Unraveling the Impact of the Oil Phase on the Physicochemical Stability and Skin Permeability of Melatonin Gel Formulations
Abstract
1. Introduction
2. Results and Discussion
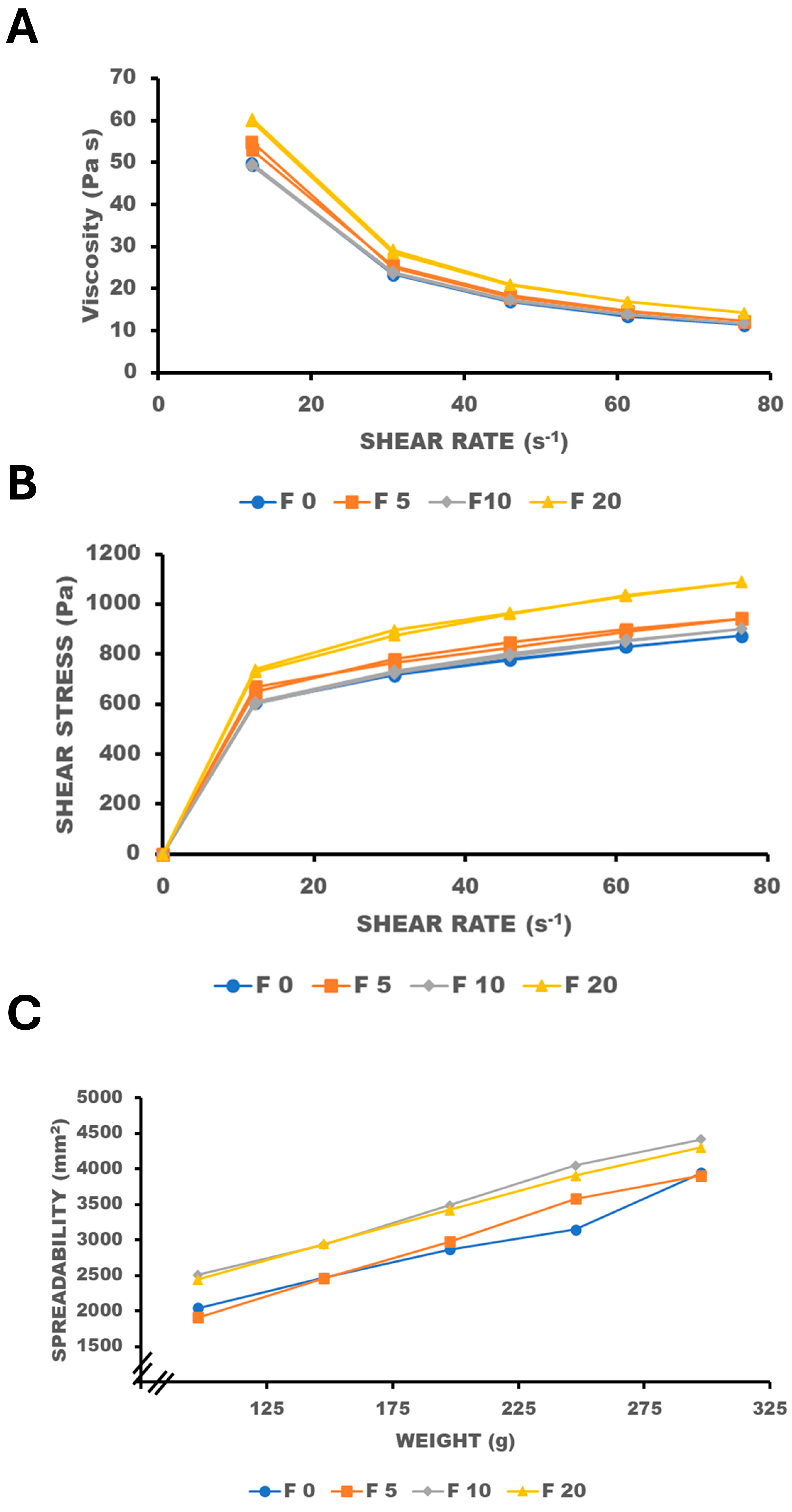
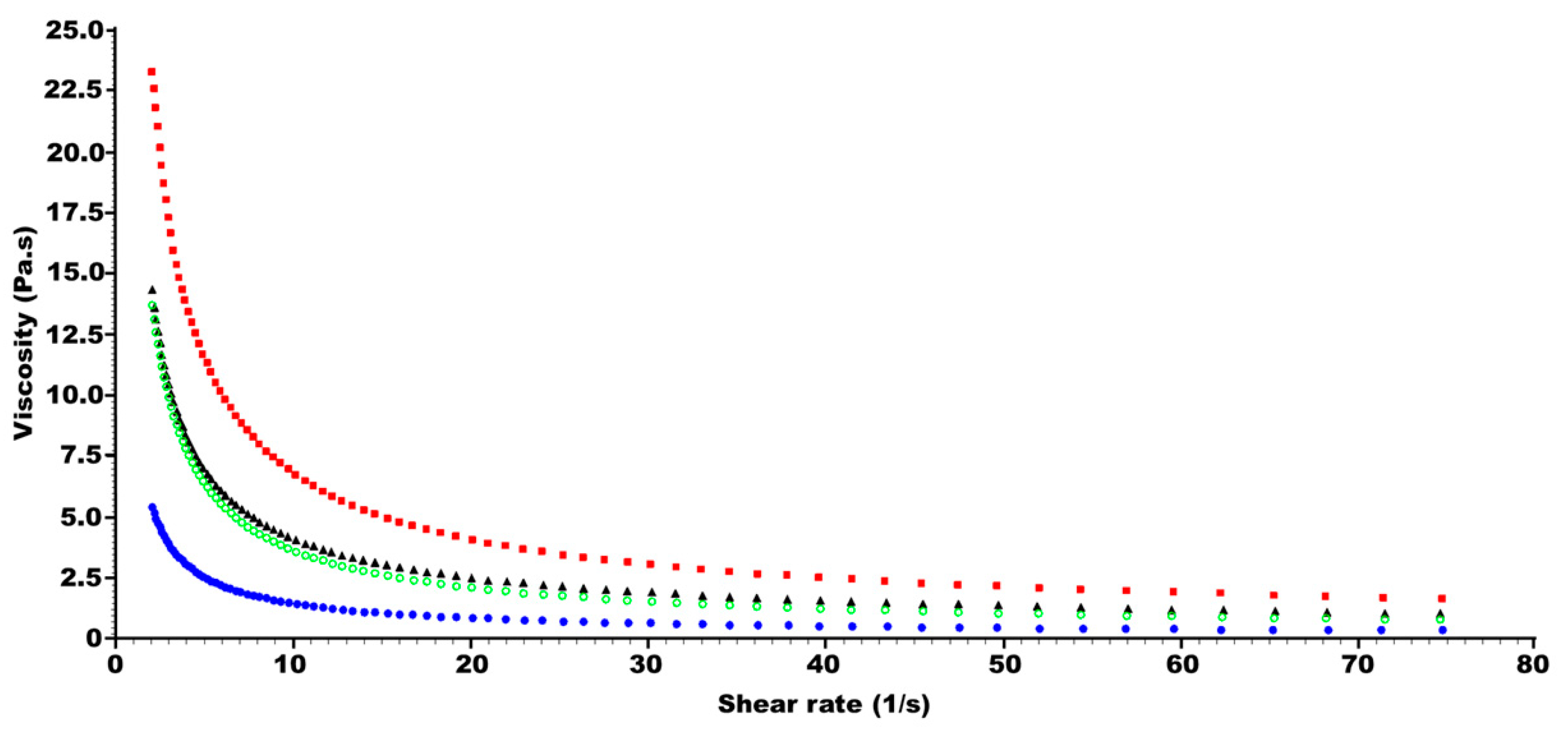
2.1. Effect of Oil Phase on Viscosity and Spreadability
2.2. Effect of Oil Phase on Skin Permeability
2.3. Effect of Oil Phase on Skin Adhesion
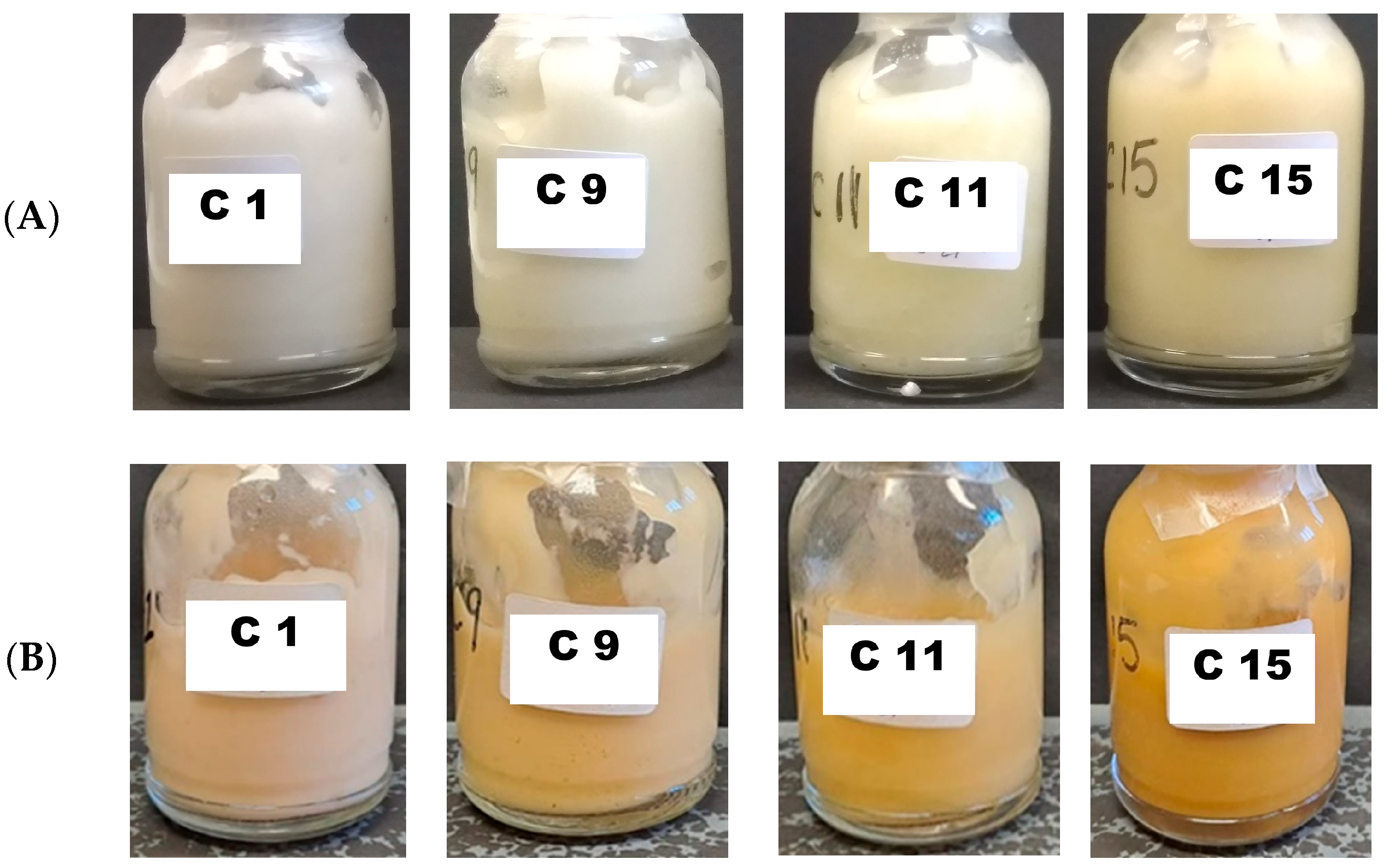
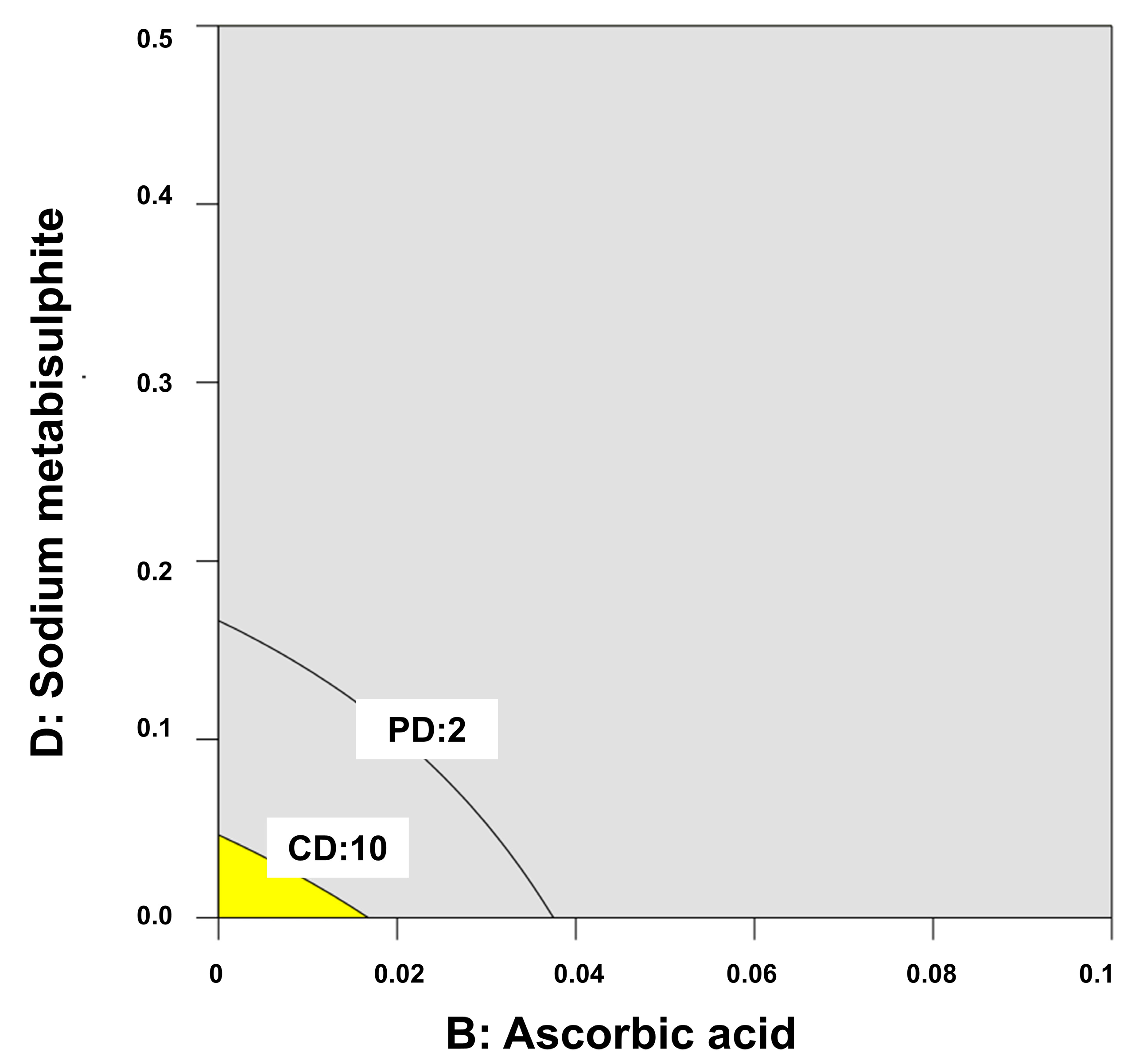
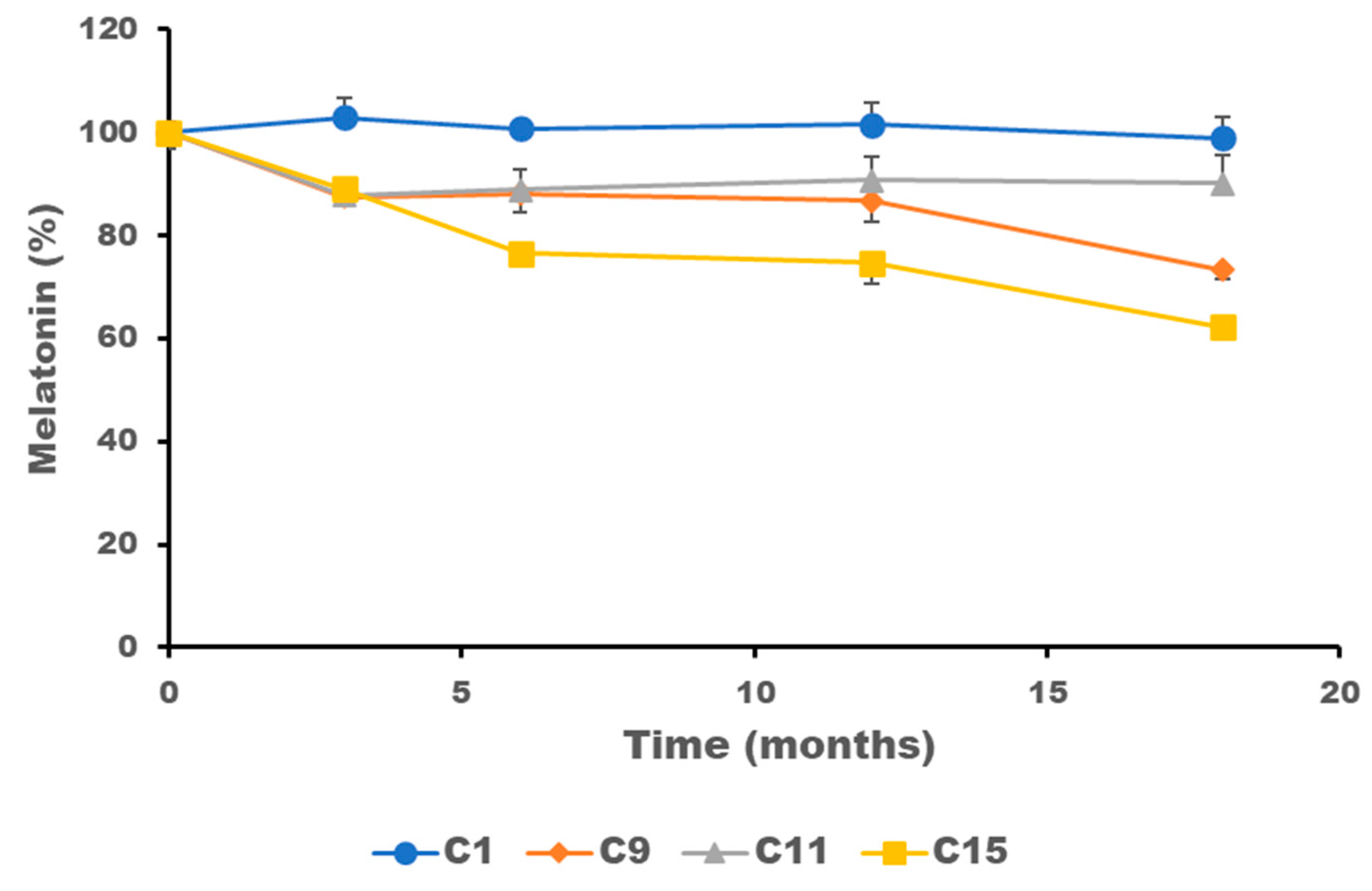
2.4. Stability of Melatonin Topical Formulations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Topical Melatonin Formulations with Different Oily Phase Ratio
4.3. Rheology of Topical Melatonin Formulations with Different Oily Phase Ratio
