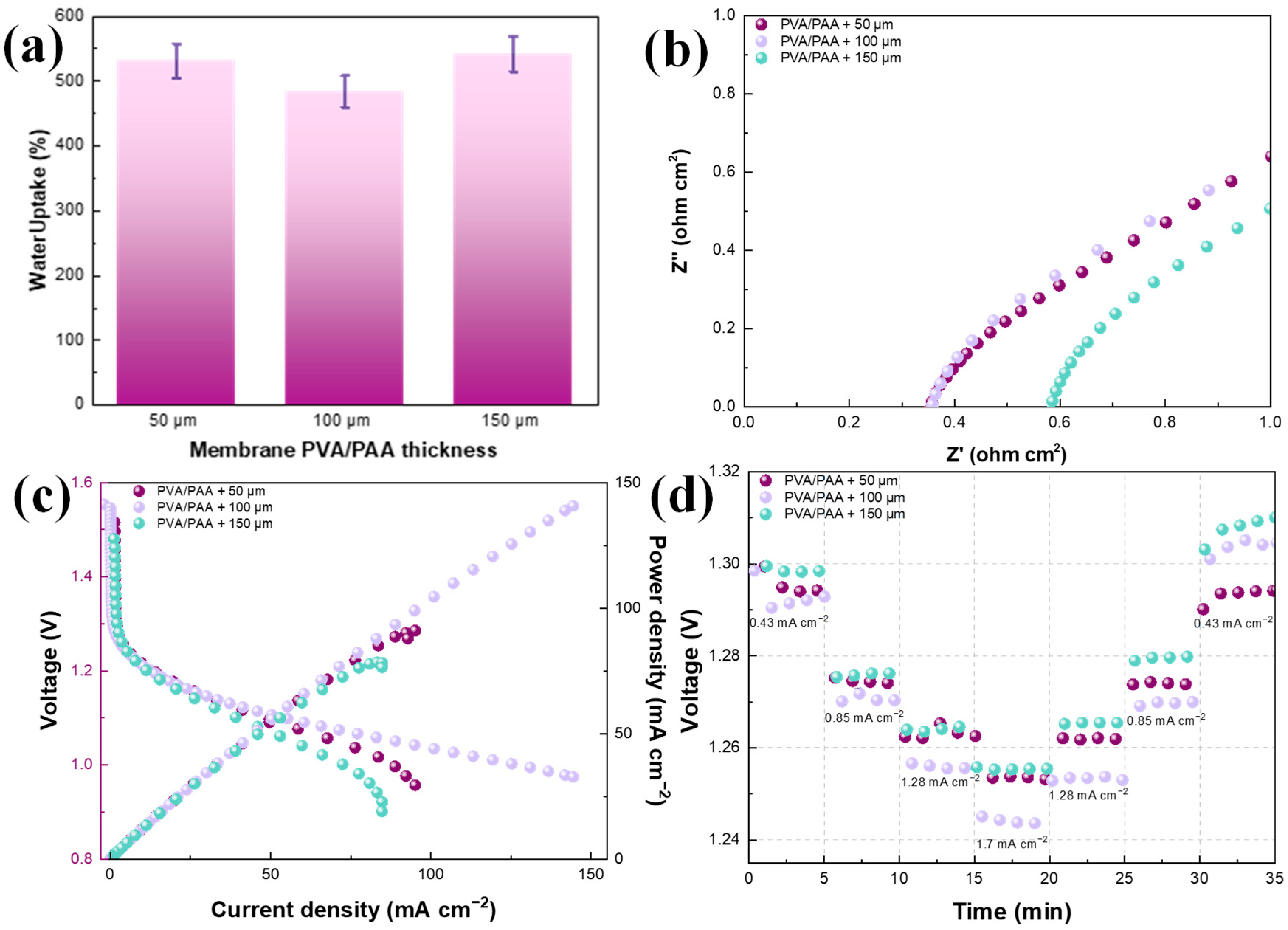
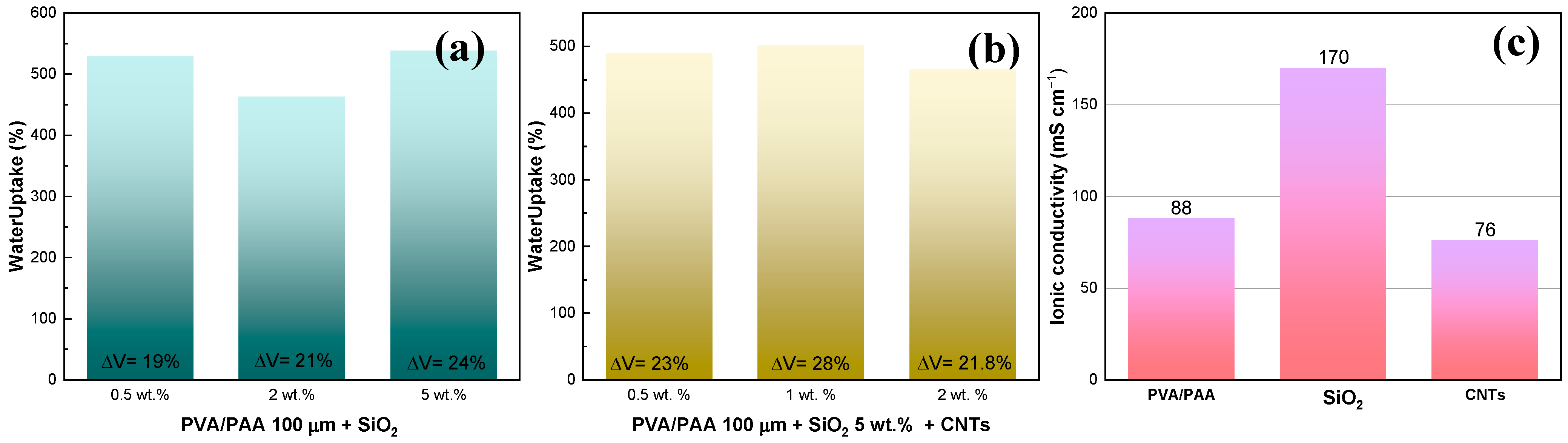
Physicochemical Results
3. Conclusions
4. Materials and Methods
4.1. Synthesis of PVA/PAA Membranes
4.2. Synthesis of SiO2 and CNTs
4.3. Physicochemical and Electrochemical Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caramia, V.; Bozzini, B. Materials science aspects of zinc-air batteries: A review. Mater. Renew. Sustain. Energy 2014, 3, 28. [Google Scholar] [CrossRef]
- Iturrondobeitia, M.; Akizu-Gardoki, O.; Amondarain, O.; Minguez, R.; Lizundia, E. Environmental impacts of aqueous zinc ion batteries based on life cycle assessment. Adv. Sustain. Syst. 2022, 6, 2100308. [Google Scholar] [CrossRef]
- Li, Q.; Li, A.; Liu, S.; Fu, Q.; Xu, Y.; Dai, J.; Li, P.; Xu, S. Cytotoxicity of biodegradable zinc and its alloys: A systematic review. J. Funct. Biomater. 2023, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.R.; Iruin, E.; Comenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Hosseini, S.; Soltani, S.M.; Li, Y.-Y. Current status and technical challenges of electrolytes in zinc–air batteries: An in-depth review. Chem. Eng. J. 2021, 408, 127241. [Google Scholar] [CrossRef]
- Wang, K.; Pei, P.; Ma, Z.; Chen, H.; Xu, H.; Chen, D.; Wang, X. Dendrite growth in the recharging process of zinc-air batteries. J. Mater. Chem. A 2015, 3, 22648–22655. [Google Scholar] [CrossRef]
- Tamtaji, M.; Kim, M.G.; Li, Z.; Cai, S.; Wang, J.; Galligan, P.R.; Hung, F.-F.; Guo, H.; Chen, S.; Luo, Z.; et al. High-throughput screening of dual atom catalysts for oxygen reduction and evolution reactions and rechargeable zinc-air battery. Nano Energy 2024, 126, 109634. [Google Scholar] [CrossRef]
- Cui, M.; Xu, B.; Shi, X.; Zhai, Q.; Dou, Y.; Li, G.; Bai, Z.; Ding, Y.; Sun, W.; Liu, H.; et al. Metal-organic framework-derived single-atom catalysts for electrocatalytic energy conversion applications. J. Maer. Chem. A 2024, 12, 18921–18947. [Google Scholar] [CrossRef]
- Sun, J.; Wang, N.; Qiu, Z.; Xing, L.; Du, L. Recent progress of non-noble metal catalysts for oxygen electrode in Zn-air batteries: A mini review. Catalysts 2022, 12, 843. [Google Scholar] [CrossRef]
- Song, D.; Hu, C.; Gao, Z.; Yang, B.; Li, Q.; Zhan, X.; Tong, X.; Tian, J. Metal-organic frameworks (MOFs) derived materials used in Zn-air battery. Materials 2022, 15, 5837. [Google Scholar] [CrossRef]
