Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings
Abstract
:1. Introduction
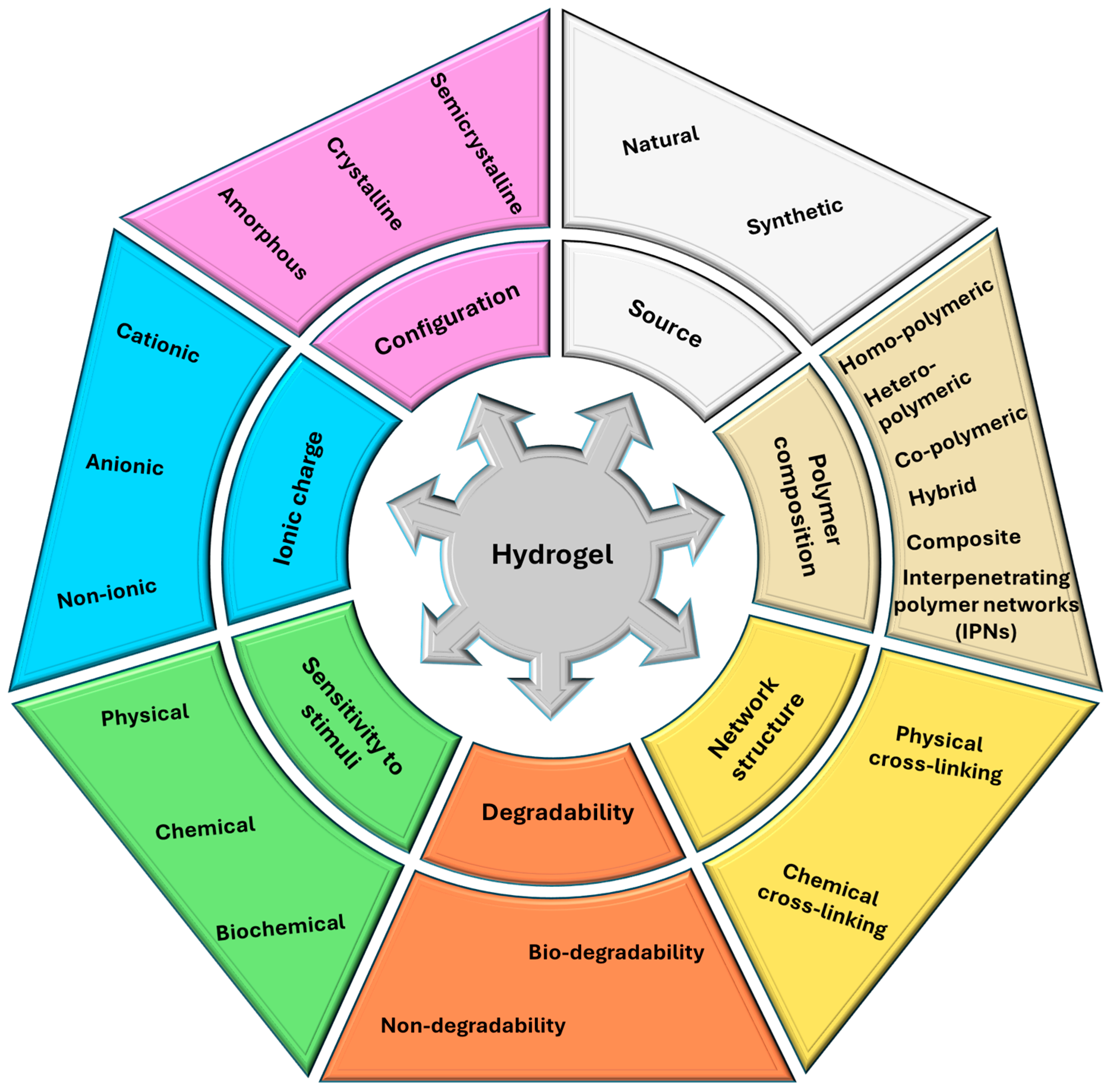
2. Key Innovations in Hydrogels
2.1. Recent Materials
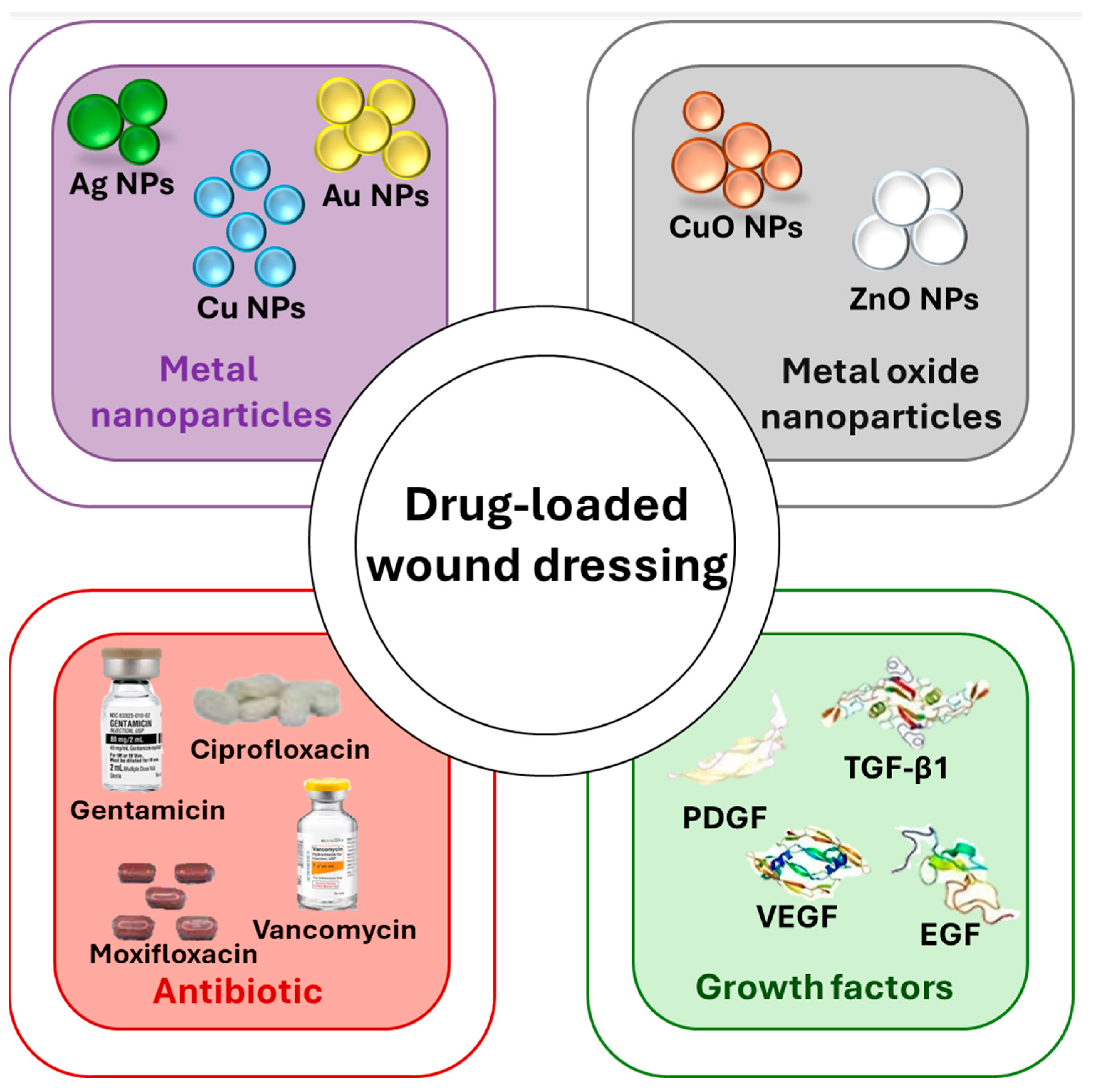
2.2. Drug Loaded Hydrogels
2.2.1. Antimicrobial Agents
2.2.2. Growth Factors
2.3. Applications in Recent Studies
2.4. Challenges Addressed
3. Progress in Foam Dressings
3.1. Material Advances
3.2. Innovative Applications
3.3. Enhanced Properties
4. Cutting-Edge Antimicrobial Dressings
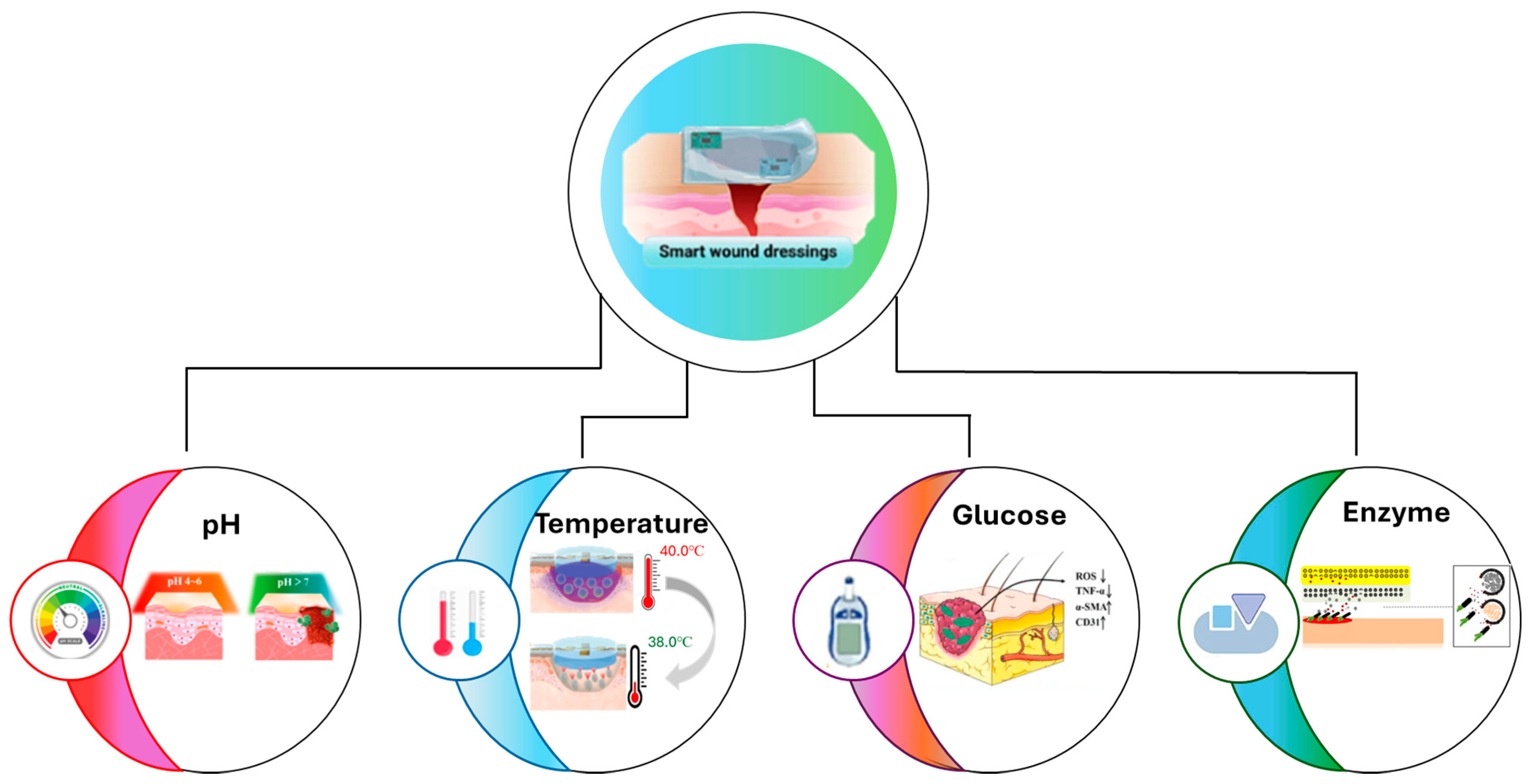
4.1. Recent Innovations
4.1.1. pH-Responsive
4.1.2. Temperature-Responsive
4.1.3. Glucose-Responsive
4.1.4. Enzyme-Responsive
| Smart Wound Dressing | Stimuli Response | Active Components | Targets Wound Type | Benefit | References |
|---|---|---|---|---|---|
| 3D printed alginate wound dressings doped with calcium phosphate nanoparticles (CaP NPs) | pH-responsive | selenium nanoparticles, Rhodamine B, Bacitracin | Under basic condition | 3D printability is adaptable to various sizes and shapes | [151] |
| Cellulose-functionalized-Graphene oxide | pH-responsive | Curcumin | Chronic wounds, burn | The curcumin was realized in a controllable manner | [152] |
| Zwitterionic chitosan and dialdehyde starch with silymarin and levofloxacin | Temperature-responsive | Silymarine, levofloxacin | Third-degree burn wound | Outstanding shape retention, flexibility abilities, self-healing, wound closure acceleration, inflammation reduction | [153] |
| Hydrogel based on poloxamers with bio-AgNPs | Temperature-responsive | Bio silver nanoparticle | Chronic infection | Mucoadhesive strength, silver ions release above 37 °C, efficient against Gram-negative, Gram-positive, and fungi | [154] |
| tea polyphenol silver nanoparticles/phenylboronic acid grafted onto hyaluronic acid–glycol chitosan hydrogels | Glucose-responsive | Tea polyphenol silver nanoparticles | Bacterially diabetic wound | Biological safety, good biocompatibility, shape adaptability, highly efficient antibacterial, anti-inflammatory, collagen deposition and vascularization promotion, and removability | [155] |
| Metal–organic drug-loaded hydrogel | Glucose-responsive | Zinc ions and deferoxamine mesylate | Diabetic ulcers | Easily applied on the wound surface, zinc ions and deferoxamine mesylate were realized at excessive glucose | [156] |
| Supramolecular peptide (Nap-Gly-Phe-Phe-Phe-Gly-Val-Asp-CONH2) hydrogel doped with nanoparticles | Enzyme-responsive | small interfering RNA-loaded NPs | Diabetic wounds | Improve diabetic healing, regulation of diabetic wound-associated genes | [157] |
4.2. Biofilm Management
4.3. Broader Applications
4.4. Concerns and Solutions
5. Recent Trends in Interdisciplinary Approaches
6. Comparative Analysis of Recent Advances
Hydrogels vs. Foams vs. Antimicrobial Dressings
7. Future Directions
7.1. Challenges
7.2. Opportunities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niculescu, A.-G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Liang, Z.; Lai, P.; Zhang, J.; Lai, Q.; He, L. Impact of moist wound dressing on wound healing time: A meta-analysis. Int. Wound J. 2023, 20, 4410–4421. [Google Scholar] [CrossRef]
- El-Sherbeni, S.A.; Negm, W.A. The wound healing effect of botanicals and pure natural substances used in in vivo models. Inflammopharmacology 2023, 31, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, D.; Feng, G.; Xu, F.-J. A hydrogel wound dressing ideally designed for chronic wound care. Matter 2023, 6, 1060–1062. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Rajeshkumar, S. Nanomaterials for wound healing: Current status and futuristic frontier. Biomed. Technol. 2024, 6, 26–45. [Google Scholar] [CrossRef]
- Burian, E.A.; Sabah, L.; Kirketerp-Møller, K.; Ibstedt, E.; Fazli, M.M.; Gundersen, G. The Safety and Antimicrobial Properties of Stabilized Hypochlorous Acid in Acetic Acid Buffer for the Treatment of Acute Wounds—A Human Pilot Study and In Vitro Data. Int. J. Low. Extrem. Wounds 2021, 22, 369–377. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef] [PubMed]
- Jonidi Shariatzadeh, F.; Currie, S.; Logsetty, S.; Spiwak, R.; Liu, S. Enhancing wound healing and minimizing scarring: A comprehensive review of nanofiber technology in wound dressings. Prog. Mater. Sci. 2025, 147, 101350. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Gherasim, O.; Niculescu, A.-G.; Grumezescu, A.M.; Neacșu, I.A.; Chircov, C.; Vasile, B.Ș.; Oprea, O.C.; Andronescu, E.; Stan, M.S.; et al. A Microfluidic Approach for Synthesis of Silver Nanoparticles as a Potential Antimicrobial Agent in Alginate–Hyaluronic Acid-Based Wound Dressings. Int. J. Mol. Sci. 2023, 24, 11466. [Google Scholar] [CrossRef]
- Moreira, T.D.; Martins, V.B.; da Silva Júnior, A.H.; Sayer, C.; de Araújo, P.H.H.; Immich, A.P.S. New insights into biomaterials for wound dressings and care: Challenges and trends. Prog. Org. Coat. 2024, 187, 108118. [Google Scholar] [CrossRef]
- Kuddushi, M.; Shah, A.A.; Ayranci, C.; Zhang, X. Recent advances in novel materials and techniques for developing transparent wound dressings. J. Mater. Chem. B 2023, 11, 6201–6224. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Niazi, M.; Ramakrishna, S. The evolution of wound dressings: From traditional to smart dressings. Polym. Adv. Technol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Vachhrajani, V.; Khakhkhar, P. Introduction. In Science of Wound Healing and Dressing Materials; Vachhrajani, V., Khakhkhar, P., Eds.; Springer: Singapore, 2020; pp. 1–10. [Google Scholar]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Z.; Xu, Y.; Qian, X.-N.; Chen, W.; Qiu, J. Antimicrobial efficiency and cytocompatibility of resveratrol and naringin as chemical decontaminants on SLA surface. Microbiol. Spectr. 2024, 12, e03679-23. [Google Scholar] [CrossRef]
- Law, S.K.; Liu, C.W.; Tong, C.W.; Au, D.C. Potential of Resveratrol to Combine with Hydrogel for Photodynamic Therapy against Bacteria and Cancer—A Review. Biomedicines 2024, 12, 2095. [Google Scholar] [CrossRef]
- Rutuja, M.H. Exploring the Epidemiology, Pathophysiology, and Treatment Approaches for Dog Bites in India. In Proceedings of the 5th International Scientific Conference, Stockholm, Sweden, 21–23 December 2023; p. 108. [Google Scholar]
- Jambholkar, P.C.; Choudhari, S.G.; Sharma, M. Louis Pasteur: A Legacy Unmasked. Cureus 2024, 16, e68080. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.R.; Singh, G.; Mohapatra, A.; Keshetty, S.S. Ignaz Phillip Semmelweis: The Unrecognized Pioneer of Aseptic Practices. Cureus 2024, 16, e68350. [Google Scholar] [CrossRef] [PubMed]
