Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications
Abstract
1. Introduction
2. Types of Ionizing Radiation
2.1. E-Beam Radiation
2.2. Gamma Radiation
2.3. Comparison Between E-Beam and Gamma Irradiation Technology
3. Radiation-Assisted Material Preparation Methods
3.1. Radiation-Induced Crosslinking
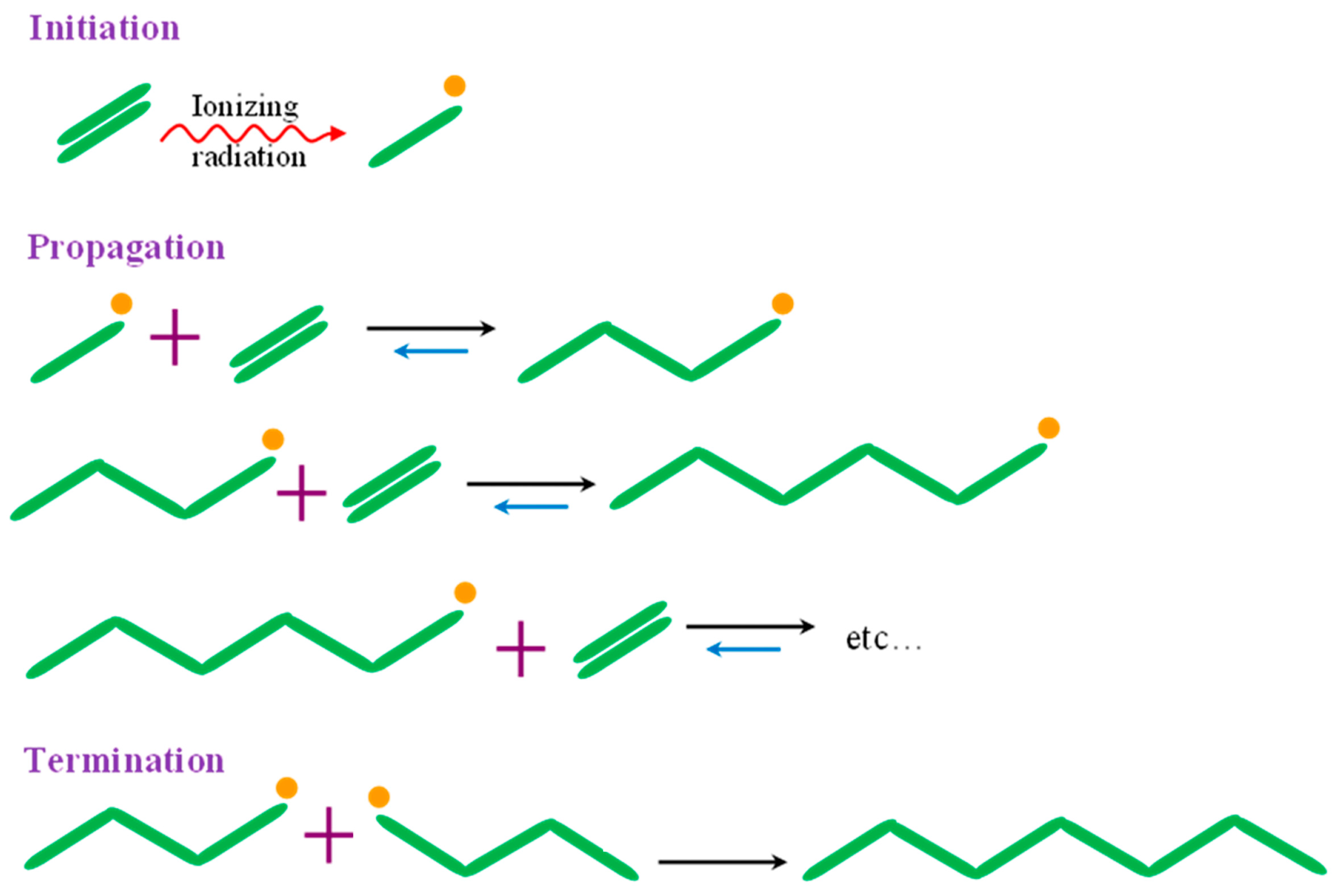
3.2. Radiation-Induced Polymerization
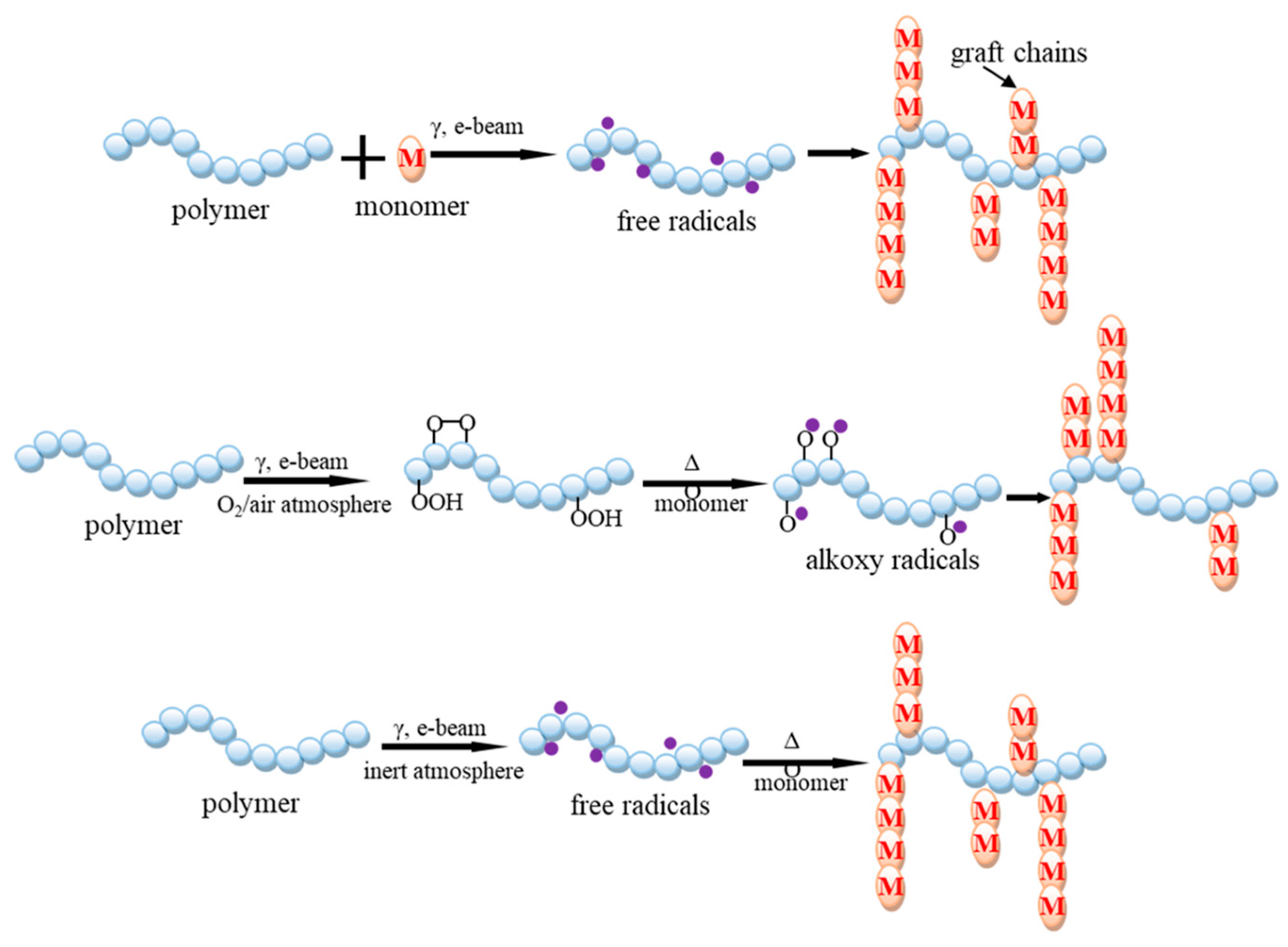
3.3. Radiation-Induced Grafting
3.4. Radiation-Induced Degradation
4. Impact of Ionizing Radiation on the Formation of Polymeric Hydrogels for TE
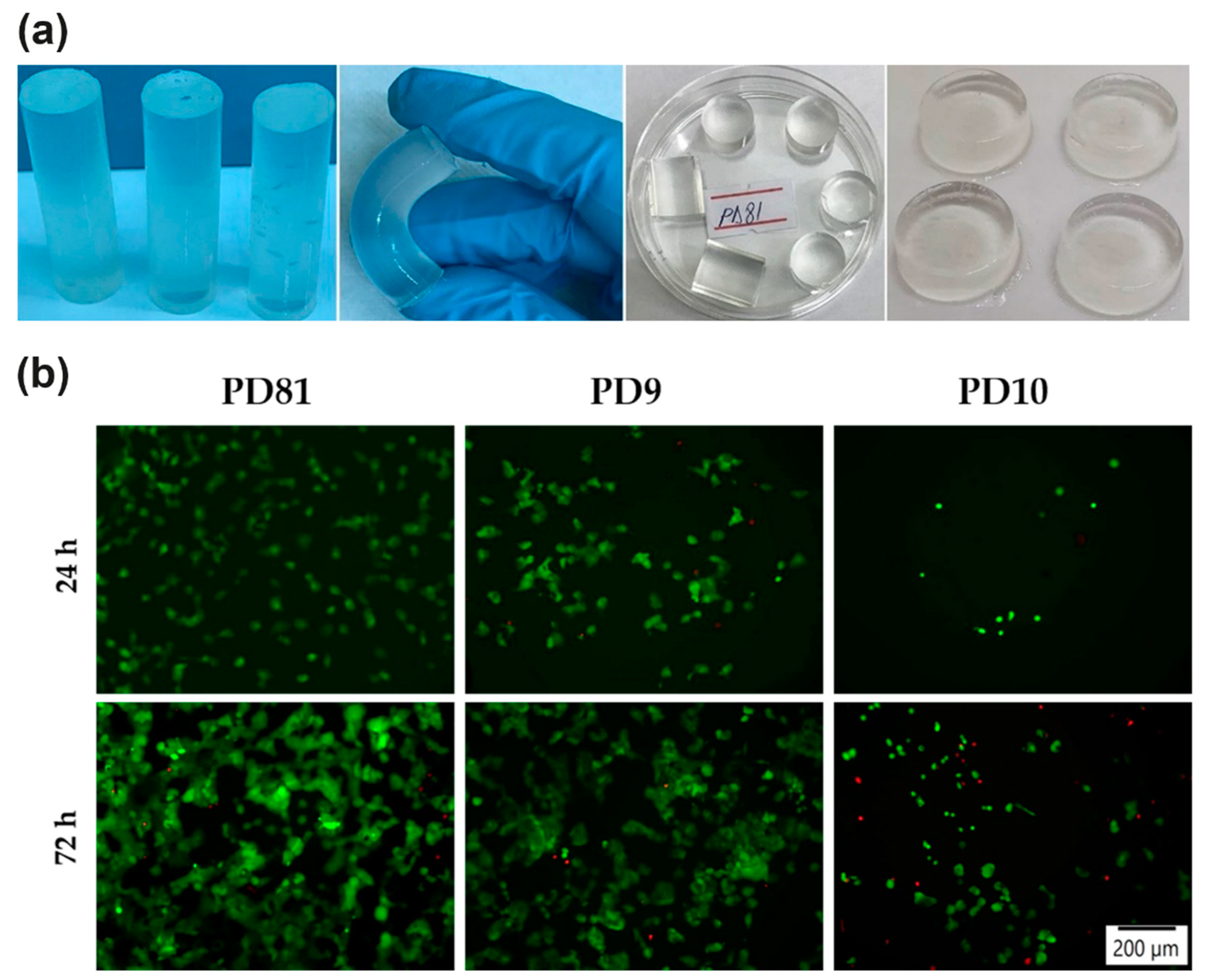
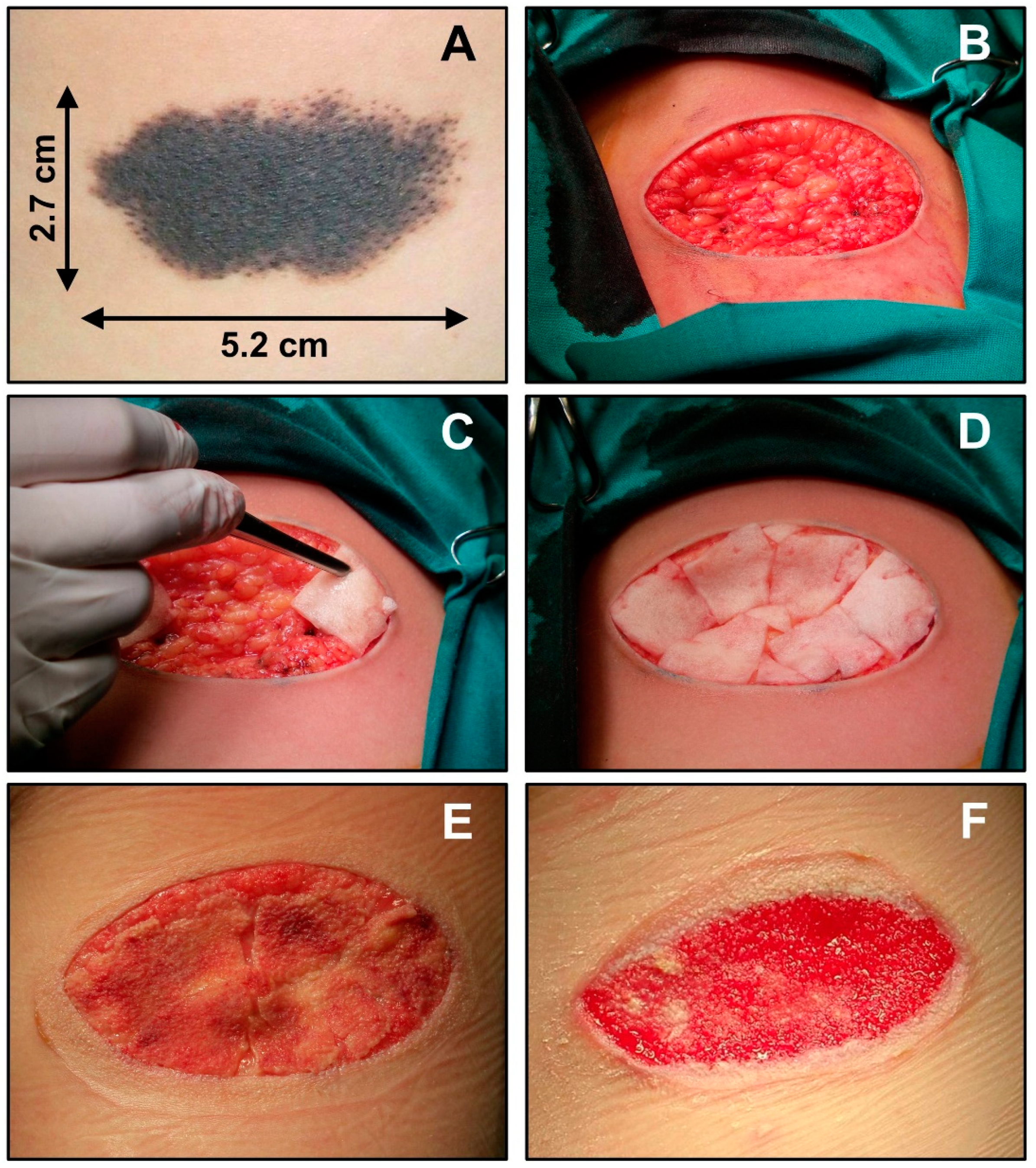
4.1. E-Beam-Irradiation-Based Hydrogel Synthesis for TE
4.2. Gamma-Irradiation-Based Hydrogel Synthesis for TE
4.3. Regulatory Considerations and Clinical Translation of Radiation-Synthesized Hydrogels
5. Conclusions, Challenges, and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghorbani, M.; Roshangar, L. Construction of Collagen/Nanocrystalline Cellulose Based-Hydrogel Scaffolds: Synthesis, Characterization, and Mechanical Properties Evaluation. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 142–148. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Liu, S. Physically Cross-Linked Gellan Gum/Hydrophobically Associated Polyacrylamide Double Network Hydrogel for Cartilage Repair. Eur. Polym. J. 2022, 167, 111074. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- More, A.P.; Chapekar, S. Irradiation Assisted Synthesis of Hydrogel: A Review. Polym. Bull. 2024, 81, 5839–5908. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulański, P. Synthesis of Hydrogels by Irradiation of Polymers in Aqueous Solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Kadlubowski, S. Radiation-Induced Synthesis of Nanogels Based on Poly(N-Vinyl-2-Pyrrolidone)—A Review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Ulański, P.; Olejnik, A.K.; Nowicka, G.; Panczenko-Kresowska, B.; Rosiak, J.M. Diet Supplement Based on Radiation-modified Chitosan and Radiation-synthesized Polyvinylpyrrolidone Microgels: Influence on the Liver Weight in Rats Fed a Fat- and Cholesterol-rich Diet. J. Appl. Polym. Sci. 2007, 105, 169–176. [Google Scholar] [CrossRef]
- Ulanski, P.; von Sonntag, C. OH-Radical-Induced Chain Scission of Chitosan in the Absence and Presence of Dioxygen. J. Chem. Soc. Perkin Trans. 2 2000, 10, 2022–2028. [Google Scholar] [CrossRef]
- Raza, M.A.; Park, S.H. Irradiated Ch/GG/PVP-based Stimuli-responsive Hydrogels for Controlled Drug Release. J. Appl. Polym. Sci. 2020, 137, 49041. [Google Scholar] [CrossRef]
- Raza, M.A.; Lim, Y.-M.; Lee, S.-W.; Seralathan, K.-K.; Park, S.H. Synthesis and Characterization of Hydrogels Based on Carboxymethyl Chitosan and Poly(Vinylpyrrolidone) Blends Prepared by Electron Beam Irradiation Having Anticancer Efficacy, and Applications as Drug Carrier for Controlled Release of Drug. Carbohydr. Polym. 2021, 258, 117718. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Jeong, J.-O.; Park, S.H. State-of-the-Art Irradiation Technology for Polymeric Hydrogel Fabrication and Application in Drug Release System. Front. Mater. 2021, 8, 769436. [Google Scholar] [CrossRef]
- Raza, M.A.; Kim, S.-A.; Kim, D.I.; Song, M.-K.; Han, S.S.; Park, S.H. Synthesis of Carboxymethyl Chitosan–Guar Gum–Poly(Vinylpyrrolidone) Ternary Blended Hydrogels with Antibacterial/Anticancer Efficacy and Drug Delivery Applications. J. Biomater. Sci. Polym. Ed. 2024, 35, 1706–1725. [Google Scholar] [CrossRef] [PubMed]
- Manas, D.; Manas, M.; Mizera, A.; Stoklasek, P.; Navratil, J.; Sehnalek, S.; Drabek, P. The High Density Polyethylene Composite with Recycled Radiation Cross-Linked Filler of RHDPEx. Polymers 2018, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Arora, S. Exploring the Impact of Gamma Rays and Electron Beam Irradiation on Physico-Mechanical Properties of Polymers & Polymer Composites: A Comprehensive Review. Nucl. Instrum. Methods Phys. Res. B 2024, 549, 165297. [Google Scholar] [CrossRef]
- Cota, S.S.; Vasconcelos, V.; Senne, M., Jr.; Carvalho, L.L.; Rezende, D.B.; Côrrea, R.F. Changes in Mechanical Properties Due to Gamma Irradiation of High-Density Polyethylene (HDPE). Braz. J. Chem. Eng. 2007, 24, 259–265. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Abdeen, Z.I. Radiation Crosslinking of Polyurethanes: Characterization by FTIR, TGA, SEM, XRD, and Raman Spectroscopy. J. Polym. 2016, 2016, 9802514. [Google Scholar] [CrossRef]
- Park, J.S.; Lim, Y.M.; Nho, Y.C. Preparation of High Density Polyethylene/Waste Polyurethane Blends Compatibilized with Polyethylene-Graft-Maleic Anhydride by Radiation. Materials 2015, 8, 1626–1635. [Google Scholar] [CrossRef]