4.4. In Vitro Skin Permeation
4.5. Skin Adhesion
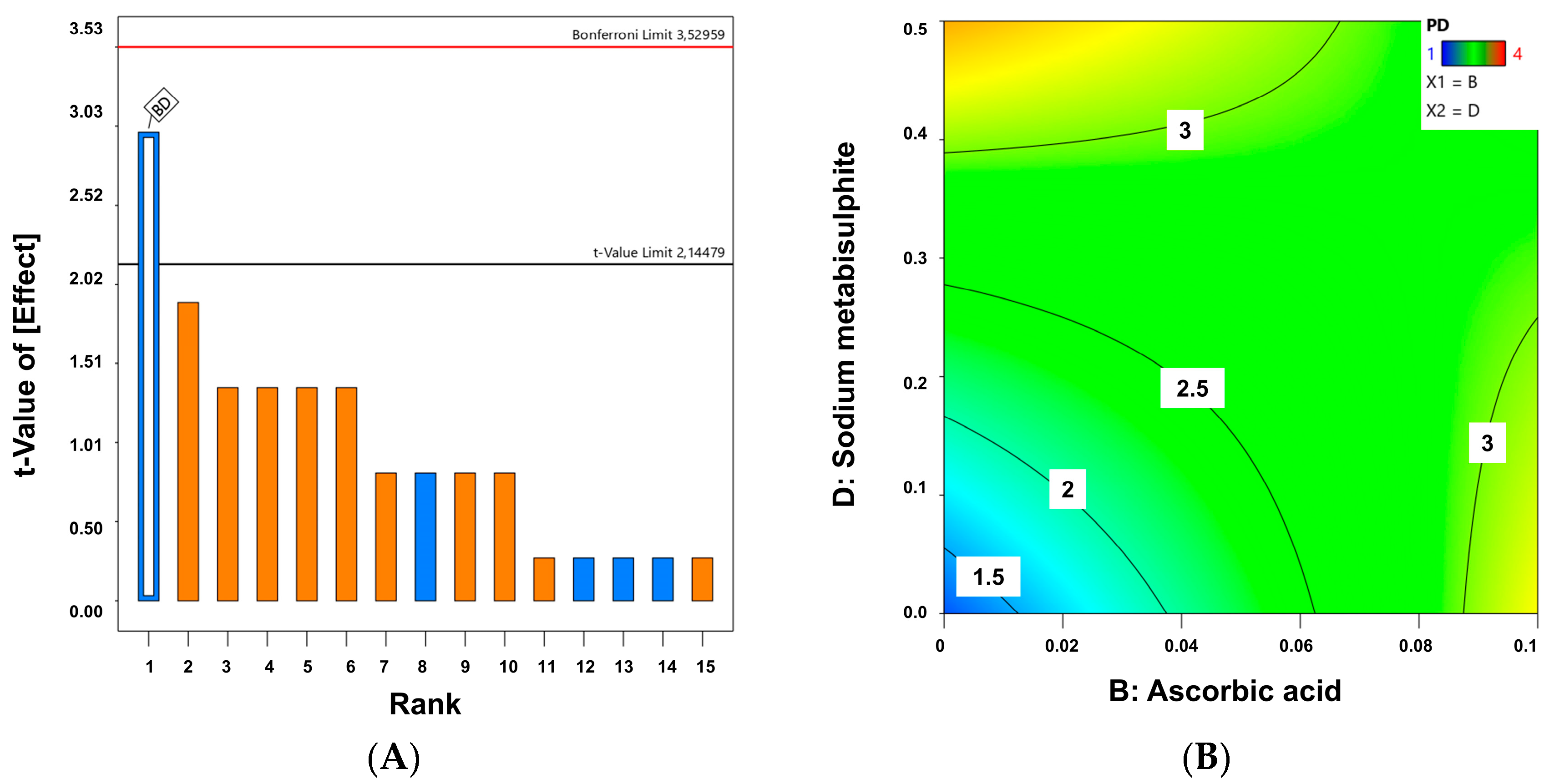
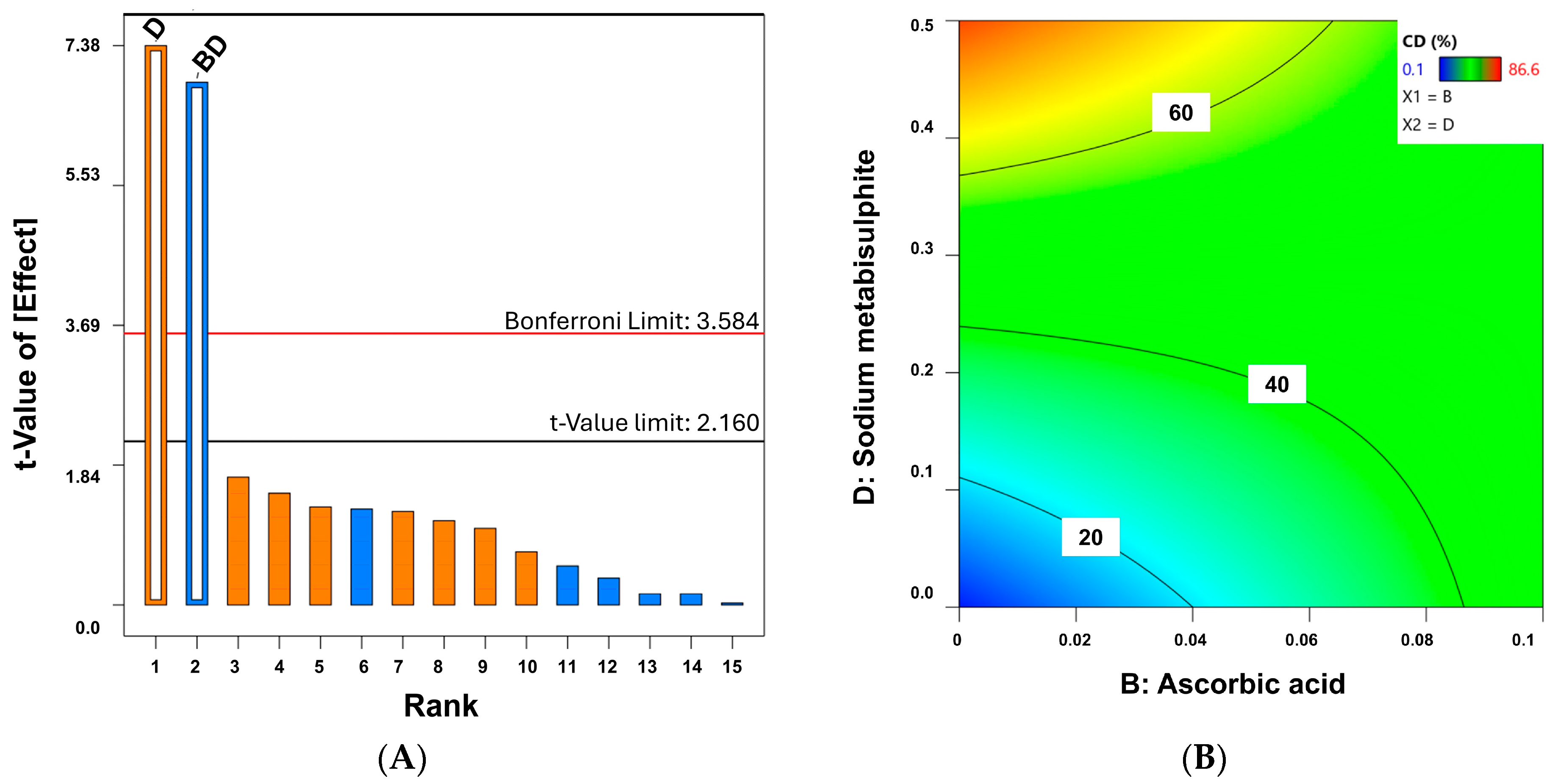
4.6. Quality by Design for Optimization of Physicochemical Stability of Melatonin Topical Formulations
4.7. Physical Characterization
4.8. Chemical Characterization by HPLC Melatonin Quantification
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnal-Forne, M.; Molina-Garcia, T.; Ortega, M.; Marcos-Garces, V.; Molina, P.; Ferrandez-Izquierdo, A.; Sepulveda, P.; Bodi, V.; Rios-Navarro, C.; Ruiz-Sauri, A. Changes in human skin composition due to intrinsic aging: A histologic and morphometric study. Histochem. Cell Biol. 2024, 162, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.K.; Bohm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczynski, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef] [PubMed]
- Day, D.; Burgess, C.M.; Kircik, L.H. Assessing the Potential Role for Topical Melatonin in an Antiaging Skin Regimen. J. Drugs Dermatol. 2018, 17, 966–969. [Google Scholar] [PubMed]
- Morreale, M.; Livrea, M.A. Synergistic effect of glycolic acid on the antioxidant activity of alpha-tocopherol and melatonin in lipid bilayers and in human skin homogenates. Biochem. Mol. Biol. Int. 1997, 42, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.C.; Garcia-Segura, J.C.; Delgado, D.C.; Romero, M.N.; Salgado, E.C.; Llorens, L.P. Compounded Melatonin Cream for the Prevention and Treatment of Radiation Dermatitis: A Case Report. Int. J. Pharm. Compd. 2022, 26, 6–8. [Google Scholar]
- Ongoren, B.; Kara, A.; Casettari, L.; Tiboni, M.; Lalatsa, A.; Sanz-Perez, A.; Gonzalez-Burgos, E.; Romero, A.; Juberias, A.; Torrado, J.J.; et al. Leveraging 3D-printed microfluidic micromixers for the continuous manufacture of melatonin loaded SNEDDS with enhanced antioxidant activity and skin permeability. Int. J. Pharm. 2024, 663, 124536. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Ramos, E.; Gil-Martin, E.; De Los Rios, C.; Egea, J.; Lopez-Munoz, F.; Pita, R.; Juberias, A.; Torrado, J.J.; Serrano, D.R.; Reiter, R.J.; et al. Melatonin as Modulator for Sulfur and Nitrogen Mustard-Induced Inflammation, Oxidative