- Solanki, D.; Vinchhi, P.; Patel, M.M. Design Considerations, Formulation Approaches, and Strategic Advances of Hydrogel Dressings for Chronic Wound Management. ACS Omega 2023, 8, 8172–8189. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Hu, L.; Liao, X.; He, J.; Liu, F. Advances of antimicrobial dressings loaded with antimicrobial agents in infected wounds. Front. Bioeng. Biotechnol. 2024, 12, 1431949. [Google Scholar] [CrossRef]
- Wathoni, N.; Suhandi, C.; Elamin, K.M.; Lesmana, R.; Hasan, N.; Mohammed, A.F.A.; El-Rayyes, A.; Wilar, G. Advancements and Challenges of Nanostructured Lipid Carriers for Wound Healing Applications. Int. J. Nanomed. 2024, 19, 8091–8113. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, B.; Lou, Z.; Han, W.; Wang, L. The advancement of intelligent dressings for monitoring chronic wound infections. Chem. Eng. J. 2024, 484, 149643. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Kasai, R.D.; Radhika, D.; Archana, S.; Shanavaz, H.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. A review on hydrogels classification and recent developments in biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1059–1069. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Liang, X.; Huang, C.; Liu, H.; Chen, H.; Shou, J.; Cheng, H.; Liu, G. Natural hydrogel dressings in wound care: Design, advances, and perspectives. Chin. Chem. Lett. 2024, 35, 109442. [Google Scholar] [CrossRef]
- Angaria, N.; Saini, S.; Hussain, M.S.; Sharma, S.; Singh, G.; Khurana, N.; Kumar, R. Natural polymer-based hydrogels: Versatile biomaterials for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 1550–1568. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Ahmadian, N.; Wheatley, S.; Alizadeh Sardroud, H.; Nasrollah, S.A.S.; Naseri, E.; Ahmadi, A. Advancements in 3D-printable polysaccharides, proteins, and synthetic polymers for wound dressing and skin scaffolding—A review. Int. J. Biol. Macromol. 2024, 266, 131207. [Google Scholar] [CrossRef]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef]
- Yasin, S.N.; Said, Z.; Halib, N.; Rahman, Z.A.; Mokhzani, N.I. Polymer-Based Hydrogel Loaded with Honey in Drug Delivery System for Wound Healing Applications. Polymers 2023, 15, 3085. [Google Scholar] [CrossRef]
- Aminzai, M.T.; Patan, A. Recent Applications and Evaluation of Metal Nanoparticle–Polymer Hybrids as Chronic Wound Dressings. J. Nanomater. 2024, 2024, 3280349. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers 2022, 14, 3806. [Google Scholar] [CrossRef] [PubMed]
- Serpico, L.; Dello Iacono, S.; Cammarano, A.; De Stefano, L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels 2023, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Kolipaka, T.; Pandey, G.; Abraham, N.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Rajinikanth, P.S.; Tickoo, V.; Srivastava, S. Stimuli-responsive polysaccharide-based smart hydrogels for diabetic wound healing: Design aspects, preparation methods and regulatory perspectives. Carbohydr. Polym. 2024, 324, 121537. [Google Scholar] [CrossRef] [PubMed]
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Naghib, S.M.; Amiri, S.; Mozafari, M.R. Stimuli-responsive chitosan-based nanocarriers for drug delivery in wound dressing applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100497. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, L.; Dai, Q.; Hu, X.; Shen, Y. Multifunctional antibacterial hydrogels for chronic wound management. Biomater. Sci. 2024, 12, 2460–2479. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles incorporated hydrogels for delivery of antimicrobial agents: Developments and trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Firmansyah, Y.; Sidharta, V.M.; Wijaya, L.; Tan, S.T. Unraveling the Significance of Growth Factors (TGF-β, PDGF, KGF, FGF, Pro Collagen, VEGF) in the Dynamic of Wound Healing. Asian J. Med. Health 2024, 22, 49–61. [Google Scholar] [CrossRef]
- Nasra, S.; Patel, M.; Shukla, H.; Bhatt, M.; Kumar, A. Functional hydrogel-based wound dressings: A review on biocompatibility and therapeutic efficacy. Life Sci. 2023, 334, 122232. [Google Scholar] [CrossRef] [PubMed]
- Cherri, M.; Stergiou, P.S.; Ahmadian, Z.; Povolotsky, T.L.; Thongrom, B.; Fan, X.; Mohammadifar, E.; Haag, R. Redox-Responsive Hydrogels Loaded with an Antibacterial Peptide as Controlled Drug Delivery for Healing Infectious Wounds. Adv. Healthc. Mater. 2024, 13, 2401289. [Google Scholar] [CrossRef]
- Liu, T.; Lu, Y.; Zhan, R.; Qian, W.; Luo, G. Nanomaterials and nanomaterials-based drug delivery to promote cutaneous wound healing. Adv. Drug Deliv. Rev. 2023, 193, 114670. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Calderon, L.; Yus, C.; Remirez de Ganuza, C.; Paesa, M.; Landa, G.; Tapia, E.; Pérez, E.; Perez, M.; Sebastian, V.; Irusta, S.; et al. Combinatorial wound dressings loaded with synergistic antibiotics in the treatment of chronic infected wounds. Chem. Eng. J. 2023, 476, 146679. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite Hydrogels with Embedded Silver Nanoparticles and Ibuprofen as Wound Dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Sayed, M.M.E.; Mohsen, D.; Fagir, M.H.; El Dein, D.K. Green Synthesis of Silver Nanoparticles Loaded Hydrogel for Wound Healing; Systematic Review. Gels 2023, 9, 530. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Alawam, K.A.; Binshaya, A.S.; Alduraywish, S.A. Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules 2023, 28, 3241. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Kimura, A.H.; de Camargo, L.C.; Gonçalves, M.C.; da Silva, J.V.; Risso, W.E.; de Andrade, F.G.; Zaia, C.T.; Lonni, A.A.; dos Reis Martinez, C.B.; et al. Hydrogel-Containing Biogenic Silver Nanoparticles: Antibacterial Action, Evaluation of Wound Healing, and Bioaccumulation in Wistar Rats. Microorganisms 2023, 11, 1815. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q. Silver Nanoparticles Incorporated Chitosan Hydrogel as a Potential Dressing Material for Diabetic Wound Healing in Nursing Care. Ind. J. Pharm. Edu. Res. 2024, 58, 139–144. [Google Scholar] [CrossRef]
- Vaniushenkova, A.A.; Poberezhniy, D.Y.; Panyukova, N.S.; Morozov, A.N.; Kalenov, S.V.; Belov, A.A. The Co-Use of Various Forms of Silver and Proteases in the Development of New Dressing Biomaterials for Wound Healing. Biointerface Res. Appl. Chem. 2024, 14, 119. [Google Scholar]
- Pemmadi, R.V.; Alhakamy, N.A.; Asfour, H.Z.; Kotta, S.; Alfaleh, M.A.; Sunnapu, P.; Uk, I.; Pottail, L.; Chithambharan, A.; Yogananthan, D.; et al. Enhancing antibacterial efficacy and accelerating infectious wound healing in rats using biogenic metal nanoparticles from marine Bacillus subtilis. Front. Mar. Sci. 2024, 11, 1284813. [Google Scholar] [CrossRef]
- Fu, W.; Sun, S.; Cheng, Y.; Ma, J.; Hu, Y.; Yang, Z.; Yao, H.; Zhang, Z. Opportunities and challenges of nanomaterials in wound healing: Advances, mechanisms, and perspectives. Chem. Eng. J. 2024, 495, 153640. [Google Scholar] [CrossRef]