- Phuc, N.H.H.; Tu, T.A.; Loc, L.C.; Viet, C.X.; Phuong, P.T.T.; Tri, N.; Thang, L.V. A review of bifunctional catalysts for Zinc-air batteries. Nanoenergy Adv. 2023, 3, 13–47. [Google Scholar] [CrossRef]
- E Tang, C.; Wang, B.; Wang, H.-F.; Zhang, Q. Defect engineering toward atomic Co-Nx-C in hierarchical graphene for rechargeable flexible solid Zn-air batteries. Adv. Mater. 2017, 29, 1703185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, W. Study on failure mechanism on rechargeable alkaline zinc–Air battery during charge/discharge cycles at different depths of discharge. Front. Chem. 2023, 11, 1121215. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xing, Z.; Jin, Z.; Liu, C.; Ge, J.; Zhu, J.; Wang, Y.; Zhao, X.; Chen, Z. Preferentially engineering FeN4 edge sites onto graphitic nanosheets for highly active and durable oxygen electrocatalysis in rechargeable Zn-air batteries. Adv. Mater. 2020, 32, 2004900. [Google Scholar] [CrossRef] [PubMed]
- Soursos, N.; Kottis, T.; Premeti, V.; Zafeiropoulos, J.; Govatsi, K.; Sygellou, L.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D.; Lianos, P. Study of suitability of corncob biochar as electrocatalyst for Zn-air batteries. Batteries 2024, 10, 209. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Xiao, S.; Zhu, A.; Zhang, Y.; Zeng, X.; Dong, P. Metal-organic-framework-derived nitrogen-doped carbon-matrix-encapsulating Co0.5Ni0.5 alloy as bifunctional oxygen electrocatalyst for zinc-air batteries. Materials 2024, 17, 2629. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, R.; He, C.; Xia, C.; Guo, W.; Xu, Z.-L.; Xia, B.Y. Advanced polymer-based electrolytes in zinc-air batteries. eScience 2022, 2, 453–466. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Shang, N.; Wang, K.; Zuo, Y.; Wei, M.; Wang, H.; Zhong, D.; Pei, P. Advances in polymer electrolytes for solid-state zinc–air batteries. Mater. Chem. Front. 2023, 7, 3994–4018. [Google Scholar] [CrossRef]
- Tan, M.J.; Li, B.; Chee, P.; Ge, X.; Liu, Z.; Zong, Y.; Loh, X.J. Acrylamide-derived freestanding polymer gel electrolyte for flexible metal-air batteries. J. Power Sources 2018, 400, 566–571. [Google Scholar] [CrossRef]