- Yasin, T.; Khan, S.; Shafiq, M.; Gill, R. Radiation Crosslinking of Styrene-Butadiene Rubber Containing Waste Tire Rubber and Polyfunctional Monomers. Radiat. Phys. Chem. 2015, 106, 343–347. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Tong, X.; Yang, F. Engineering Interpenetrating Network Hydrogels as Biomimetic Cell Niche with Independently Tunable Biochemical and Mechanical Properties. Biomaterials 2014, 35, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Ashiku, S.K.; Randolph, M.A.; Vacanti, C.A. Tissue Engineered Cartilage. Mater. Sci. Forum 1997, 250, 129–150. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–Origin Polymers as Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, A.G. Radiation Technologies: The Future Is Today. Radiat. Phys. Chem. 2023, 213, 111233. [Google Scholar] [CrossRef]
- Kurashima, S.; Satoh, T.; Saitoh, Y.; Yokota, W. Irradiation Facilities of the Takasaki Advanced Radiation Research Institute. Quantum Beam Sci. 2017, 1, 2. [Google Scholar] [CrossRef]
- Şener Raman, T.; Kuehnert, M.; Daikos, O.; Scherzer, T.; Krömmelbein, C.; Mayr, S.G.; Abel, B.; Schulze, A. A Study on the Material Properties of Novel PEGDA/Gelatin Hybrid Hydrogels Polymerized by Electron Beam Irradiation. Front. Chem. 2023, 10, 1094981. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, I.; Poursaleh, A.M.; Khalafi, H. Design Study of Double-Layer Beam Trajectory Accelerator Based on the Rhodotron Structure. Nucl. Instrum. Methods Phys. Res. A 2016, 828, 72–80. [Google Scholar] [CrossRef]
- Krist, P.; Bíla, J.; Granja, C.; Leroy, C. Microtron Modelling and Control. AIP Conf. Proc. 2010, 1204, 185–187. [Google Scholar]
- Martin, D.I.; Jianu, A.; Bestea, V.; Toma, M.; Oproiu, C.; Marghitu, S. Control System for ALID-7 Linear Accelerator Used in Radiation Processing. Rev. Sci. Instrum. 1999, 70, 2984–2987. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Niculescu, M.; Ighigeanu, D. Obtaining a New Type of Polyelectrolyte Based on Acrylamide and Hydrolyzed Collagen by Electron Beam Irradiation. Polym. Bull. 2017, 74, 1299–1326. [Google Scholar] [CrossRef]
- Ticoş, D.; Scurtu, A.; Oane, M.; Diplaşu, C.; Giubega, G.; Călina, I.; Ticoş, C.M. Complementary Dosimetry for a 6 MeV Electron Beam. Results Phys. 2019, 14, 102377. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, Z.; Zhao, H.; Tang, F.; Lu, J.; Wei, Q.; Zhang, X. Electron Beam Irradiation Modification of Collagen Membrane. Biomaterials 2006, 27, 15–23. [Google Scholar] [CrossRef]
- Henley, E.M.; Dash, J.G. Electromagnetic Waves. In Physics Around Us; World Scientific: London, UK, 2012; pp. 253–268. [Google Scholar]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D. Effects of Sterilization on Poly(Ethylene Glycol) Hydrogels. J. Biomed. Mater. Res. A 2008, 87, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.d.S.; Islam, M.; Hasan, M.d.K.; Nam, K.-W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Karim, A.A. Impact of Radiation Processing on Starch. Compr. Rev. Food Sci. Food Saf. 2009, 8, 44–58. [Google Scholar] [CrossRef]
- Sonnier, R.; Leroy, E.; Clerc, L.; Bergeret, A.; Lopez-Cuesta, J.M. Polyethylene/Ground Tyre Rubber Blends: Influence of Particle Morphology and Oxidation on Mechanical Properties. Polym. Test. 2007, 26, 274–281. [Google Scholar] [CrossRef]
- Naikwadi, A.T.; Sharma, B.K.; Bhatt, K.D.; Mahanwar, P.A. Gamma Radiation Processed Polymeric Materials for High Performance Applications: A Review. Front. Chem. 2022, 10, 837111. [Google Scholar] [CrossRef]
- Mohamady Ghobashy, M.; Awad, A.; Elhady, M.A.; Elbarbary, A.M. Silver Rubber-Hydrogel Nanocomposite as PH-Sensitive Prepared by Gamma Radiation: Part I. Cogent Chem. 2017, 3, 1328770. [Google Scholar] [CrossRef]
- Abdel-Ghaffar, A.M. Radiation Synthesis and Modification of Biopolymers and Polymeric Composites for Biomedical Applications. Polym. Polym. Compos. 2023, 31, 09673911231166636. [Google Scholar] [CrossRef]
- Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Baik, S.-H. Humectants Effect on Aqueous Fluids Absorption of γ-Irradiated PVA Hydrogel Followed by Freeze Thawing. Radiat. Phys. Chem. 2010, 79, 650–653. [Google Scholar] [CrossRef]
- El-damhougy, T.K.; Ahmed, A.S.I.; Gaber, G.A.; Mazied, N.A.; Bassioni, G. Radiation Synthesis for a Highly Sensitive Colorimetric Hydrogel Sensor-Based p(AAc/AMPS)-TA for Metal Ion Detection. Results Mater. 2021, 9, 100169. [Google Scholar] [CrossRef]
- Miller, R.B. Overview of Food Irradiation Technology and Concepts. In Electronic Irradiation of Foods; Springer: Boston, MA, USA, 2005; pp. 17–42. [Google Scholar]
- Makuuchi, K.; Cheng, S. Basic Concepts of Radiation Processing. In Radiation Processing of Polymer Materials and Its Industrial Applications; Wiley: New York, NY, USA, 2012; pp. 1–25. [Google Scholar]