- Babu, P.J.; Tirkey, A.; Paul, A.A.; Kristollari, K.; Barman, J.; Panda, K.; Sinha, N.; Babu, B.R.; Marks, R.S. Advances in nano silver-based biomaterials and their biomedical applications. Eng. Regen. 2024, 5, 326–341. [Google Scholar] [CrossRef]
- Rafi, R.; Zulfiqar, S.; Asad, M.; Zeeshan, R.; Zehra, M.; Khalid, H.; Akhtar, N.; Yar, M. Smart wound dressings based on carbon doped copper nanoparticles for selective bacterial detection and eradication for efficient wound healing application. Mater. Today Commun. 2023, 35, 105914. [Google Scholar] [CrossRef]
- Shtapenko, O.; Syrvatka, V.; Horbay, R.; Dzen, Y.; Slyvchuk, O.; Gromyko, O.; Gevkan, I. Evaluation of Antimicrobial Activity and in Vivo Wound Healing Properties of Copper Nanoparticles and Copper Nanoparticles Nanoemulsion. Biointerface Res. Appl. Chem. 2024, 14, 15. [Google Scholar]
- Zheng, Q.; Chen, C.; Liu, Y.; Gao, J.; Li, L.; Yin, C.; Yuan, X. Metal Nanoparticles: Advanced and Promising Technology in Diabetic Wound Therapy. Int. J. Nanomed. 2024, 19, 965–992. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Deng, L.; Shen, Z.; Huang, Q.; Shah, N.G.; Chen, W.; Zhang, Y.; Wang, X.; Yu, L.; et al. Antibacterial and antioxidative hydrogel dressings based on tannic acid-gelatin/oxidized sodium alginate loaded with zinc oxide nanoparticles for promoting wound healing. Int. J. Biol. Macromol. 2024, 279, 135177. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Mirmasoudi, S.S.; Pourmohammadi-Bejarpasi, Z.; Feizkhah, A.; Mobayen, M.; Hedayati, M.; Sadeghi, M.; Esmailzadeh, M.; Mirkatoul, F.B.; Jamshidi, S. Polyacrylic acid/ polyvinylpyrrolidone hydrogel wound dressing containing zinc oxide nanoparticles promote wound healing in a rat model of excision injury. Heliyon 2023, 9, e19230. [Google Scholar] [CrossRef]
- Adel Alawadi, H.; Andarzbakhsh, K.; Rastegari, A.; Mohammadi, Z.; Aghsami, M.; Saadatpour, F. Chitosan–Aloe Vera Composition Loaded with Zinc Oxide Nanoparticles for Wound Healing: In Vitro and In Vivo Evaluations. IET Nanobiotechnol. 2024, 2024, 6024411. [Google Scholar] [CrossRef]
- Samsulkahar, N.F.; Hadi, A.A.; Shamsuddin, M.; Nik, N.A.N. Biosynthesis of Gold Nanoparticles Using Strobilanthes crispa Aqueous Leaves Extract and Evaluation of Its Antibacterial Activity. Biointerface Res. Appl. Chem. 2023, 13, 63. [Google Scholar]
- Dong, Y.; Wang, Z.; Wang, J.; Sun, X.; Yang, X.; Liu, G. Mussel-inspired electroactive, antibacterial and antioxidative composite membranes with incorporation of gold nanoparticles and antibacterial peptides for enhancing skin wound healing. J. Biol. Eng. 2024, 18, 3. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Q.; Liu, S.; Lin, D.; Zhao, H.; Liu, X.; Zhou, G. Research progress on antimicrobial hydrogel dressing for wound repair. Eur. Polym. J. 2023, 197, 112372. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, H.; Wang, X.; Dong, S.; Guo, L.; Zhang, S.; Yang, X.; Liu, C.; Jiang, X.; Kan, M.; et al. Advances in preparation and application of antibacterial hydrogels. J. Nanobiotechnol. 2023, 21, 300. [Google Scholar] [CrossRef]
- Wang, X.; Song, R.; Johnson, M.A.S.; Shen, P.; Zhang, N.; Lara-Sáez, I.; Xu, Q.; Wang, W. Chitosan-Based Hydrogels for Infected Wound Treatment. Macromol. Biosci. 2023, 23, 2300094. [Google Scholar] [CrossRef]
- Tamahkar, E.; Özkahraman, B.; Süloğlu, A.K.; İdil, N.; Perçin, I. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Technol. 2020, 58, 101536. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Esmaeili, Z.; Eftekhari, B.S.; Khosravimelal, S.; Alehosseini, M.; Orive, G.; Dolatshahi-Pirouz, A.; Pal Singh Chauhan, N.; Janmey, P.A.; Hashemi, A.; et al. Antibacterial smart hydrogels: New hope for infectious wound management. Mater. Today Bio 2022, 17, 100499. [Google Scholar] [CrossRef]
- Mullin, J.A.; Rahmani, E.; Kiick, K.L.; Sullivan, M.O. Growth factors and growth factor gene therapies for treating chronic wounds. Bioeng. Transl. Med. 2024, 9, e10642. [Google Scholar] [CrossRef]
- He, Y.; Yang, W.; Zhang, C.; Yang, M.; Yu, Y.; Zhao, H.; Guan, F.; Yao, M. ROS/pH dual responsive PRP-loaded multifunctional chitosan hydrogels with controlled release of growth factors for skin wound healing. Int. J. Biol. Macromol. 2024, 258, 128962. [Google Scholar] [CrossRef]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef]
- Shariati, A.; Moradabadi, A.; Azimi, T.; Ghaznavi-Rad, E. Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci. Rep. 2020, 10, 1032. [Google Scholar] [CrossRef]
- Boateng, J.S.; Hafezi, F.; Tabriz, A.G.; Douroumis, D. 3D printed composite dressings loaded with human epidermal growth factor for potential chronic wound healing applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104684. [Google Scholar] [CrossRef]
- Bodnar, R.J. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv. Wound Care 2012, 2, 24–29. [Google Scholar] [CrossRef]
- Yalçıntaş, Y.M.; Duman, H.; López, J.M.M.; Portocarrero, A.C.M.; Lombardo, M.; Khallouki, F.; Koch, W.; Bordiga, M.; El-Seedi, H.; Raposo, A.; et al. Revealing the Potency of Growth Factors in Bovine Colostrum. Nutrients 2024, 16, 2359. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Tehrany, P.M.; Rahmanian, P.; Rezaee, A.; Ranjbarpazuki, G.; Sohrabi Fard, F.; Asadollah Salmanpour, Y.; Zandieh, M.A.; Ranjbarpazuki, A.; Asghari, S.; Javani, N.; et al. Multifunctional and theranostic hydrogels for wound healing acceleration: An emphasis on diabetic-related chronic wounds. Environ. Res. 2023, 238, 117087. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H.; Yang, P.; Shen, X.; Hu, Y.; Cheng, Y.; Yao, H.; Zhang, Z. Nano-oxygenated hydrogels for locally and permeably hypoxia relieving to heal chronic wounds. Biomaterials 2022, 282, 121401. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, P.; Cao, R.; Wu, J.; Ji, H.; Wang, M.; Hu, C.; Huang, P.; Wang, X. Exudate Absorbing and Antimicrobial Hydrogel Integrated with Multifunctional Curcumin-Loaded Magnesium Polyphenol Network for Facilitating Burn Wound Healing. ACS Nano 2023, 17, 22355–22370. [Google Scholar] [CrossRef]
- Goh, M.; Du, M.; Peng, W.R.; Saw, P.E.; Chen, Z. Advancing burn wound treatment: Exploring hydrogel as a transdermal drug delivery system. Drug Deliv. 2024, 31, 2300945. [Google Scholar] [CrossRef]
- Shi, W.; Song, N.; Huang, Y.; Wang, W.; He, C.; Zhao, W.; Zhao, C. Enhanced thermal conductive and moisturizing hydrogels by constructing 3D networks of BN-OH and CNT-OH in alignment for burn therapy. Mater. Des. 2023, 233, 112239. [Google Scholar] [CrossRef]
- Fateme, F. 3D-printed hydrogels dressings with bioactive borate glass for continuous hydration and treatment of second-degree burns. Int. J. Bioprint. 2023, 9, 0118. [Google Scholar]
- Activheal® Hydrogel Is an Effective Method for Hydrating Dry Necrotic and Sloughy Wounds. Available online: https://activheal.com/wound-care-dressing-range/hydrogel-dressing/ (accessed on 17 January 2025).