- Liu, H.; Xie, W.; Huang, Z.; Yao, C.; Han, Y.; Huang, W. Recent advances in flexible Zn-air batteries: Materials for electrodes and electrolytes. Small Methods 2022, 6, 2101116. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, H.; Cao, Y.; Ai, X. Preparation and electrochemical characterization of the alkaline polymer gel electrolyte polymerized from acrylic acid and KOH solution. Electrochim. Acta 2004, 49, 2533–2539. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. Alkaline Zn-air and Al-air cells based on novel solid PVA/PAA polymer electrolyte membranes. J. Membr. Sci. 2006, 280, 802–808. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Chung, H.-J.; Ivey, D.G. A study of alkaline gel polymer electrolytes for rechargeable zinc–air batteries. Electrochim. Acta 2019, 327, 135021. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Clark, M.P.; Xiong, M.; Chung, H.-J.; Ivey, D.G. A tri-electrode configuration for zinc-air batteries using gel polymer electrolytes. Electrochim. Acta 2020, 357, 136865. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Aasen, D.; Zhalmuratova, D.; Labbe, M.; Chung, H.-J.; Ivey, D.G. Compositional effect of gel polymer electrolyte and battery design for zinc-air batteries. Batt. Supercaps 2020, 3, 917–927. [Google Scholar] [CrossRef]
- Song, Z.; Ding, J.; Liu, B.; Liu, X.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. A rechargeable Zn-air battery with high energy efficiency and long life enabled by a highly water-retentive gel electrolyte with reaction modifier. Adv. Mat. 2020, 32, 1908127. [Google Scholar] [CrossRef]
- Kuruahmet, D.; Singil, M.M.; Guler, A.; Yildirim, S.; Gungor, H.; Uzun, E.; Alkan, E.; Guler, M.O.; Akbulut, H. Enhancing the electrochemical properties of silicon nanoparticles by graphene-based aerogels. Energy Technol. 2023, 11, 2201503. [Google Scholar] [CrossRef]
- Singh, K.K.; Chaudhary, S.K.; Venugopal, R.; Gaurav, A. Bulk synthesis of multi-walled carbon nanotubes by AC arc discharge method. Proc. ImechE Part N J. Nanomater. Nanoeng. Nanosyst. 2017, 231, 141–151. [Google Scholar] [CrossRef]
- Lu, C.; Lin, F.; Shao, H.; Bi, S.; Chen, N.; Shao, G.; Jiang, J. Carboxylated carbon nanotube/polyimide films with low thermal expansion coefficient and excellent mechanical properties. Polymers 2022, 14, 4565. [Google Scholar] [CrossRef]
- Janudin, N.; Abdullah, N.; Yasin, F.M.; Yaacob, M.H.; Ahmad, M.Z.; Abdullah, L.C.; Othman, R.N.I.R.; Syah, N.A.A.; Kasim, N.A.M. Carbon nanotubes-based gas sensors in detection of methane gas at room temperature. Zulfaqar Int. J. Def. Eng. Technol. 2018, 1, 76–84. [Google Scholar]