Stress and DNA Damage: Molecular Therapeutics. Antioxidants 2023, 12, 397. [Google Scholar] [CrossRef]
- USP-NF. Pharmacopoeia-National Formulary [USP 38 NF 33]; Md United States Pharmacopeial Convention: Rockville, MD, USA, 2015. [Google Scholar]
- Metta, S.; Amir, K.M.; Lakshmi, M.S.; Devi, P.; Shruthi, K. A Review: Pharmaceutical Gels and Its Types with Prominence Role of Its Drug Delivery Systems. Int. J. Res. Adn Anal. Rev. 2023, 10, 686–701. [Google Scholar]
- Eccleston, G.M. Emulsions and creams. In Aulton’s Pharmaceutics. The Design and Manufacture of Medicines, 5th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier: Edinburgh, UK, 2018; pp. 446–475. [Google Scholar]
- Spir, L.G.; Ataide, J.A.; De Lencastre Novaes, L.C.; Moriel, P.; Mazzola, P.G.; De Borba Gurpilhares, D.; Silveira, E.; Pessoa, A., Jr.; Tambourgi, E.B. Application of an aqueous two-phase micellar system to extract bromelain from pineapple (Ananas comosus) peel waste and analysis of bromelain stability in cosmetic formulations. Biotechnol. Prog. 2015, 31, 937–945. [Google Scholar] [CrossRef]
- Eros, I.; Soosne-Csanyi, E.; Selmeczi, B. Influence of viscosity on drug release from ointments, creams, gels and emulsions. Acta Pharm. Hung. 1994, 64, 57–61. [Google Scholar] [PubMed]
- Pemulen Technical Data Sheet. Available online: https://espanol.lubrizol.com/Personal-Care/Products/Product-Finder/Products-Data/Pemulen-TR-1-polymer (accessed on 6 March 2024).