- Hydrogel Wound Dressing 3M™ Tegaderm™ 15 Gram Gel/Amorphous Sterile. Available online: https://mms.mckesson.com/product/493621/Solventum-Corporation-91110 (accessed on 17 January 2025).
- Flaminal® Hydro/Forte. Available online: https://www.flenhealth.com/products/flaminal#PRODUCTS (accessed on 17 January 2025).
- Hydrosorb® Gel. Available online: https://www.hartmann.info/ro-ro/products/tratamentul-plagilor/pansamente-hidroactive/pansamente-cu-hidrogel-%E2%80%90-amarphous-gel/hydrosorb%C2%AE-gel (accessed on 17 January 2025).
- Fitostimoline®. Available online: https://fitostimolineperte.it/applications/?lang=en (accessed on 17 January 2025).
- HydroTac®. Available online: https://www.hartmann.info/ro-ro/products/tratamentul-plagilor/pansamente-hidroactive/foam-wound-dressings/hydrotac%C2%AE#uses (accessed on 17 January 2025).
- INTRASITE◊ Gel Hydrogel Wound Dressing. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/intrasite-gel-ppl#reference-materials (accessed on 17 January 2025).
- Briefing, M.I. Oxyzyme and Iodozyme 2-Layer Hydrogel Wound Dressings with Iodine for Treating Chronic Wounds. NICE. Available online: https://www.nice.org.uk/advice/mib11/chapter/technology-overview (accessed on 17 January 2025).
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers 2023, 15, 2000. [Google Scholar] [CrossRef]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel Wound Dressings Accelerating Healing Process of Wounds in Movable Parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef]
- Rodrigues, M.; Thilagavati, G. Development and study of textile-based hydrogel wound dressing material. Ind. Textila 2022, 73, 40–47. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Shi, J.; Liu, W.; Kritchenkov, A.S.; Van Vlierberghe, S.; Wang, L.; Liu, W.; Gao, J. Cotton Fabric-Reinforced Hydrogels with Excellent Mechanical and Broad-Spectrum Photothermal Antibacterial Properties. Polymers 2024, 16, 1346. [Google Scholar] [CrossRef]
- Koc, U.; Aykut, Y.; Eren, R. Natural fibers woven fabric reinforced hydrogel composites for enhanced mechanical properties. J. Ind. Text. 2020, 51, 6315S–6332S. [Google Scholar] [CrossRef]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Zafar, M.S.; Ahmad, S.; Afzal, A.; Nawab, Y.; Rasheed, A.; Ulker, Z. Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers 2021, 13, 4098. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chen, K.; Qiu, H. Preparation and properties of antibacterial composite hydrogels based on polyvinyl alcohol, chitosan, and nano-metal oxide. Cellulose 2024, 31, 3607–3622. [Google Scholar] [CrossRef]
- Mushtaq, F.; Ashfaq, M.; Anwar, F.; Ayesha, B.T.; Latif, H.S.; Khalil, S.; Sarwar, H.S.; Khan, M.I.; Sohail, M.F.; Maqsood, I. Injectable Chitosan–Methoxy Polyethylene Glycol Hybrid Hydrogel Untangling the Wound Healing Behavior: In Vitro and In Vivo Evaluation. ACS Omega 2024, 9, 2145–2160. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Ghilan, A.; Rusu, D.; Simionescu, N.; Tartau, L.M. Nanostructured hyaluronic acid-based hydrogels encapsulating synthetic/ natural hybrid nanogels as promising wound dressings. Biochem. Eng. J. 2022, 179, 108341. [Google Scholar] [CrossRef]
- Gefen, A.; Alves, P.; Beeckman, D.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Swanson, T.; Woo, K. Mechanical and contact characteristics of foam materials within wound dressings: Theoretical and practical considerations in treatment. Int. Wound J. 2023, 20, 1960–1978. [Google Scholar] [CrossRef]
- Raepsaet, C.; Alves, P.; Cullen, B.; Gefen, A.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Santamaria, N.; Sharpe, A.; Swanson, T.; et al. Clinical research on the use of bordered foam dressings in the treatment of complex wounds: A systematic review of reported outcomes and applied measurement instruments. J. Tissue Viability 2022, 31, 514–522. [Google Scholar] [CrossRef]
- Hargis, A.; Yaghi, M.; Bermudez, N.M.; Gefen, A. Foam Dressings for Wound Healing. Curr. Dermatol. Rep. 2024, 13, 28–35. [Google Scholar] [CrossRef]
- Holloway, S.; Harding, K.G. Wound dressings. Surgery 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Gefen, A.; Alves, P.; Beeckman, D.; Cullen, B.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Santamaria, N.; Swanson, T.; Woo, K.; Söderström, B.; et al. Fluid handling by foam wound dressings: From engineering theory to advanced laboratory performance evaluations. Int. Wound J. 2024, 21, e14674. [Google Scholar] [CrossRef]
- Gefen, A.; Alves, P.; Beeckman, D.; Cullen, B.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Santamaria, N.; Sharpe, A.; Swanson, T.; et al. How Should Clinical Wound Care and Management Translate to Effective Engineering Standard Testing Requirements from Foam Dressings? Mapping the Existing Gaps and Needs. Adv. Wound Care 2022, 13, 34–52. [Google Scholar] [CrossRef]
- Huang, C.-C. Design and Characterization of a Bioinspired Polyvinyl Alcohol Matrix with Structural Foam-Wall Microarchitectures for Potential Tissue Engineering Applications. Polymers 2022, 14, 1585. [Google Scholar] [CrossRef]
- Ma, Q.; Rejab, M.R.M.; Siregar, J.P.; Guan, Z. A review of the recent trends on core structures and impact response of sandwich panels. J. Compos. Mater. 2021, 55, 2513–2555. [Google Scholar] [CrossRef]
- Trucillo, P.; Di Maio, E. Classification and Production of Polymeric Foams among the Systems for Wound Treatment. Polymers 2021, 13, 1608. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Chen, S.-H.; Chen, R.-F.; Liu, K.-F.; Kuo, Y.-R.; Wang, C.-K.; Lee, T.-M.; Wang, Y.-H. A Multifunctional Polyethylene Glycol/Triethoxysilane-Modified Polyurethane Foam Dressing with High Absorbency and Antiadhesion Properties Promotes Diabetic Wound Healing. Int. J. Mol. Sci. 2023, 24, 12506. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Derman, S. Properties of Ideal Wound Dressing. J. Fac. Pharm. Ank. Univ. 2023, 47, 1119–1131. [Google Scholar] [CrossRef]
- Feng, F.; Zhao, Z.; Li, J.; Huang, Y.; Chen, W. Multifunctional dressings for wound exudate management. Prog. Mater. Sci. 2024, 146, 101328. [Google Scholar] [CrossRef]
- Gong, X.; Wang, F.; Yang, J.; Du, H.; Jiang, M.; Tan, M.; Chen, G.; Chen, Z. Engineered composite dressing with exudate management capabilities for the process of entire wound healing. Mater. Today Commun. 2024, 39, 108557. [Google Scholar] [CrossRef]
- Lungulescu, E.-M.; Fierascu, R.C.; Stan, M.S.; Fierascu, I.; Radoi, E.A.; Banciu, C.A.; Gabor, R.A.; Fistos, T.; Marutescu, L.; Popa, M.; et al. Gamma Radiation-Mediated Synthesis of Antimicrobial Polyurethane Foam/Silver Nanoparticles. Polymers 2024, 16, 1369. [Google Scholar] [CrossRef]
- Susrutha, M.J.; Khan, S.; Branham, S.K.; Pande, A.H. Types of Wound Dressings and Materials used in Mild to Moderately Exuding Wounds: A Review. Int. J. Health Technol. Innov. 2022, 1, 52–59. [Google Scholar] [CrossRef]
- Yamane, T.; Nakagami, G.; Yoshino, S.; Shimura, M.; Kitamura, A.; Kobayashi-Hattori, K.; Oishi, Y.; Nishijima, Y.; Minematsu, T.; Sanada, H. Hydrocellular foam dressings promote wound healing associated with decrease in inflammation in rat periwound skin and granulation tissue, compared with hydrocolloid dressings. Biosci. Biotechnol. Biochem. 2015, 79, 185–189. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- 3M™ Tegaderm™ Silicone Border Foam Dressing. Available online: https://www.3m.co.uk/3M/en_GB/p/d/b00035966/ (accessed on 22 January 2025).
- ALLEVYN: Bordered Dressings. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/allevyn-border-global-new#productfeatures (accessed on 22 January 2025).
- ACTIVHEAL. Available online: https://activheal.com/wound-care-dressing-range/foam-dressing/ (accessed on 22 January 2025).
- Sorbact® Foam Dressing. Available online: https://sorbact.com/product/sorbact-foam-dressing/ (accessed on 22 January 2025).