- Al-Assaf, S. The Radiation Chemistry of Polysaccharides; International Atomic Energy Agency: Vienna, Austria, 2017; ISBN 9789201015167. [Google Scholar]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, M.A.; Bulone, D.; Veres, M.; Spinella, A.; Spadaro, G.; Dispenza, C. Structure of E-Beam Sculptured Poly(N-Vinylpyrrolidone) Networks across Different Length-Scales, from Macro to Nano. Polymer 2013, 54, 54–64. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network Structure Studies on γ–Irradiated Collagen–PVP Superabsorbent Hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Leonard, D.J.; Pick, L.T.; Farrar, D.F.; Dickson, G.R.; Orr, J.F.; Buchanan, F.J. The Modification of PLA and PLGA Using Electron-beam Radiation. J. Biomed. Mater. Res. A 2009, 89, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Medina, E.; Licea-Claveríe, A.; Cornejo-Bravo, J.M.; Arndt, K.F. Effect of Method of Preparation on Properties of Temperature and PH-Sensitive Gels: Chemical Crosslinking versus Irradiation with e-Beam. React. Funct. Polym. 2007, 67, 67–80. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Nanostructuring of Polymers by Controlling of Ionizing Radiation-Induced Free Radical Polymerization, Copolymerization, Grafting and Crosslinking by RAFT Mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.K.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface Functionalization of Biomaterials by Radical Polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Chmielewski, A.G. Advances in Radiation Chemistry of Polymers—Proceedings of the a Technical Meeting Held in Notre Dame, IN, USA, 13–17 September 2003; IAEA: Vienna, Austria, 2004; ISBN 9201125046. [Google Scholar]
- Sun, Y.; Chmielewski, A.G. Applications of Ionizing Radiation in Materials Processing; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; ISBN 9788393393589. [Google Scholar]
- Stannett, V.T. Radiation Grafting—State-of-the-Art. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1990, 35, 82–87. [Google Scholar] [CrossRef]
- Seman, N.; Tarmizi, Z.I.; Ali, R.R.; Salleh, M.S.N. Modified Surface Polymer Prepared by Using Radiation-Induced Grafting Co-Polymerization Method and Its Application: A Mini Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1091, 012065. [Google Scholar] [CrossRef]
- Nisar, S.; Pandit, A.H.; Nadeem, M.; Pandit, A.H.; Rizvi, M.M.A.; Rattan, S. γ-Radiation Induced L-Glutamic Acid Grafted Highly Porous, PH-Responsive Chitosan Hydrogel Beads: A Smart and Biocompatible Vehicle for Controlled Anti-Cancer Drug Delivery. Int. J. Biol. Macromol. 2021, 182, 37–50. [Google Scholar] [CrossRef]
- Gaylord, N.G.; Adler, G. Radiation Chemistry of Polymeric Systems High Polymers, A. Chapiro, Ed., Interscience, New York, 1962, xvi + 712 pp. $21.00. J. Polym. Sci. Part A 1963, 1, 2237. [Google Scholar] [CrossRef]
- Henglein, A.J.W.T.; Spinks, R.J. Woods: An Introduction to Radiation Chemistry, Third Edition, John-Wiley and Sons, Inc., New York, Toronto 1990. ISBN 0-471-61403-3. 574 Seiten, Preis: DM 91, 45. Berichte Bunsenges. Phys. Chem. 1991, 95, 451. [Google Scholar] [CrossRef]
- Adamus-Wlodarczyk, A.; Wach, R.; Ulanski, P.; Rosiak, J.; Socka, M.; Tsinas, Z.; Al-Sheikhly, M. On the Mechanisms of the Effects of Ionizing Radiation on Diblock and Random Copolymers of Poly(Lactic Acid) and Poly(Trimethylene Carbonate). Polymers 2018, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, G.; Alessi, S.; Dispenza, C. Ionizing Radiation-Induced Crosslinking and Degradation of Polymers. Appl. Ioniz. Radiat. Mater. Process. 2017, 1, 167–182. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Yang, J.; Rao, L.; Wang, Y.; Zhao, Y.; Liu, D.; Wang, Z.; Fu, L.; Wang, Y.; Yang, X.; Li, Y.; et al. Recent Advances in Smart Hydrogels Prepared by Ionizing Radiation Technology for Biomedical Applications. Polymers 2022, 14, 4377. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Demeter, M.; Scărișoreanu, A.; Călina, I. State of the Art of Hydrogel Wound Dressings Developed by Ionizing Radiation. Gels 2023, 9, 55. [Google Scholar] [CrossRef]
- Haema, K.; Oyama, T.G.; Kimura, A.; Taguchi, M. Radiation Stability and Modification of Gelatin for Biological and Medical Applications. Radiat. Phys. Chem. 2014, 103, 126–130. [Google Scholar] [CrossRef]
- Haryanto; Singh, D.; Han, S.S.; Son, J.H.; Kim, S.C. Poly(Ethylene Glycol) Dicarboxylate/Poly(Ethylene Oxide) Hydrogel Film Co-Crosslinked by Electron Beam Irradiation as an Anti-Adhesion Barrier. Mater. Sci. Eng. C 2015, 46, 195–201. [Google Scholar] [CrossRef]