- de Lima, J.A.; Paines, T.C.; Motta, M.H.; Weber, W.B.; Dos Santos, S.S.; Cruz, L.; da Silva, C.B. Novel Pemulen/Pullulan blended hydrogel containing clotrimazole-loaded cationic nanocapsules: Evaluation of mucoadhesion and vaginal permeation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Milic-Askrabic, J.; Vuleta, G.; Ibric, S.; Stupar, M. The Influence of Processing Variables on Performance of O/W Emulsion Gels Based on Polymeric Emulsifier (Pemulen (R)TR-2NF). Int. J. Cosmet. Sci. 1999, 21, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Haywood, A.; Burrell, A.; Breda, K.; George, R.; Testa, C.; Norris, R. Stability of melatonin in an extemporaneously compounded sublingual solution and hard gelatin capsule. Int. J. Pharm. Compd. 2009, 13, 170–174. [Google Scholar]
- Johnson, C.E.; Cober, M.P.; Thome, T.; Rouse, E. Stability of an extemporaneous alcohol-free melatonin suspension. Am. J. Health Syst. Pharm. 2011, 68, 420–423. [Google Scholar] [CrossRef]
- Pegoraro, N.S.; Camponogara, C.; Cruz, L.; Oliveira, S.M. Oleic acid exhibits an expressive anti-inflammatory effect in croton oil-induced irritant contact dermatitis without the occurrence of toxicological effects in mice. J. Ethnopharmacol. 2021, 267, 113486. [Google Scholar] [CrossRef]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Optimized formulation for topical administration of clotrimazole using Pemulen polymeric emulsifier. Drug Dev. Ind. Pharm. 2011, 37, 559–568. [Google Scholar] [CrossRef]
- Frei, G.; Haimhoffer, A.; Csapo, E.; Bodnar, K.; Vasvari, G.; Nemes, D.; Lekli, I.; Gyongyosi, A.; Bacskay, I.; Feher, P.; et al. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. [Google Scholar] [CrossRef]
- Palakkal, S.; Cortial, A.; Frusic-Zlotkin, M.; Soroka, Y.; Tzur, T.; Nassar, T.; Benita, S. Effect of cyclosporine A-tempol topical gel for the treatment of alopecia and anti-inflammatory disorders. Int. J. Pharm. 2023, 642, 123121. [Google Scholar] [CrossRef]
- Marriot, C. Rheology. In Aulton’s Pharmaceutics. The Design and Manufacture of Medicines, 5th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier: Edinburgh, UK, 2018; pp. 93–113. [Google Scholar]
- Kikwai, L.; Kanikkannan, N.; Babu, R.J.; Singh, M. Effect of vehicles on the transdermal delivery of melatonin across porcine skin in vitro. J. Control. Release 2002, 83, 307–311. [Google Scholar] [CrossRef]
- Rodriguez-Torrado, M.; Kara, A.; Torrado, S.; Romero, A.; Juberias, A.; Torrado, J.J.; Serrano, D.R. In Vitro and In Vivo Characteristics of Olive Oil as Excipient for Topical Administration. Pharmaceutics 2022, 14, 2615. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Oh, Y.K.; Kim, C.K. Effects of vehicles and enhancers on transdermal delivery of melatonin. Int. J. Pharm. 2001, 212, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Loffler, M. Rheology of cream gels and o/w emulsions stabilized by a polymeric sulphonic acid. Eur. Cosmet. 2001, 11, 26–29. [Google Scholar]
- Tehcnical Data Sheet of Pemulen. Introducing Pemulen® Polymeric Emulsifiers. Available online: https://www.lubrizol.com/-/media/Lubrizol/Health/TDS/TDS-114_Introducing_Pemulen_Polymeric_Emulsifiers.pdf (accessed on 21 August 2024).
- Lopez-Castillo, C.; Rodriguez-Fernandez, C.; Cordoba, M.; Torrado, J.J. Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with gamma-Cyclodextrins. Molecules 2018, 23, 3349. [Google Scholar] [CrossRef] [PubMed]
- Panyatip, P.; Nunthaboot, N.; Puthongking, P. In Silico ADME, Metabolism Prediction and Hydrolysis Study of Melatonin Derivatives. Int. J. Tryptophan Res. 2020, 13, 1178646920978245. [Google Scholar] [CrossRef]
- Tegeli, V.S.; Thorat, Y.S.; Shivsharan, U.S.; Gajeli, G.B.; Kumbhar, S.T.; Chougule, G.K. Pemulen as a versatile emulsifier. Int. J. Drug Formul. Res. 2011, 2, 52–63. [Google Scholar]
- Chickering, D.E.; Mathiowitz, E. Definitions, mechanisms, and theories of bioadhesion. In Bioadhesive Drug Delivery Systems (Fundamentals, Novel Approaches and Development); Mathiowitz, E., Chickering, D.E., III, Lehr, C.M., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 1–10. [Google Scholar]
- Goodrich, B.F. The science of rheology: Pharmaceutically applied. Chem. Eng. News 1991, 69. [Google Scholar]
- Szucs, M.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Vaghi, P.; Szabo-Revesz, P.; Eros, I. Mucoadhesive behaviour of emulsions containing polymeric emulsifier. Eur. J. Pharm. Sci. 2008, 34, 226–235. [Google Scholar] [CrossRef]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the skin: Understanding formulation and efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Chapter 15—Natural Antioxidants in Cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar]
- Pujol, I.; Pastor, F.J.; dos Santos Lazera, M.; Guarro, J. Evaluation of the Neo-Sensitabs diffusion method for determining the antifungal susceptibilities of Cryptococcus gattii isolates, using three different agar media. Rev. Iberoam. De Micol. 2008, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Gordo, M.J.; Matji, A.; Gonzalez, S.; Lalatsa, A.; Torrado, J.J. Tuning the Transdermal Delivery of Hydroquinone upon Formulation with Novel Permeation Enhancers. Pharmaceutics 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, R.; Statts, L.; de Jesus, J.A.; Dea-Ayuela, M.A.; Bautista, L.; Simao, R.; Bolas-Fernandez, F.; Ballesteros, M.P.; Laurenti, M.D.; Passero, L.F.D.; et al. Ultradeformable Lipid Vesicles Localize Amphotericin B in the Dermis for the Treatment of Infectious Skin Diseases. ACS Infect. Dis. 2020, 6, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Millipore, M. Strat-M Membrane. Available online: https://www.merckmillipore.com/ES/es/product/Strat-M-Membrane-for-Transdermal-Diffusion-Testing,MM_NF-C112892 (accessed on 18 July 2019).