- PermaFoam® Classic. Available online: https://www.hartmann.info/en-au/products/wound-management/hydroactive-wound-dressings/foam-wound-dressings/permafoam%C2%AE-classic (accessed on 22 January 2025).
- Mepilex Border Ag. Available online: https://www.molnlycke.us/products-solutions/mepilex-border-ag/ (accessed on 22 January 2025).
- Chen, Y.; Liang, Y.; Liu, J.; Yang, J.; Jia, N.; Zhu, C.; Zhang, J. Optimizing microenvironment by integrating negative pressure and exogenous electric fields via a flexible porous conductive dressing to accelerate wound healing. Biomater. Sci. 2021, 9, 238–251. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, M.; Lee, G.-J.; Lim, S.I. A review of recent advances in nanotechnology for the delivery of therapeutics in wound healing. J. Pharm. Investig. 2024, 55, 33–54. [Google Scholar] [CrossRef]
- Alencar, M.S.F.; Silva, G.H.C.; Silva, A.A.d.; Azevedo, A.K.R.d.; Pereira, M.A.; Nascimento, J.C.C.d.; Santos, P.S.d.; Campos, J.d.S.; Bernardes, M.S.V.; Caparroz, D.P.P.d.D.; et al. Evidence-based practices on nanotechnology in wound treatment. Cad. Pedagógico 2024, 21, e10234. [Google Scholar] [CrossRef]
- Fauzian, F.; Garmana, A.N.; Mauludin, R. Applications of nanotechnology-based drug delivery system for delivering natural products into acute and chronic wounds: A review. Biointerface Res. Appl. Chem. 2023, 13, 426. [Google Scholar]
- Yadav, R.; Kumar, R.; Kathpalia, M.; Ahmed, B.; Dua, K.; Gulati, M.; Singh, S.; Singh, P.J.; Kumar, S.; Shah, R.M.; et al. Innovative approaches to wound healing: Insights into interactive dressings and future directions. J. Mater. Chem. B 2024, 12, 7977–8006. [Google Scholar] [CrossRef]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. pH-Responsive wound dressings: Advances and prospects. Nanoscale Horiz. 2023, 8, 422–440. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. pH factors in chronic wound and pH-responsive polysaccharide-based hydrogel dressings. Int. J. Biol. Macromol. 2024, 279, 135118. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart wound dressing for advanced wound management: Real-time monitoring and on-demand treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, J.; Wu, X.; Zeng, M.; Tian, Y.; Wu, K.; Wei, D.; Sun, J.; Guo, Z.; Fan, H. Flexible and temperature-responsive hydrogel dressing for real-time and remote wound healing monitoring. J. Mater. Chem. B 2023, 11, 4934–4945. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Jia, X.; Dou, Z.; Zhang, Y.; Li, F.; Xing, B.; Hu, Z.; Li, X.; Liu, Z.; Yang, W.; Liu, Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics 2023, 15, 2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Newton, M.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, Y.; Li, Q.; Yang, K.; Sun, L.; Xu, H.; Wang, R. Intelligent design and medical applications of antimicrobial hydrogels. Colloid Interface Sci. Commun. 2023, 53, 100696. [Google Scholar] [CrossRef]
- Huang, T.; Sun, Z.; Heath, D.E.; O’Brien-Simpson, N.; O’Connor, A.J. 3D printed and smart alginate wound dressings with pH-responsive drug and nanoparticle release. Chem. Eng. J. 2024, 492, 152117. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Khan, M.U.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Fathi, A.; Gholami, M.; Motasadizadeh, H.; Malek-Khatabi, A.; Sedghi, R.; Dinarvand, R. Thermoresponsive in situ forming and self-healing double-network hydrogels as injectable dressings for silymarin/levofloxacin delivery for treatment of third-degree burn wounds. Carbohydr. Polym. 2024, 331, 121856. [Google Scholar] [CrossRef]
- Wunnoo, S.; Bilhman, S.; Waen-ngoen, T.; Yawaraya, S.; Paosen, S.; Lethongkam, S.; Kaewnopparat, N.; Voravuthikunchai, S.P. Thermosensitive hydrogel loaded with biosynthesized silver nanoparticles using Eucalyptus camaldulensis leaf extract as an alternative treatment for microbial biofilms and persistent cells in tissue infections. J. Drug Deliv. Sci. Technol. 2022, 74, 103588. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, X.; Chen, Y.; Chang, H.; Lu, D.; Pei, D.; Geng, Z.; Zeng, Z.; Guo, C.; Huang, J.; et al. Dual Glucose/ROS-Sensitive Injectable Adhesive Self-Healing Hydrogel with Photothermal Antibacterial Activity and Modulation of Macrophage Polarization for Infected Diabetic Wound Healing. ACS Mater. Lett. 2023, 5, 3142–3155. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-responsive multifunctional metal–organic drug-loaded hydrogel for diabetic wound healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Zeng, G.; Mao, N.; Li, N.; Li, L.; Xu, X.; Yan, L. Supramolecular peptide hydrogel doped with nanoparticles for local siRNA delivery and diabetic wound healing. Chem. Eng. J. 2023, 457, 141244. [Google Scholar] [CrossRef]
- Ye, Y.; Ghrayeb, M.; Miercke, S.; Arif, S.; Müller, S.; Mascher, T.; Chai, L.; Zaburdaev, V. Residual cells and nutrient availability guide wound healing in bacterial biofilms. Soft Matter 2024, 20, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Squyres, G.R.; Newman, D.K. Biofilms as more than the sum of their parts: Lessons from developmental biology. Curr. Opin. Microbiol. 2024, 82, 102537. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burn. Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Darvishi, S.; Tavakoli, S.; Kharaziha, M.; Girault, H.H.; Kaminski, C.F.; Mela, I. Advances in the sensing and treatment of wound biofilms. Angew. Chem. 2022, 134, e202112218. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Sedighi, O.; Bednarke, B.; Sherriff, H.; Doiron, A.L. Nanoparticle-Based Strategies for Managing Biofilm Infections in Wounds: A Comprehensive Review. ACS Omega 2024, 9, 27853–27871. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef]
- Ibne Shoukani, H.; Nisa, S.; Bibi, Y.; Ishfaq, A.; Ali, A.; Alharthi, S.; Kubra, K.t.; Zia, M. Green synthesis of polyethylene glycol coated, ciprofloxacin loaded CuO nanoparticles and its antibacterial activity against Staphylococcus aureus. Sci. Rep. 2024, 14, 21246. [Google Scholar] [CrossRef]
- Masoudi, M.; Mashreghi, M.; Zenhari, A.; Mashreghi, A. Combinational antimicrobial activity of biogenic TiO2 NP/ZnO NPs nanoantibiotics and amoxicillin-clavulanic acid against MDR-pathogens. Int. J. Pharm. 2024, 652, 123821. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Canalejas, A.; Baelo, A.; Herbera, S.; Blanco-Cabra, N.; Vukomanovic, M.; Torrents, E. 3D spatial organization and improved antibiotic treatment of a Pseudomonas aeruginosa–Staphylococcus aureus wound biofilm by nanoparticle enzyme delivery. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Ousey, K.; Rippon, M.G.; Rogers, A.A.; Totty, J.P. Considerations for an ideal post-surgical wound dressing aligned with antimicrobial stewardship objectives: A scoping review. J. Wound Care 2023, 32, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Niederstätter, I.M.; Schiefer, J.L.; Fuchs, P.C. Surgical Strategies to Promote Cutaneous Healing. Med. Sci. 2021, 9, 45. [Google Scholar] [CrossRef]
- Explore Atrauman® Ag. Available online: https://www.hartmann.info/en-hk/brands/l/hk/for-wound-care/atrauman-ag (accessed on 23 January 2025).
- Suprasorb A + Ag. Available online: https://www.bandagesplus.com/wound-care/wound-dressings/antimicrobial-dressings/suprasorb-a-ag?srsltid=AfmBOopUsWSz-7TJXBkdbHK7NvmKil_a7KH5e2s-u-oio-3JH_jm2yqj (accessed on 23 January 2025).
- Aquacel AG+ Extra Silver Hydrofiber Wound Dressing. Available online: https://medicaldressings.co.uk/aquacel-ag-extra-silver-hydrofiber-wound-dressing/ (accessed on 23 January 2025).
- Telfa AMD Non-Adherent. Available online: https://products.mediq.co.uk/portfolio/telfa-amd-non-adherent/ (accessed on 23 January 2025).
- 3M™ Silvercel™ Hydro-Alginate Antimicrobial Dressing with Silver. Available online: https://www.3m.com.ro/3M/ro_RO/p/d/b5005265076/ (accessed on 23 January 2025).
- Askina® Calgitrol® Ag+. Available online: https://catalogs.bbraun.com/en-01/p/PRID00002996/askina-calgitrol-ag-plus-antimicrobial-cmc-fiber-silver-wound-dressing (accessed on 23 January 2025).
- Cutimed® Sorbion® Sorbact®. Available online: https://www.cutimed.co.uk/products/manage-infection-exudate/bacteria-binding-superabsorbent-dressing/cutimed-sorbion-sorbact/cutimed-sorbion-sorbact (accessed on 23 January 2025).
- Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. [Google Scholar] [CrossRef]
- Barroso, A.; Mestre, H.; Ascenso, A.; Simões, S.; Reis, C. Nanomaterials in wound healing: From material sciences to wound healing applications. Nano Sel. 2020, 1, 443–460. [Google Scholar] [CrossRef]
- Dong, F.; Li, S. Wound Dressings Based on Chitosan-Dialdehyde Cellulose Nanocrystals-Silver Nanoparticles: Mechanical Strength, Antibacterial Activity and Cytotoxicity. Polymers 2018, 10, 673. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, F.; Rafiq, M.; Yu, B.; Cong, H.; Shen, Y. Preparation of antimicrobial peptides and their combination with hydrogels for wound healing applications. Int. J. Biol. Macromol. 2024, 274, 133494. [Google Scholar] [CrossRef]
- Shi, S.; Dong, H.; Chen, X.; Xu, S.; Song, Y.; Li, M.; Yan, Z.; Wang, X.; Niu, M.; Zhang, M.; et al. Sustained release of alginate hydrogel containing antimicrobial peptide Chol-37(F34-R) in vitro and its effect on wound healing in murine model of Pseudomonas aeruginosa infection. J. Vet. Sci. 2023, 24, e44. [Google Scholar] [CrossRef]
- de Oliveira, K.B.; Leite, M.L.; Melo, N.T.; Lima, L.F.; Barbosa, T.C.; Carmo, N.L.; Melo, D.A.; Paes, H.C.; Franco, O.L. Antimicrobial Peptide Delivery Systems as Promising Tools Against Resistant Bacterial Infections. Antibiotics 2024, 13, 1042. [Google Scholar] [CrossRef]
- Drayton, M.; Kizhakkedathu, J.N.; Straus, S.K. Towards Robust Delivery of Antimicrobial Peptides to Combat Bacterial Resistance. Molecules 2020, 25, 3048. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.; Zainab, L.; Naheed, A.; Ahmad Qureshi, H.; Bibi, H.S.; Khalid, A.; Tehreem, N. Nanotechnology-based Approaches for Efficient Wound Monitoring and Healing: Wound Monitoring and Healing. Pak. Biomed. J. 2023, 6, 10–18. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Yao, L.; Chen, S.; Wang, X.; Luo, Y.; Lyu, H.; Yu, Y.; Zhou, P.; Zhou, Y. Research trends and hot topics of wearable sensors in wound care over past 18 years: A bibliometric analysis. Heliyon 2024, 10, e38762. [Google Scholar] [CrossRef] [PubMed]
- Prakashan, D.; Kaushik, A.; Gandhi, S. Smart sensors and wound dressings: Artificial intelligence-supported chronic skin monitoring—A review. Chem. Eng. J. 2024, 497, 154371. [Google Scholar] [CrossRef]
- Du, C.; Liu, J.; Fikhman, D.A.; Dong, K.S.; Monroe, M.B.B. Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant and Antimicrobial Properties for Traumatic Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 809361. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, N.; Ni, C.; Cao, R.; Hu, L.; Chen, J.; Zhao, Q.; Xie, T.; Liu, Z. Geometrically adaptive porous shape memory polymers towards personalized biomedical devices. Chem. Eng. J. 2024, 484, 149394. [Google Scholar] [CrossRef]
- Vakil, A.U.; Ramezani, M.; Monroe, M.B.B. Antimicrobial Shape Memory Polymer Hydrogels for Chronic Wound Dressings. ACS Appl. Bio Mater. 2022, 5, 5199–5209. [Google Scholar] [CrossRef]
- Dikmetas, D.N.; Devecioglu, D.; Özünal, Z.G.; Demiroz, A.; Yavuz, E.; Sirkeci, C.B.; Karbancioglu-Guler, F.; Kahveci, D. From waste to remedy: Extraction and utilization of food waste-derived bioactive components in wound healing. Trends Food Sci. Technol. 2024, 145, 104347. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Famurewa, A.C.; Oprea, E. Natural Bioactive Compounds and Human Health. Molecules 2024, 29, 3372. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-derived polysaccharides and their therapeutic potential in wound healing application—A review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef]
- Stenlund, P.; Enstedt, L.; Gilljam, K.M.; Standoft, S.; Ahlinder, A.; Lundin Johnson, M.; Lund, H.; Millqvist Fureby, A.; Berglin, M. Development of an All-Marine 3D Printed Bioactive Hydrogel Dressing for Treatment of Hard-to-Heal Wounds. Polymers 2023, 15, 2627. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Otero, A.; Coutinho, P. Application of microalgae and microalgal bioactive compounds in skin regeneration. Algal Res. 2021, 58, 102395. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Arndt, T.; Chatterjee, U.; Shilkova, O.; Francis, J.; Lundkvist, J.; Johansson, D.; Schmuck, B.; Greco, G.; Nordberg, Å.E.; Li, Y.; et al. Tuneable Recombinant Spider Silk Protein Hydrogels for Drug Release and 3D Cell Culture. Adv. Funct. Mater. 2024, 34, 2303622. [Google Scholar] [CrossRef]
- Branković, M.; Zivic, F.; Grujovic, N.; Stojadinovic, I.; Milenkovic, S.; Kotorcevic, N. Review of Spider Silk Applications in Biomedical and Tissue Engineering. Biomimetics 2024, 9, 169. [Google Scholar] [CrossRef]
- Ornithopoulou, E.; Åstrand, C.; Gustafsson, L.; Crouzier, T.; Hedhammar, M. Self-Assembly of RGD-Functionalized Recombinant Spider Silk Protein into Microspheres in Physiological Buffer and in the Presence of Hyaluronic Acid. ACS Appl. Bio Mater. 2023, 6, 3696–3705. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent advances in decellularized biomaterials for wound healing. Mater. Today Bio 2023, 19, 100589. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Li, M.; Nie, L.; Wei, Q.; Okoro, O.V.; Jafari, H.; Wang, S.; Deng, J.; Chen, J.; et al. Bioactive wound dressing based on decellularized tendon and GelMA with incorporation of PDA-loaded asiaticoside nanoparticles for scarless wound healing. Chem. Eng. J. 2023, 466, 143016. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Jaiswal, R.; Sherje, A.P. Recent advances in biopolymer-based smart hydrogel for wound healing. J. Drug Deliv. Sci. Technol. 2024, 99, 105990. [Google Scholar] [CrossRef]
- Lopes, A.I.; Pintado, M.M.; Tavaria, F.K. Plant-Based Films and Hydrogels for Wound Healing. Microorganisms 2024, 12, 438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Cheng, H.; Zhu, J.; Li, X.; Ye, S.; Li, X. Hydrogel-based dressings designed to facilitate wound healing. Mater. Adv. 2024, 5, 1364–1394. [Google Scholar] [CrossRef]
- Rajput, J.H.; Rathi, V.; Mukherjee, A.; Yadav, P.; Gupta, T.; Das, B.; Poundarik, A. A novel polyurethane-based silver foam dressing with superior antimicrobial action for management of infected chronic wounds. Biomed. Mater. 2025, 20, 015005. [Google Scholar] [CrossRef]
- Raepsaet, C.; Alves, P.; Cullen, B.; Gefen, A.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Santamaria, N.; Sharpe, A.; Swanson, T.; et al. The development of a core outcome set for clinical effectiveness studies of bordered foam dressings in the treatment of complex wounds. J. Tissue Viability 2023, 32, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kushare, A.; Gupta, M.N.; Ambre, P. Advanced Dressings for Chronic Wound Management. ACS Appl. Bio Mater. 2024, 7, 2660–2676. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ou, Q.; Chen, K.; Liang, C.; Zeng, X.; Lin, D.; Lin, L. Foam dressing and micropower vacuum dressing promote diabetic foot ulcer wound healing by activating the PI3K/AKT/mTOR pathway in rats. J. Biomater. Appl. 2024, 39, 40–47. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef]
- Sussman, G. An update on wound management. Aust. Prescr. 2023, 46, 29–35. [Google Scholar] [CrossRef]
- Meredith, K.; Forbes, L.E. Antimicrobial Activity of Silver-Containing Surgical Dressings in an In vitro Direct Inoculation Simulated Wound Fluid Model Against a Range of Gram-Positive and Gram-Negative Bacteria. Surg. Infect. 2023, 24, 637–644. [Google Scholar] [CrossRef]
- Swetha Menon, N.P.; Kamaraj, M.; Anish Sharmila, M.; Govarthanan, M. Recent progress in polysaccharide and polypeptide based modern moisture-retentive wound dressings. Int. J. Biol. Macromol. 2024, 256, 128499. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Sun, Q.; Xiao, D.; Zhang, M.; Gao, S.; Guo, B.; Lin, Y. Tetrahedral framework nucleic acids/hyaluronic acid-methacrylic anhydride hybrid hydrogel with antimicrobial and anti-inflammatory properties for infected wound healing. Int. J. Oral Sci. 2024, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Perluxo, J.D.; da Silva, A.I.C.; Cardoso, R.P.; da Conceição, M.O.T.; Pinhati, F.R.; Rosa, D.S.; Mulinari, D.R. A Novel Sustainable Antimicrobial Polyurethane foam Castor Oil-based. J. Inorg. Organomet. Polym. Mater. 2024, 34, 2488–2500. [Google Scholar] [CrossRef]
- Han, J.; Liu, H.; Cheng, J.; Wang, X.; Xu, C.; Zhang, F.; Dong, X. A chondroitin sulfate-based temperature-responsive hydrogel with antimicrobial properties for epidermal wound repair in diabetic patients. Eur. Polym. J. 2025, 222, 113588. [Google Scholar] [CrossRef]
- Lan, Z.; Kar, R.; Chwatko, M.; Shoga, E.; Cosgriff-Hernandez, E. High porosity PEG-based hydrogel foams with self-tuning moisture balance as chronic wound dressings. J. Biomed. Mater. Res. Part A 2023, 111, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Orhan, B.; Karadeniz, D.; Kalaycıoğlu, Z.; Kaygusuz, H.; Torlak, E.; Erim, F.B. Foam-based antibacterial hydrogel composed of carboxymethyl cellulose/polyvinyl alcohol/cerium oxide nanoparticles for potential wound dressing. Int. J. Biol. Macromol. 2025, 291, 138924. [Google Scholar] [CrossRef]
- Lan, Z.; Guo, L.; Fletcher, A.; Ang, N.; Whitfield-Cargile, C.; Bryan, L.; Welch, S.; Richardson, L.; Cosgriff-Hernandez, E. Antimicrobial hydrogel foam dressing with controlled release of gallium maltolate for infection control in chronic wounds. Bioact. Mater. 2024, 42, 433–448. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L.; Gill, E.J. Advancements and Challenges in Self-Healing Hydrogels for Wound Care. Gels 2024, 10, 241. [Google Scholar] [CrossRef]