- Haryanto; Singh, D.; Huh, P.H.; Kim, S.C. Hyperbranched Poly(Glycidol)/Poly(Ethylene Oxide) Crosslinked Hydrogel for Tissue Engineering Scaffold Using E-beams. J. Biomed. Mater. Res. A 2016, 104, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.; Hietschold, P.; Krömmelbein, C.; Kunschmann, T.; Konieczny, R.; Knolle, W.; Mierke, C.T.; Zink, M.; Mayr, S.G. Design of Biomimetic Collagen Matrices by Reagent-Free Electron Beam Induced Crosslinking: Structure-Property Relationships and Cellular Response. Mater. Des. 2019, 168, 107606. [Google Scholar] [CrossRef]
- Demeter, M.; Meltzer, V.; Călina, I.; Scărișoreanu, A.; Micutz, M.; Albu Kaya, M.G. Highly Elastic Superabsorbent Collagen/PVP/PAA/PEO Hydrogels Crosslinked via e-Beam Radiation. Radiat. Phys. Chem. 2020, 174, 108898. [Google Scholar] [CrossRef]
- Dehghan-Niri, M.; Vasheghani-Farahani, E.; Baghaban Eslaminejad, M.; Tavakol, M.; Bagheri, F. Physicomechanical, Rheological and in Vitro Cytocompatibility Properties of the Electron Beam Irradiated Blend Hydrogels of Tyramine Conjugated Gum Tragacanth and Poly (Vinyl Alcohol). Mater. Sci. Eng. C 2020, 114, 111073. [Google Scholar] [CrossRef] [PubMed]
- Krömmelbein, C.; Mütze, M.; Konieczny, R.; Schönherr, N.; Griebel, J.; Gerdes, W.; Mayr, S.G.; Riedel, S. Impact of High-Energy Electron Irradiation on Mechanical, Structural and Chemical Properties of Agarose Hydrogels. Carbohydr. Polym. 2021, 263, 117970. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, C.; Vroman, I.; Coqueret, X.; Alix, S. Effects of Electron Beam Irradiation on 3D-Printed Biopolymers for Bone Tissue Engineering. J. Compos. Sci. 2021, 5, 182. [Google Scholar] [CrossRef]
- Riedel, S.; Ward, D.; Kudláčková, R.; Mazur, K.; Bačáková, L.; Kerns, J.G.; Allinson, S.L.; Ashton, L.; Koniezcny, R.; Mayr, S.G.; et al. Electron Beam-Treated Enzymatically Mineralized Gelatin Hydrogels for Bone Tissue Engineering. J. Funct. Biomater. 2021, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Krömmelbein, C.; Xie, X.; Seifert, J.; Konieczny, R.; Friebe, S.; Käs, J.; Riedel, S.; Mayr, S.G. Electron Beam Treated Injectable Agarose/Alginate Beads Prepared by Electrospraying. Carbohydr. Polym. 2022, 298, 120024. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Lei, F.; Dohlman, C.H.; Gonzalez-Andrades, M.; Guild, C.; Paschalis, E.I.; Chodosh, J. Critical Media Attributes in E-Beam Sterilization of Corneal Tissue. Acta Biomater. 2022, 138, 218–227. [Google Scholar] [CrossRef]
- Ling, Y.; Chen, L.; Huang, M.; Zhou, C.; Yang, L.; Niu, H.; Su, L.; Yang, Y.; Pirraco, R.P.; Reis, R.L.; et al. A Novel Method for the Preparation of Poly (Acrylamide-Co-Acrylonitrile) Upper Critical Solution Temperature Thermosensitive Hydrogel by the Partial Dehydration of Acrylamide Grafted Polypropylene Sheets. Gels 2022, 8, 345. [Google Scholar] [CrossRef]
- Demeter, M.; Negrescu, A.M.; Calina, I.; Scarisoreanu, A.; Albu Kaya, M.; Micutz, M.; Dumitru, M.; Cimpean, A. Synthesis, Physicochemical Characteristics, and Biocompatibility of Multi-Component Collagen-Based Hydrogels Developed by E-Beam Irradiation. J. Funct. Biomater. 2023, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Niri, M.; Vasheghani-Farahani, E.; Eslaminejad, M.B.; Tavakol, M.; Bagheri, F. Preparation of Gum Tragacanth/Poly (Vinyl Alcohol)/Halloysite Hydrogel Using Electron Beam Irradiation with Potential for Bone Tissue Engineering. Carbohydr. Polym. 2023, 305, 120548. [Google Scholar] [CrossRef] [PubMed]
- Šrámková, P.; Kučka, J.; Kroneková, Z.; Lobaz, V.; Šlouf, M.; Mičušík, M.; Šepitka, J.; Kleinová, A.; Chorvát, D.; Mateášik, A.; et al. Electron Beam Irradiation as a Straightforward Way to Produce Tailorable Non-Biofouling Poly(2-Methyl-2-Oxazoline) Hydrogel Layers on Different Substrates. Appl. Surf. Sci. 2023, 625, 157061. [Google Scholar] [CrossRef]
- Demeter, M.; Călina, I.; Scărișoreanu, A.; Mitran, V.; Popa, M.; Cîmpean, A.; Chifiriuc, M.C.; Micutz, M.; Matei, E.; Mitu, B. Biocompatible and Antimicrobial Chitosan/PVP/PEO/PAA/AgNP Composite Hydrogels Synthesized by e-Beam Cross-Linking. Radiat. Phys. Chem. 2024, 216, 111391. [Google Scholar] [CrossRef]
- Cairns, M.-L.; Sykes, A.; Dickson, G.R.; Orr, J.F.; Farrar, D.; Dumba, A.; Buchanan, F.J. Through-Thickness Control of Polymer Bioresorption via Electron Beam Irradiation. Acta Biomater. 2011, 7, 548–557. [Google Scholar] [CrossRef] [PubMed]
- López-Barriguete, J.E.; Flores-Rojas, G.G.; López-Saucedo, F.; Isoshima, T.; Bucio, E. Improving Thermo-Responsive Hydrogel Films by Gamma Rays and Loading of Cu and Ag Nanoparticles. J. Appl. Polym. Sci. 2021, 138, 49841. [Google Scholar] [CrossRef]