- Yakymchuk, O.; Perepelytsina, O.; Dobrydnev, A.; Sydorenko, M.V. Effect of single-walled carbon nanotubes on tumor cells viability and formation of multicellular tumor spheroids. Nano Express 2015, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.V.; Nate, M.M.; Kurup, M.B.; Bambole, V.A.; Sabharwal, S. Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. NIMP-B 2005, 237, 585–592. [Google Scholar] [CrossRef]
- Lee, J.; Isobe, T.; Senna, M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J. Colloid Int. Sci. 1996, 177, 490–494. [Google Scholar] [CrossRef]
- Omkaram, I.; Chakradhar, R.P.S.; Rao, J.L. EPR, optical, infrared and Raman studies of VO2+ ions in polyvinylalcohol films. Phys. B Cond. Matter 2007, 388, 318–325. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Mat Hussin, R.; Saion, E.; Mat Yunus, W.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Lertsarawut, P.; Hemvichian, K.; Rattanawongwiboon, T.; Suwunmala, P. Dye adsorbent prepared by radiation-induced graft polymerization of acrylic acid onto carboxymethyl cellulose. J. Phys. Conf. Ser. 2019, 1285, 012023. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Par, J.-S.; Kim, E.J.; Jeong, S.-I.; Lee, J.Y.; Lim, Y.-M. Preparation of radiation cross-linked poly(acrylic acid) hydrogel containing metronidazole with enhanced antibacterial activity. Int. J. Mol. Sci. 2020, 21, 187. [Google Scholar] [CrossRef]
- Manavi-Tehrani, I.; Rabiee, M.; Parviz, M.; Tahriri, M.R.; Fahimi, Z. Preparation, characterization and controlled release investigation of biocompatible pH-sensitive PVA/PAA hydrogels. Macromol. Symp. 2010, 296, 457–465. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, H.; Choi, J.; Choi, S.-J.; Yoon, K.R. Internally connected porous PVA/PAA membrane with cross-aligned nanofiber network for facile and long-lasting ion transport in zinc–air batteries. Energy Storage Mater. 2024, 71, 103594. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Sergent, N.; Berthomé, G.; Chatenet, M. Huge instability of Pt/C catalysts in alkaline medium. ACS Catal. 2015, 5, 4819–4824. [Google Scholar] [CrossRef]