- Zubair, M.; Hussain, A.; Shahzad, S.; Arshad, M.; Ullah, A. Emerging trends and challenges in polysaccharide derived materials for wound care applications: A review. Int. J. Biol. Macromol. 2024, 270, 132048. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C.; Suryadevara, N.K.; Nag, A. Wearable Sensors for Healthcare: Fabrication to Application. Sensors 2022, 22, 5137. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.-C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.-C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2023, 41, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Fayne, R.A.; Borda, L.J.; Egger, A.N.; Tomic-Canic, M. The Potential Impact of Social Genomics on Wound Healing. Adv. Wound Care 2019, 9, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Darwin, E.; Tomic-Canic, M. Healing Chronic Wounds: Current Challenges and Potential Solutions. Curr. Dermatol. Rep. 2018, 7, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent Developments in Antibacterial Therapy: Focus on Stimuli-Responsive Drug-Delivery Systems and Therapeutic Nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef] [PubMed]
- Encarnação, R.; Manuel, T.; Palheira, H.; Neves-Amado, J.; Alves, P. Artificial Intelligence in Wound Care Education: Protocol for a Scoping Review. Nurs. Rep. 2024, 14, 627–640. [Google Scholar] [CrossRef]
- Ganesan, O.; Morris, M.X.; Guo, L.; Orgill, D. A review of artificial intelligence in wound care. Artif. Intell. Surg. 2024, 4, 364–375. [Google Scholar] [CrossRef]
- Talwar, A.; Shen, C.; Shin, J.H. Applications of Artificial Intelligence in Wound Care. ScieRxiv 2024. [Google Scholar] [CrossRef]
- Barakat-Johnson, M.; Jones, A.; Burger, M.; Leong, T.; Frotjold, A.; Randall, S.; Kim, B.; Fethney, J.; Coyer, F. Reshaping wound care: Evaluation of an artificial intelligence app to improve wound assessment and management amid the COVID-19 pandemic. Int. Wound J. 2022, 19, 1561–1577. [Google Scholar] [CrossRef]
- Swerdlow, M.; Guler, O.; Yaakov, R.; Armstrong, D.G. Simultaneous Segmentation and Classification of Pressure Injury Image Data Using Mask-R-CNN. Comput. Math. Methods Med. 2023, 2023, 3858997. [Google Scholar] [CrossRef]
- Chan, K.S.; Chan, Y.M.; Tan, A.H.M.; Liang, S.; Cho, Y.T.; Hong, Q.; Yong, E.; Chong, L.R.C.; Zhang, L.; Tan, G.W.L.; et al. Clinical validation of an artificial intelligence-enabled wound imaging mobile application in diabetic foot ulcers. Int. Wound J. 2022, 19, 114–124. [Google Scholar] [CrossRef]
- Moradifar, F.; Sepahdoost, N.; Tavakoli, P.; Mirzapoor, A. Multi-functional dressings for recovery and screenable treatment of wounds: A review. Heliyon 2025, 11, e41465. [Google Scholar] [CrossRef]


| Clinical Trial ID | Official Title | Intervention/Treatment | Enrollment | Study Completion | Phase |
|---|---|---|---|---|---|
| NCT02361931 | Prospective, Multicenter, Single-blind, Randomized, Controlled Clinical Trial on Safety and Efficacy of a Novel Topical Formulation Containing Erythropoietin for the Treatment of Diabetic Foot Ulcers | Drug: A hydrogel containing erythropoietin Drug: Hydrogel (as a part of SOC) | 20 | 12.06.2018 | Phase 1 Phase 2 |
| NCT05607979 | The RENEW Study: (Restoring Tissue and Evaluating Novel Treatments for Efficacy in Wounds): A Non-Inferiority Study | Drug: Lavior Diabetic Wound Gel Drug: Smith & Nephew Solosite Gel Hydrogel Wound Dressing | 75 | 01.05.2024 | Phase 2 Phase 3 |
| NCT04834245 | Evaluation of Diabetic Foot Wound Healing Using Hydrogel/Nano Silver-based Dressing vs. Traditional Dressing: A Prospective Randomized Control Stud | Procedure: Hydrogel/nano silver-based dressing | 30 | 30.12.2019 | Not applicable |
| NCT01143727 | Comparison of SANTYL vs. Hydrogel in Debridement of Inflamed Diabetic Foot Ulcers | Drug: Santyl Drug: Tegaderm Hydrogel | 20 | 10.2020 | Phase 4 |
| NCT03700580 | Application of a Double Blind Clinical Trial Protocol for Evaluation of Healing Action of P1G10, From V Cundinamarcensis to Chronic Neuropathic Wounds in Diabetic Foot Ulcers. | Drug: Hydrogel treatment Drug: P1G10 | 50 | 15.10.2016 | Phase 2 |
| NCT01427569 | A Randomized, Placebo-controlled, Double-blind Phase II Study to Evaluate the Efficacy of IZN-6D4 Gel for the Treatment of Diabetic Foot Ulcers | Drug: IZN-6D4 Gel Other: Placebo hydrogel | 82 | 08.2015 | Phase 2 |
| NCT05661474 | The Effects of Fitostimoline® Hydrogel Versus Saline Gauze Dressing in Patients With Diabetic Foot Ulcers: a Monocentric, Two-arm, Open-label, Randomized, Controlled Trial. | Drug: Fitostimoline ® hydrogel group Drug: Saline gauze group | 40 | 12.12.2022 | Phase 4 |
| NCT03816618 | The Healing Effects Of Honey and Hydrogel Products On The Diabetic Foot | Drug: Medihoney Gel in A Tube Drug: Hydrogel Drug: Fucidin Ointment | 120 | 13.02.2023 | Early Phase 1 |
| NCT02111291 | Clinical Outcomes Associated With Enzymatic Debridement of Diabetic Foot Ulcers for Up To 12 Weeks With Clostridial Collagenase (Santyl®) Ointment | Biological: Collagenase SANTYL® Ointment Biological: Hydrogel (if needed) and foam dressing | 215 | 12.2015 | Phase 4 |
| Clinical Trial ID | Official Title | Intervention/Treatment | Enrollment | Study Completion | Phase | Location |
|---|---|---|---|---|---|---|
| NCT05877638 | Randomized Controlled Trial Assessing a Novel Glycopolymer Compound in the Treatment of Superficial Partial-Thickness Burns | Device: SynePure Wound Cleanser and Catasyn Advanced Technology Hydrogel Drug: SILVADENE Cream 1% (silver sulfadiazine) | 2 | 20.06.2024 | Not applicable | Louisiana, USA |
| NCT03190655 | Efficacy and Safety of Aluminaid Versus Hydrogel Wound Dressings in the Treatment of Partial Thickness Burns | Device: Aluminaid Device: Hydrogel | 6 | 05.03.2018 | Not applicable | Jakarta, Indonesia |
| NCT01062191 | Flexible Hydrogel Nanoparticle Wound Dressing Allows Greater Joint Range of Motion Compared to Typical Sodium Carboxymethylcellulose Dressing | Device: Aquacel AG, typical carboxymethylcellulose dressing Device: Altrazeal Flexible Hydrogel Nanoparticle Wound Dressing | 3 | 10.2008 | Not applicable | Texas, USA |
| NCT03674151 | Randomized-controlled Trial of Wound Healing, Pain, Microbiology, Handling and Thrift of Different Wound Dressings in Patients With Split-skin Grafted Third Degree Burns | Device: Silver Nylon dressing Device: Manuka-Honey Device: Povidone-Iod (PVP-Iod) Device: Hydrogel | 20 | 12.2025 (estimated) | Not applicable | Schleswig-Holstein, Germany |
| NCT02394873 | A Phase 1 Clinical Study to Evaluate the Safety of Allogeneic Adipose-derived Stem Cells in the Subjects With Deep Second-degree Burn Wound | Biological: ALLO-ASC-DFU | 5 | 10.2015 | Phase 1 | Seoul, South Korea |
| NCT01499264 | Efficacy of MySkin Patch for the Healing of Burn Wounds: a Randomised Controlled Trial | Device: MySkin patch Device: Traditional Dressing | 120 | 20.2014 | Phase 3 | Milano and Varese, Italy |
| NCT03183622 | A Follow-up Study to Evaluate the Safety for the Patients With ALLO-ASC-DFU Treatment in Phase 1 Clinical Trial of ALLO-ASC-BI-101 | Biological: ALLO-ASC-DFU | 5 | 12.2017 | - | Seoul, South Korea |
| NCT04601532 | Randomized Controlled Trial Assessing a Novel Glycopolymer Compound in the Treatment of Superficial Partial-thickness Burns | Device: Catasyn™ Advanced Technology Hydrogel and SynePure™ Wound Cleanser Drug: Silver Sulfadiazine | 26 | 08.12.2022 | Phase 4 | Pennsylvnia, USA |
| NCT03113747 | Safety and Efficacy Evaluation of Tissue Engineered Construct Based on Allogeneic Adipose-derived Multipotent Mesenchymal Stromal Cells and Platelet-poor Plasma Fibrin Hydrogel to Treat the Patients With Burn Wounds | Biological: ALLO-ASCs | 20 | 26.12.2018 | Phase 1 Phase 2 | Kyiv, Ukraine |
| NCT03183648 | A Follow-up Study to Evaluate the Safety for the Patients With ALLO-ASC-DFU Treatment in Phase 2 Clinical Trial of ALLO-ASC-BI-201 | Biological: ALLO-ASC-DFU | 14 | 06.2023 | - | Seoul, South Korea |
| Commercial Product | Producer | Dressing Type | Type of Wound | Reference |
|---|---|---|---|---|
| Activheal Hydrogel | Advanced Medical Solutions Ltd. | Amorphous hydrogel | Pressure ulcers, Leg ulcers, Diabetic ulcers, Cavity wounds, Graft and Donor sites, Lacerations and abrasions, Post op surgical wounds | [94] |
| 3M™ Tegaderm™ Hydrogel | Solventum Corporation | Amorphous hydrogel | Pressure ulcers, Arterial ulcers, Venous ulcers, Neuropathic ulcers, Post-operative surgical wounds, Abrasions, Lacerations | [95] |
| Flaminal | Flen Health | Antimicrobial hydrogel | Superficial and deep partial burns, Infected wounds, diabetic foot ulcers, Leg ulcers, Pressure ulcers, Neonates and Pediatrics, Oncologic wounds, Wounds from Radiotherapy, Skin tears, Post-surgical wounds, Traumatic wounds | [96] |
| Hydrosorb gel | PAUL HARTMANN AG | Amorphous hydrogel | Ulcers, Decubitus ulcers | [97] |
| Fitostimoline® idrogel | Damor | Antimicrobial hydrogel | Ulcers, Wounds, Sores, Scalds, Abrasions, First- degree burns, Second-degree burns | [98] |
| HydroTac® | PAUL HARTMANN AG | Sheet of hydrogel | Leg ulcers, Diabetic foot syndrome, Decubital ulcer, Burns up to grade 2A, Skin graft donor sites | [99] |
| INTRASITE GEL | Smith & Nephew Medical Ltd. | Antimicrobial hydrogel | Pressure sores, Leg ulcers, Diabetic foot ulcers, Malignant wounds, Burns, Surgical wounds, Scalds, Lacerations, Grazes, Amputations | [100] |
| Iodozyme | Crawford Healthcare Ltd. | Two-layer hydrogel | External wounds, Moderately exuding, Dry wounds, Non-exuding, Under compression therapy, Infected wounds | [101] |
| Oxyzyme | Crawford Healthcare Ltd. | Two-layer hydrogel | External wounds, Moderately exuding, Dry wounds, Non-exuding, Under compression therapy, Mildly or non-infected wounds | [101] |
| Clinical Trial ID | Official Title | Intervention/Treatment | Enrollment | Study Completion | Phase | Location |
|---|---|---|---|---|---|---|
| NCT01036438 | A Double-blind, Comparative, Superiority, Multi-centre Investigation Evaluating the Efficacy of an Absorbent Foam Dressing Containing Silver (Mepilex Ag) Versus the Same Dressing Without Silver Used on Subjects With Venous Leg Ulcers or Mixed Ulcers | Device: Mepilex Ag Device: Mepilex without Ag | 201 | 10.2013 | Phase 4 | 44 locations from (Czech Republic, France, Germany, Netherlands) |