- Gujjar, S.; Tyagi, A.; Sainger, S.; Bharti, P.; Nain, V.; Sood, P.; Jayabal, P.; Sharma, J.C.; Sharma, P.; Rajput, S.; et al. Biocompatible Human Placental Extracellular Matrix Derived Hydrogels. Adv. Biol. 2024, 8, e2300349. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Cho, Y.; Choi, D.H.; Park, M.J.; Yang, J.H.; Chung, S. Gamma Irradiation Exposure for Collapsed Cell Junctions and Reduced Angiogenesis of 3-D in Vitro Blood Vessels. Sci. Rep. 2021, 11, 18230. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Silva, J.C.; Figueiredo, L.; Ferreira, F.C.; Kotov, N.A.; Colaço, R.; Serro, A.P. High-Performance Bilayer Composites for the Replacement of Osteochondral Defects. Biomater. Sci. 2022, 10, 5856–5875. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Sartore, L. Effects of Gamma Sterilization on the Physicomechanical and Thermal Properties of Gelatin-Based Novel Hydrogels. Polym. Eng. Sci. 2019, 59, 2533–2540. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, N.; Zeng, J.; Liang, Z.; Qi, Z.; Jiang, H.; Chen, H.; Liu, X. Chondrogenic Differentiation of Adipose-Derived Stromal Cells Induced by Decellularized Cartilage Matrix/Silk Fibroin Secondary Crosslinking Hydrogel Scaffolds with a Three-Dimensional Microstructure. Polymers 2023, 15, 1868. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, S.R.; El-Samanody, E.S.A.; Khatab, M.; Abdel-Monem, Y.K.; Korraa, S.; Senna, M.M.H. Scaffold Fabrication for Tissue Engineering Based on Radiation Copolymerization of Polymer Blends: (I) Zein/Poly (Vinyl Butyral) Blends. Polym. Eng. Sci. 2023, 63, 3507–3518. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A. Radiation Synthesis of Hydrogels with Silver Nanoparticles for Use as an Antimicrobial Burn Wound Dressing. Polym. Sci. Ser. B 2022, 64, 188–197. [Google Scholar] [CrossRef]
- Jeong, J.O.; Jeong, S.I.; Lim, Y.M.; Park, J.S. Effective BMP-2 Release and Mineralization on a Graphene Oxide/Polyvinylpyrrolidone Hydrogel Forming Poly (ε-Caprolactone) Nanofibrous Scaffolds. Materials 2022, 15, 8642. [Google Scholar] [CrossRef]
- Peng, W.; Lu, X.; Wu, J.; Wang, Y.; Zhu, X.; Ouyang, H.; Li, L.; Wu, J.; Liu, Y.; Bao, J. Autoclaving PHEMA-Based Hydrogels Immersed in Deionized Water Has No Effect on Physicochemical Properties and Cell Behaviors. ACS Omega 2022, 7, 32038–32045. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Espona-Noguera, A.; Galvez-Martin, P.; López-Ruiz, E.; Marchal, J.A.; López-Donaire, M.L.; Zabala, A.; Ciriza, J.; Saenz-del-Burgo, L.; et al. Development, Characterization and Sterilisation of Nanocellulose-Alginate-(Hyaluronic Acid)- Bioinks and 3D Bioprinted Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2021, 126, 112160. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Chan, S.W.; Comeau, P.A.; Willett, T.L.; Yim, E.K.F. Effect of Sterilization Treatment on Mechanical Properties, Biodegradation, Bioactivity and Printability of GelMA Hydrogels. Biomed. Mater. 2020, 15, 065017. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Yu, H.; Sun, M.; Li, Z.; Zhao, F.; Ao, Y.; Chen, H. Investigation on the Structure and Mechanical Properties of Highly Tunable Elastomeric Silk Fibroin Hydrogels Cross-Linked by Î3-Ray Radiation. ACS Appl. Bio Mater. 2020, 3, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Raafat, A.I.; Kamal, H.; Sharada, H.M.; Abd elhalim, S.A.; Mohamed, R.D. Radiation Synthesis of Magnesium Doped Nano Hydroxyapatite/(Acacia-Gelatin) Scaffold for Bone Tissue Regeneration: In Vitro Drug Release Study. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2890–2906. [Google Scholar] [CrossRef]
- Singh, B.; Rajneesh. Gamma Radiation Synthesis and Characterization of Gentamicin Loaded Polysaccharide Gum Based Hydrogel Wound Dressings. J. Drug Deliv. Sci. Technol. 2018, 47, 200–208. [Google Scholar] [CrossRef]
- López-Barriguete, J.E.; Isoshima, T.; Bucio, E. Development and Characterization of Thermal Responsivehydrogel Films for Biomedical Sensor Application. Mater. Res. Express 2018, 5, 045703. [Google Scholar] [CrossRef]
- Critchley, S.; Cunniffe, G.; O’Reilly, A.; Diaz-Payno, P.; Schipani, R.; McAlinden, A.; Withers, D.; Shin, J.; Alsberg, E.; Kelly, D.J. Regeneration of Osteochondral Defects Using Developmentally Inspired Cartilaginous Templates. Tissue Eng. Part A 2019, 25, 159–171. [Google Scholar] [CrossRef]
- González-Torres, M.; Leyva-Gómez, G.; Rivera, M.; Krötzsch, E.; Rodríguez-Talavera, R.; Rivera, A.L.; Cabrera-Wrooman, A. Biological Activity of Radiation-Induced Collagen–Polyvinylpyrrolidone–PEG Hydrogels. Mater. Lett. 2018, 214, 224–227. [Google Scholar] [CrossRef]
- Weinstein-Oppenheimer, C.R.; Brown, D.I.; Coloma, R.; Morales, P.; Reyna-Jeldes, M.; Díaz, M.J.; Sánchez, E.; Acevedo, C.A. Design of a Hybrid Biomaterial for Tissue Engineering: Biopolymer-Scaffold Integrated with an Autologous Hydrogel Carrying Mesenchymal Stem-Cells. Mater. Sci. Eng. C 2017, 79, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk Fibroin/Hydroxyapatite Composite Hydrogel Induced by Gamma-Ray Irradiation for Bone Tissue Engineering. Biomater. Res. 2017, 21, s40824-017. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Castellanos, A.; Ocampo-García, B.E.; Domínguez-García, M.V.; Flores-Estrada, J.; Flores-Merino, M.V. Hydrogels Based on Poly(Ethylene Glycol) as Scaffolds for Tissue Engineering Application: Biocompatibility Assessment and Effect of the Sterilization Process. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef]