- Liu, F.; Wei, P.; Zhang, J.; Shi, M.; Hou, J.; Chen, H.; Li, Y.; Li, S. Potential-driven instability effect of carbon supports for Pt/C electrocatalysts. Carbon 2024, 216, 118562. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Y.; You, B. Dynamic electrodeposition on bubbles: An effective strategy toward porous electrocatalysts for green hydrogen cycling. Acc. Chem. Res. 2023, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Elguézabal, A.; Antúnez, W.; Alonso, G.; Paraguay-Delgado, F.; Espinosa, F.; Miki-Yoshida, M. Study of carbon nanotubes synthesis by spray pyrolysis and model of growth. Diam. Relat. Mater. 2006, 15, 1329–1335. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, H.; Shin, S.; Jang, S.; Bae, J.H.; Pang, C.; Choi, J.; Yoon, K.R. Hierarchically porous hydrogel electrolyte prepared from interpenetrating polymer networks for flexible Zn-Air batteries. Energy Storage Mater. 2023, 60, 102802. [Google Scholar] [CrossRef]
- Ambriz-Peláez, O.; Béjar, J.; Delgado, A.D.; Rodríguez-González, C.; Ramos-Castillo, C.M.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Arjona, N. NiMn layered double hydroxides with promoted surface defects as bifunctional electrocatalysts for rechargeable zinc–air batteries. FlatChem 2024, 45, 100664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Patiño, L.; Guerra-Balcázar, M.; Álvarez-Contreras, L.; Arjona, N. Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Gel Polymer Electrolyte Modified with Multi-Walled Carbon Nanotubes and SiO2 Nanospheres to Increase Rechargeability of Zn–Air Batteries. Gels 2024, 10, 587. https://doi.org/10.3390/gels10090587
Díaz-Patiño L, Guerra-Balcázar M, Álvarez-Contreras L, Arjona N. Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Gel Polymer Electrolyte Modified with Multi-Walled Carbon Nanotubes and SiO2 Nanospheres to Increase Rechargeability of Zn–Air Batteries. Gels. 2024; 10(9):587. https://doi.org/10.3390/gels10090587
Chicago/Turabian StyleDíaz-Patiño, Lucia, Minerva Guerra-Balcázar, Lorena Álvarez-Contreras, and Noé Arjona. 2024. "Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Gel Polymer Electrolyte Modified with Multi-Walled Carbon Nanotubes and SiO2 Nanospheres to Increase Rechargeability of Zn–Air Batteries" Gels 10, no. 9: 587. https://doi.org/10.3390/gels10090587
APA StyleDíaz-Patiño, L., Guerra-Balcázar, M., Álvarez-Contreras, L., & Arjona, N. (2024). Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Gel Polymer Electrolyte Modified with Multi-Walled Carbon Nanotubes and SiO2 Nanospheres to Increase Rechargeability of Zn–Air Batteries. Gels, 10(9), 587. https://doi.org/10.3390/gels10090587