- Lalatsa, A.; Emeriewen, K.; Protopsalti, V.; Skelton, G.; Saleh, G.M. Developing transcutaneous nanoenabled anaesthetics for eyelid surgery. Br. J. Ophthalmol. 2016, 100, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities Between Artificial Membrane Strat-M and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Levintova, Y.; Plakogiannis, F.M.; Bellantone, R.A. An improved in vitro method for measuring skin permeability that controls excess hydration of skin using modified Franz diffusion cells. Int. J. Pharm. 2011, 419, 96–106. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Poorahmary, B.; Škalko-Basne, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2011, 125, 180–188. [Google Scholar] [CrossRef]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef]
- Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E.; Mohammed, Y. Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics 2020, 12, 287. [Google Scholar] [CrossRef]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef]



| Membrane | Jss (µg/cm2/min) | Lag time (min) | P (cm/h) × 102 | D (cm2/h) × 103 |
|---|---|---|---|---|
| F20 | 1.13 ± 0.38 | 16.33 ± 8.52 | 6.80 ± 2.32 | 0.57 ± 0.16 |
| F0 | 1.52 ± 0.29 | 2.31 ± 0.54 | 9.12 ± 1.77 | 7.20 ± 1.59 |
| Formulation | Viscosity (Pa·s) | MV (µm) |
|---|---|---|
| C1 | 1.8 ± 0.4 | 21.2 ± 14.4 |
| C9 | 1.1 ± 0.3 | 28.3 ± 21.0 |
| C11 | 0.8 ± 0.2 | 43.8 ± 22.7 |
| C15 | 0.3 ± 0.1 | 34.0 ± 16.8 |
| Component | F0 | F5 | F10 | F20 |
|---|---|---|---|---|
| Melatonin | 0.1 | 0.1 | 0.1 | 0.1 |
| Methylparaben | 0.16 | 0.16 | 0.16 | 0.16 |
| Propylparaben | 0.04 | 0.04 | 0.04 | 0.04 |
| Ethanol 96 | 1.0 | 1.0 | 1.0 | 1.0 |
| Glycerine | 3 | 3 | 3 | 3 |
| Pemulen® TR-1 | 0.4 | 0.4 | 0.4 | 0.4 |
| Olive oil | 0 | 1.25 | 2.5 | 5 |
| Isopropyl myristate | 0 | 3.75 | 7.5 | 15 |
| Triethanolamine | pH 5.6 ± 0.3 | pH 5.6 ± 0.3 | pH 5.6 ± 0.3 | pH 5.6 ± 0.3 |
| Deionized water | Up to 100 mL | Up to 100 mL | Up to 100 mL | Up to 100 mL |
| QTPP | CMAs |
|---|---|
| Physical appearance after at least 12 months of storage (no color change or phase separation) | DL-α-tocopheryl acetate (absence or 0.05%) |
| Ascorbic acid (absence or 0.1%) | |
| Low chemical degradation (<10%) | EDTA (absence or 0.1%) |
| Sodium metabisulphite (absence or 0.5%) |
| Code Formulation | a | b | c | d | P.D. | C.D. |
|---|---|---|---|---|---|---|
| C1 | - | - | - | - | 1 | 0.1 |
| C2 | - | - | - | + | 4 | 81.1 |
| C3 | - | - | + | - | 2 | 0.5 |
| C4 | - | - | + | + | 3 | 72.7 |
| C5 | - | + | - | - | 4 | 39.4 |
| C6 | - | + | - | + | 2 | 44.9 |
| C7 | - | + | + | - | 3 | 57.2 |
| C8 | - | + | + | + | 2 | 62.5 |
| C9 | + | - | - | - | 1 | 1.4 |
| C10 | + | - | - | + | 3 | 86.6 |
| C11 | + | - | + | - | 1 | 9.1 |
| C12 | + | - | + | + | 4 | 81.6 |
| C13 | + | + | - | - | 4 | 50.5 |
| C14 | + | + | - | + | 3 | 24.3 |
| C15 | + | + | + | - | 2 | 36.2 |
| C16 | + | + | + | + | 4 | 62.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrado, J.J.; Anaya, B.J.; Kara, A.; Ongoren, B.; Esteban-Ruiz, S.; Laguna, A.; Guillén, A.; Saro, M.G.; Serrano, D.R. Unraveling the Impact of the Oil Phase on the Physicochemical Stability and Skin Permeability of Melatonin Gel Formulations. Gels 2024, 10, 595. https://doi.org/10.3390/gels10090595
Torrado JJ, Anaya BJ, Kara A, Ongoren B, Esteban-Ruiz S, Laguna A, Guillén A, Saro MG, Serrano DR. Unraveling the Impact of the Oil Phase on the Physicochemical Stability and Skin Permeability of Melatonin Gel Formulations. Gels. 2024; 10(9):595. https://doi.org/10.3390/gels10090595
Chicago/Turabian StyleTorrado, Juan J., Brayan J. Anaya, Aytug Kara, Baris Ongoren, Sofía Esteban-Ruiz, Almudena Laguna, Alicia Guillén, Miguel G. Saro, and Dolores R. Serrano. 2024. "Unraveling the Impact of the Oil Phase on the Physicochemical Stability and Skin Permeability of Melatonin Gel Formulations" Gels 10, no. 9: 595. https://doi.org/10.3390/gels10090595
APA StyleTorrado, J. J., Anaya, B. J., Kara, A., Ongoren, B., Esteban-Ruiz, S., Laguna, A., Guillén, A., Saro, M. G., & Serrano, D. R. (2024). Unraveling the Impact of the Oil Phase on the Physicochemical Stability and Skin Permeability of Melatonin Gel Formulations. Gels, 10(9), 595. https://doi.org/10.3390/gels10090595