- Zhao, L.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C.; Kim, S.Y. Gamma Ray-Induced Synthesis of Hyaluronic Acid/Chondroitin Sulfate-Based Hydrogels for Biomedical Applications. Radiat. Phys. Chem. 2015, 106, 404–412. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Chen, W.; Xu, L.; Wei, S.; Zheng, Y.; Zhai, M. Biological Behavior of Fibroblast on Contractile Collagen Hydrogel Crosslinked by γ-Irradiation. J. Biomed. Mater. Res. A 2014, 102, 2669–2679. [Google Scholar] [CrossRef]
- Zhao, L.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C.; Kim, S.Y. Hyaluronic Acid/Chondroitin Sulfate-Based Hydrogel Prepared by Gamma Irradiation Technique. Carbohydr. Polym. 2014, 102, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Xu, L.; Wei, S.; Zhai, M. Radiation Cross-Linked Collagen/Dextran Dermal Scaffolds: Effects of Dextran on Cross-Linking and Degradation. J. Biomater. Sci. Polym. Ed. 2015, 26, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Lim, J.Y.; Park, J.S.; Gwon, H.J.; Jeong, S.I.; Lim, Y.M. Radiation-Induced Biomimetic Modification of Dual-Layered Nano/Microfibrous Scaffolds for Vascular Tissue Engineering. Biotechnol. Bioprocess Eng. 2014, 19, 118–125. [Google Scholar] [CrossRef]
- El-Hag Ali, A. In Situ Formation of Hydroxyapatite within Gelatin Based Copolymer Hydrogel Prepared by Ionizing Radiation. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 7–14. [Google Scholar] [CrossRef]
- Andersen, T.; Melvik, J.E.; Gåserød, O.; Alsberg, E.; Christensen, B.E. Ionically Gelled Alginate Foams: Physical Properties Controlled by Operational and Macromolecular Parameters. Biomacromolecules 2012, 13, 3703–3710. [Google Scholar] [CrossRef]
- Lee, E.-H.; Kamigaito, Y.; Tsujimoto, T.; Seki, S.; Uyama, H.; Tagawa, S.; Sung, M.-S. Preparation of Poly(γ-glutamic acid) Hydrogel/Apatite Composites and Their Application for Scaffold of Cell Proliferation. Sen’i Gakkaishi 2010, 66, 104–111. [Google Scholar] [CrossRef]
- Yue, Z.; Wen, F.; Gao, S.; Ang, M.Y.; Pallathadka, P.K.; Liu, L.; Yu, H. Preparation of Three-Dimensional Interconnected Macroporous Cellulosic Hydrogels for Soft Tissue Engineering. Biomaterials 2010, 31, 8141–8152. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zhao, Y.M.; Sun, H.H.; Jin, T.; Wang, Q.T.; Zhou, W.; Wu, Z.F.; Jin, Y. Novel Glycidyl Methacrylated Dextran (Dex-GMA)/Gelatin Hydrogel Scaffolds Containing Microspheres Loaded with Bone Morphogenetic Proteins: Formulation and Characteristics. J. Control. Release 2007, 118, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Hoffman, A.S. Applications of Thermally Reversible Polymers and Hydrogels in Therapeutics and Diagnostics. J. Control. Release 1987, 6, 297–305. [Google Scholar] [CrossRef]
- Pabinger, C.; Berghold, A.; Boehler, N.; Labek, G. Revision Rates after Knee Replacement. Cumulative Results from Worldwide Clinical Studies versus Joint Registers. Osteoarthr. Cartil. 2013, 21, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]



| Polymers | Ionizing Technology | Applications | Findings | References |
|---|---|---|---|---|
| GEL | E-beam; acceleration energy: 2 MeV; beam current: 2 mA; doses: 10, 20, 40, 60, 80, and 100 kGy; dose rate: 10 kGy/pass | Biological and medical applications | High gel fraction, efficient crosslinking, good swelling ability and stability | [68] |
| PEGDMA, PEO, PEGDC | E-beam; acceleration energy: 0.7 MeV; beam current: 5 and 10 mA; doses: 100, 150, 200, 250, and 300 kGy | Biomedical materials (anti-adhesive barriers) | Good biodegradability, excellent mechanical properties, adequate tissue adherence, low hemolysis capability, low toxicity | [69] |
| HPG, PEO, potassium methoxide, glycidol | E-beam; acceleration energy: 0.7 MeV; beam current: 5 and 10 mA; dose: 300 kGy | TE | Suitable water absorption capacity, good cell viability, low cytotoxicity | [70] |
| COL | E-beam; acceleration energy: 10 MeV; pulse repetition: 180 Hz; doses: 10, 20, 40, 60, and 100 kGy; dose rate: 5 kGy/min | TE, mammalian extracellular matrix-mimetic systems | Adequate mechanical properties, crosslinked structure, excellent cytocompatibility, cell viability | [71] |
| COL, PVP, PAA, PEO | E-beam; acceleration energy: 6 MeV; beam current: 10 μA; pulse repetition: 53 Hz; doses: 15, 20, and 25 kGy; dose rate: 4 kGy/min | TE, wound dressing | Excellent mechanical properties, permanent network structure, swelling capacity, stability | [72] |
| TA, GT, PVA | E-beam; acceleration energy: 10 MeV; doses: 14, 28, and 56 kGy; room temperature | TE | Higher crosslink density, interconnected pores, excellent elastic recovery, non-cytotoxicity | [73] |
| Agarose | E-beam; acceleration energy: 10 MeV; pulse repetition: 180 Hz; doses: 5, 10, 15, 20, 25, and 30 kGy | TE | Viscoelastic properties, degradation at high electron doses | [74] |
| PLA, PCL | E-beam; acceleration energy: 10 MeV; doses: 10, 15, 25, 50, 75, and 100 kGy; dose rate: 15 kGy/s | Bone TE | Adequate structural characteristics, stable network structure | [75] |
| GEL, alkaline phosphate, calcium glycerophosphate | E-beam; scanning frequency: 3 Hz; pulse repetition: 180 Hz; pulse length: 8 μs; doses: 5, 10, 15, 20, and 40 kGy | Bone TE | Good stability, increased bioactivity, excellent cytocompatibility | [76] |
| ALG, Agarose | E-beam; acceleration energy: 10 MeV; pulse repetition rate: 180 Hz; doses: 10, 20, and 30 kGy | TE | Good mechanical and dynamic properties | [77] |
| Porcine corneal tissues | E-beam; acceleration energy: 2.6 MeV; dose: 25 kGy; dose rate: 5 kGy/pass | TE | Improved structural and biomechanical properties, high biocompatibility | [78] |
| AAm, acrylonitrile, PP | E-beam; acceleration energy: 2 MeV; doses: 40, 60, 80, 100, 160, and 200 kGy; dose rate: 200 Gy/s | Biomedicine, cell harvesting | Thermo-responsive swelling, non-cytotoxicity | [79] |
| COL, PVP, PEO, CMC, CHT | E-beam; acceleration energy: 6 MeV; beam current: 5 μA; pulse repetition: 53 Hz; doses: 5, 10, 20, 30, and 40 kGy; dose rate: 4 kGy/min | TE, wound dressing | Excellent stability, elastic structure, adequate swelling properties, excellent biocompatibility | [80] |
| TG, PVA, HNT | E-beam; acceleration energy: 10 MeV; dose: 28 kGy | Bone tissue engineering | Porous microstructure, adequate degradation rate, superior mechanical properties, excellent cell viability | [81] |
| PMeOx, EnOx | E-beam; acceleration energy: 10 MeV; pulse repetition: 423 Hz; doses: 2, 5, 10, 20, 40, and 100 kGy; dose rate: 0.5 kGy/min | Living tissue | Excellent non-biofouling properties, adequate mechanical behavior, good stability | [82] |
| CS, PVP, PEO, PAA, AgNP | E-beam; acceleration energy: 6 MeV; dose: 15 kGy; dose rate: 3 kGy/min | TE, wound dressing | Significant antimicrobial activity, high biocompatibility, excellent stability and swelling capacity | [83] |
| Materials | Fabrication Method | Applications | Findings | References |
|---|---|---|---|---|
| P-ECM; enzyme | γ-sterilization; radiation doses: 5 + 2 kGy, 15 + 2 kGy, and 25 + 2 kGy | Soft tissue reconstruction | Biocompatibility, physicochemical characteristics | [86] |
| SF; dECM | γ-irradiation; dose: 60 kGy; dose rate: 196 Gy/min; room temperature | Cartilage regeneration | Higher water absorption capacity, surface wettability, and no cytotoxicity | [90] |
| Zein; PVB | γ-irradiation-induced copolymerization; doses: up to 50 kGy; dose rate: 1.33 kGy/h | TE | Thermal stability, Vero (monkey kidney cell) proliferation | [91] |
| PVP; CMC | γ-irradiation; doses: 25 kGy and 40 kGy; dose rate: 5–6 kGy/h | Antimicrobial burn wound dressing | Antimicrobial efficacy, fluid absorption ability, moisture transmission ability | [92] |
| GO; PVP; PCL | γ-irradiation; dose: 25 kGy/h | Promising for bone TE | Excellent mineralization, BMP-2 release, osteodifferentiation | [93] |
| pHEMA; AA; Fe3+ | γ-sterilization; dose: 25 kGy | Biomedical applications or TE | Good wound healing, no distinct inflammatory properties | [94] |
| Aramid or PBO; PVA; Mg-substituted ceramic (β-TCP or BCP) | γ-sterilization; dose: 25 kGy; dose rate: 5 kGy/h | Bilayer composites for the replacement of osteochondral defects | High biomimicry with natural tissues, biological behavior, adequate porosity | [88] |
| NC; ALG; HA | Autoclave, β-radiation, and γ-radiation; beam energy: 10 MeV; maximum power: 80 kW; dose: 25 kGy; dose rate: 3.2 Gy·min−1 | 3D bioprinted scaffolds for TE and regenerative medicine | Sterilization-induced biocompatibility and higher cell viability against D1-MSCs | [95] |
| ECM | Seeding of hMVECs into the 3D blood vessel model for 4 days before and after γ-irradiation; doses: 4 Gy and 8 Gy | Microfluidic chips for evaluation of γ-irradiation on angiogenic and endothelial barrier properties | Reorganization of the fiber structure of hMVECs, increase in apoptotic hMVECs | [87] |
| GEL | γ-sterilization; dose: 25 kGy; sterilization time: 3790 min | Potential for TE | Swelling, compressive modulus | [96] |
| SF | γ-radiation; doses: 45, 75, 105, 135, and 165 kGy; dose rate: 196 Gy/min; room temperature | Soft/tough tissue engineering scaffold | Tunable porosity, uniform pore structure, adjustable mechanical strength, good biocompatibility | [97] |
| Gel; PEG; HEC; CHT | γ-sterilization; dose: 25 kGy | Potential for TE | Water uptake, mechanical properties, stress relaxation response | [89] |
| Mg-doped Hap; AG; GEL | γ-irradiation source | Bone tissue regeneration | Biocompatibility, antibacterial activity, in vitro drug release | [98] |
| AG; TG; gentamicin | γ-irradiation; dose: 27.3 kGy | Wound dressing potential | Mucoadhesive, antioxidant/antibacterial activity | [99] |
| Copoly (NIPAAm/DMAAm/MMA), copoly (NIPAAm/DMAAm/EEM) | γ-rays; dose: 50 kGy; dose rate: 10.1 kGy/h; room temperature | Biomedical sensor application | Thermo-responsive hydrogel, swelling characteristics | [100] |
| RGD-functionalized γ-irradiated ALG | γ-irradiation; dose: 5 Mrad | Osteochondral defect regeneration | Resistance to hypertrophy, vascularization, and endochondral ossification | [101] |
| Cellulose; PVP; PEG | γ-irradiation; dose: 50 kGy; dose rate: 3.6 kGy/h | Connective tissue regeneration applications; terpolymer scaffolds for cell attachment | Higher cell viability of fibroblasts at low concentrations of PVP/PEG | [102] |
| GEL; CHT; HA | Freeze-drying; γ- irradiation sterilization; dose: 25 kGy | Hybrid biomaterial for TE: scaffold with autologous hydrogel carrying hMSCs | Partial biodegradation at one week, high biocompatibility, early regeneration capacity at 4 weeks, absence of rejection signs | [103] |
| SF; HAP | γ-ray irradiation; dose: 60 kGy; dose rate: 15 kGy/h | Bone TE | Increased osteogenic differentiation, improved cell adhesion and proliferation | [104] |
| RGD-functionalized γ-irradiated ALG PEGMA; GelMA; PCL | γ-ray irradiation; dose: 5 Mrad 3D bioprinting | 3D bioprinting; whole bone organ engineering | Mechanically reinforced hypertrophic cartilage templates | [105] |
| PEG; CHT | γ-sterilization; dose: 13.83 ± 0.7 kGy | TE application | Biocompatibility, hemocompatibility | [106] |
| HA; CS; PAAc | γ-irradiation; doses: 5–25 kGy; nitrogen atmosphere; room temperature | Skin TE | Gel fractions, high water content, HaCaT cell viability | [107] |
| COL | γ-irradiation; dose rate: 20 Gy/min; room temperature | Potential for TE applications | Accelerated apoptosis or L929 cell proliferation | [108] |
| HA; CS; PVA | γ-irradiation; doses: 5–25 kGy; nitrogen atmosphere; room temperature | Skin TE | High cell viability, cell growth after 7 days | [109] |
| COL; DEX | γ-irradiation; dose rate: 15 Gy/min; room temperature | Artificial dermal substitutes | Tunable mechanical strength, pore size, water absorption, controllable degradation rate | [110] |
| PLCL; GEL; AAc | γ-irradiation; dose: 10 kGy; ambient temperatures; electrospinning | Vascular TE | Significant increase in Young’s modulus, improved SMC infiltration and proliferation | [111] |
| nHA; GEL; AAc | Copolymerization and crosslinking through γ-irradiation; dose rate: 10.28 kGy/h | Bone TE scaffolds | In situ nanocomposite formation, biocompatibility | [112] |
| ALG | γ-irradiation; doses: 0–30 kGy | Potential for wound dressing | Higher flexibility, better pore structure control, hydration properties, and mechanical integrity compared to foams prepared using other techniques | [113] |
| PGA; apatite | γ-ray irradiation; doses: 40, 80, and 120 kGy; room temperature; under nitrogen | TE applications | Good cell proliferation of MC3T3-E1 cells after 7 days | [114] |
| Hydroxypropyl cellulose | γ-irradiation; dose rate: 10 kGy/h; irradiation time: 30 min | Soft TE | High water content, interconnected macroporosity, mechanical integrity, and cytocompatibility | [115] |
| PEG | γ-sterilization | Potential for TE and controlled drug delivery | Release of cyclosporine, rhodamine B | [34] |
| GMA-DEX; GEL; BMP | γ-ray irradiation; dose: 1 kGy | Potential for TE and drug delivery applications | Temperature responsiveness, BMP release | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călina, I.; Demeter, M.; Scărișoreanu, A.; Abbas, A.; Raza, M.A. Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications. Gels 2025, 11, 47. https://doi.org/10.3390/gels11010047
Călina I, Demeter M, Scărișoreanu A, Abbas A, Raza MA. Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications. Gels. 2025; 11(1):47. https://doi.org/10.3390/gels11010047
Chicago/Turabian StyleCălina, Ion, Maria Demeter, Anca Scărișoreanu, Awn Abbas, and Muhammad Asim Raza. 2025. "Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications" Gels 11, no. 1: 47. https://doi.org/10.3390/gels11010047
APA StyleCălina, I., Demeter, M., Scărișoreanu, A., Abbas, A., & Raza, M. A. (2025). Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications. Gels, 11(1), 47. https://doi.org/10.3390/gels11010047