| NCT00627094 | A Randomised, Controlled and Double-blind Clinical Investigation on the Effectiveness and Safety of a Foam Dressing Biatain Ibu Non-adhesive vs. Biatain Non-adhesive, in Painful Chronic Venous Leg Ulcers | Device: Biatain Device: Biatain Ibu | 120 | 04.2009 | Not applicable | 13 locations from (Denmark, France, Germany, Spain) |
| NCT02167815 | A Multicenter, Post Marketing Clinical Follow up (PMCF) Investigation to Evaluate the Performance and Safety of a Soft Silicone Foam Dressing and to Evaluate the Performance of Standard Care in Exuding Venous Leg Ulcers | Device: Mepilex XT Other: standard care | 30 | 07.2015 | Not applicable | 3 locations from Prague, Třinec, Jihlava, Czech Republic |
| NCT05608317 | A Prospective, Open, Multi-Center, Interventional, Non-Comparative Clinical Investigation to Follow the Progress of Exuding Venous Leg Ulcers Using a Non-Bordered Foam Dressing | Device: ALLEVYN Non-Adhesive | 20 | 30.11.2024 (estimated) | Not applicable | California, Florida, Pennsylvania, USA |
| NCT03900455 | Effectiveness of the Use of a Polyurethane Foam Multilayer Dressing in the Sacral Area, in Addition to Standard Healthcare, to Prevent the Onset of Pressure Ulcer in Patients at Risk. Multicentric Randomized Controlled Trial | Device: Hydrocellular polyurethane foam multilayer dressing Procedure: Standard preventive care | 711 | 19.03.2020 | Not applicable | 9 locations from Alessandria, Bologna, Cesena, Pavia, Reggio Emilia, Roma, Trento, Verona, Italy |
| Clinical Trial ID | Official Title | Intervention/Treatment | Enrollment | Study Completion | Study Type | Location |
|---|---|---|---|---|---|---|
| NCT03662997 | A Prospective, Randomized, Controlled Study Using Cross-Over Design to Evaluate and Compare 3 Multi-Layered Foam Dressings for the Management of Chronic Wounds | Device: Bordered Five-Layer Foam Dressing Device: Hydropolymer Foam Dressing Device: Hydrocellular Multi-Layer Foam Dressing | 40 | 15.11.2019 | Interventional | 5 locations: California (2), New Jersey (1), Pennsylvania (2), USA |
| NCT03877484 | A Prospective, Multi-center, Post-Market Clinical Follow-Up Study to Evaluate the Safety and Effectiveness of ALLEVYN Gentle Border | Device: ALLEVYN Gentle border | 43 | 19.11.2021 | Observational | 6 locations: France (1), Germany (4), United Kingdom (1) |
| NCT03900455 | Effectiveness of the Use of a Polyurethane Foam Multilayer Dressing in the Sacral Area, in Addition to Standard Healthcare, to Prevent the Onset of Pressure Ulcer in Patients at Risk. Multicentric Randomized Controlled Trial | Device: Hydrocellular polyurethane foam multilayer dressing Procedure: Standard preventive care | 711 | 19.03.2020 | Interventional | 9 locations in Italy |
| NCT03596112 | The Difference in Wound Size Reduction Comparing Two Frequently Used Wound Dressings in Everyday Care—a Randomized Controlled Trail | Other: application of a polyacrylate wound pad Other: Hydrocellular foam | 77 | 31.05.2020 | Interventional | Onex, Switzerland |
| NCT02692482 | Effectiveness of the Use of a New Polyurethane Foam Multilayer Dressing in the Sacral Area to Prevent the Onset of Pressure Sores in the Elderly With Hip Fractures. Randomized Controlled Trial. | Device: hydrocellular polyurethane foam multilayer dressing Procedure: standard care | 359 | 12.2016 | Interventional | Bologna, Italy |
| Commercial Product | Producer | Dressing Type | Type of Wound | Reference |
|---|---|---|---|---|
| HydroTac® | PAUL HARTMANN AG | PU foam with hydrogel sheet | Small to moderate exudate amount wounds, leg ulcers, diabetic foot syndrome, decubital ulcer, burns up to grade 2A, skin graft donor sites | [99] |
| 3M™ Tegaderm™ Silicone Foam Border | Solventum Corporation | Multi-layer foam dressing | Arterial ulcers, Donor sites, Superficial partial thickness burns, Pressure ulcers, Surgical wounds, Venous leg ulcer | [130] |
| ALLEVYN | Smith + Nephew | Hydrocellular foam | Burn wounds, Surgical wounds, Skin tears, Donor sites, Diabetic foot ulcers, Venous leg ulcers, Pressure injuries | [131] |
| ActivHeal® Foam Adhesive | ActivHeal | Two-layer foam dressing | Pressure ulcers, Leg ulcers, Diabetic ulcers, Post-operative surgical, Cavity wounds (secondary dressing), Lacerations and abrasions, Graft wounds, Donor sites, Superficial, partial thickness burns | [132] |
| Sorbact® Foam Dressing | Abigo Medical AB | bacteria- and fungi foam dressing | Clean, contaminated, colonized, or infected wounds, Surgical wounds, traumatic wounds, Pressure ulcers, Diabetic ulcers, Foot ulcers, Leg ulcers | [133] |
| PermaFoam® Classic | PAUL HARTMANN AG | Hydrophilic polymer foam dressing | Venous ulcers, Pressure ulcers II-IV, Diabetic foot ulcers, Abrasions, Incisions, Donor sites | [134] |
| Mepilex® Border Ag | Mölnlycke Health Care AB | Antimicrobial foam dressing | Pressure ulcers, Leg ulcers, Foot ulcers, Partial thickness burns, Traumatic wounds, Surgical wounds | [135] |
| Clinical Trial ID | Official Title | Intervention/Treatment | Enrollment | Study Completion | Phase |
|---|---|---|---|---|---|
| NCT00981110 | Surgical Sites Infections Following Colorectal Cancer Surgery. A Randomized Prospective Trial Comparing Standard and Advanced Antimicrobial Dressing Containing Ionic Silver. | Device: AQUAGEL Ag Hydrofiber Wound Dressing Device: Mepore Self-adhesive absorbent dressing | 120 | 12.2010 | Phase 3 |
| NCT00203541 | Effects of a New Antimicrobial Dressing on Wound Healing and Incidence of Sternal Wound Infections in Subjects Who Have Undergone Cardiac Surgical Procedures Requiring Median Sternotomy | Device: TELFA™ A.M.D. Island dressing | 1100 | 06.2006 | Not applicable |
| NCT03402945 | A Cluster-randomized Factorial Crossover Trial, Comparing Antibiotic Mono-prophylaxis With Cefazolin vs. Dual-prophylaxis With Cefazolin Plus Vancomycin and Conventional Wound Dressing vs. Prevena Negative-pressure Wound Management | Device: Prevena Drug: Cefazolin Drug: Vancomycin Other: standard wound dressing | 4107 | 12.2024 (estimated) | Phase 4 |
| NCT03284749 | Randomised Controlled Trial on the Effect of Copper Impregnated Dressings and Maternity Pads on the Healing of Obstetric Wounds and Wound Infection | Other: Copper impregnated wound dressing Other: Normal wound dressing Other: Copper impregnated maternity pads Other: Normal maternity pads | 774 | 19.12.2017 | Not applicable |
| Commercial Product | Producer | Dressing Type | Type of Wound | Reference |
|---|---|---|---|---|
| Atrauman Ag | Paul Hartmann AG | Silver-containing dressing | Ulcus cruris, Diabetic leg ulcer, Decubitus, Acute burns (up to 2nd degree) | [171] |
| Suprasorb A + Ag | Lohmann & Rauscher | Silver-containing dressing | Venous leg ulcers, Arterial ulcers, Diabetic ulcers, Pressure ulcers | [172] |
| Aquacel AG+ Extra | ConvaTec | Silver-containing dressing | Diabetic foot ulcers, Leg ulcers, Venous stasis ulcers, Arterial ulcers, Leg ulcers of mixed etiology, Pressure ulcers/sores, Surgical wounds, Traumatic wounds, Fungoides-cutaneous tumors, Fungating carcinoma, Kaposi’s sarcoma, Angiosarcoma, Cutaneous metastasis | [173] |
| Telfa | Cardinal Health | Non-adherent antimicrobial dressings | Lightly draining wounds, Burns, Skin grafts, Donor sites, Abrasions, Surgical incisions, Chronic wounds | [174] |
| 3M™ Silvercel™ | 3M | Silver-containing dressing | Chronic wounds with moderate to high levels of exudate | [175] |
| Askina® Calgitrol® Ag+ | B. Braun SE | Sil-ver-containing dressing | Leg ulcers, Pressure ulcers, Diabetic ulcers, Post-operative, Wounds left to heal by secondary intent, Donor sites, Abrasions, Lacerations, Partial thickness burns | [176] |
| Cutimed® Sorbion® Sorbact® | Abigo Medical AB | Antimicrobial dressings | Pressure ulcers, Leg ulcers, Diabetic foot ulcers, Surgical wounds, Traumatic wounds | [177] |
| Type of Dressings | Composition | Patient Comfort | Properties | Recent Innovation | Targeted Wounds | References |
|---|---|---|---|---|---|---|
| Hydrogels | Natural, synthetic polymers | Easy dressing removal | Absorb excess exudate, good biological compatibility, good hemostatic, tissue repair stimulation, gas exchange, strongly adhere to | Bioactive agents’ incorporation | Acute and chronic wounds | [36,74,202,203,204,205] |
| Foam Dressings | Polyurethane, silicone resins | Depending on the exudate amount, it can be worn for one to seven days with silicone adhesive, easily removed, lower pain risk and skin stripped | Absorb high to moderate wound exudate, maintain moisture, control gaseous exchange, effective thermal insulation, cushioning, protection | Antimicrobial agent incorporation | Acute and chronic wounds | [115,206,207,208,209] |
| Antimicrobial Dressings | Different antimicrobial agents | Good tolerability | Effective against various types of bacteria, fungi, fungi, viruses, low- to moderate-exuding wounds | Stimuli response smart dressing | Diabetic wound, surgical site infections | [144,210,211,212] |
| Hybrid System | Components | Benefits | Reported Efficacy | Application Areas | Reference |
|---|---|---|---|---|---|
| Hydrogels and antimicrobial dressing | Hyaluronic acid-methacrylic anhydride, antimicrobial peptides, tetrahedral framework nucleic acid | Antimicrobial activity, anti-inflammatory | Broad-spectrum antibacterial properties, cell migration promotion, anti-inflammatory properties, reduced scarring | Infected wound | [215] |
| Antimicrobial polyurethane foam | Polyurethane foam, castor oil, zinc oxide | Antibacterial activity | Improvement of thermal and mechanical properties, antimicrobial activity against (P. aeruginosa) | Hospital infections | [216] |
| Temperature-responsive hydrogel | Temperature-responsive hydrogel, oxidized chondroitin sulfate, gelatin, silver nanoparticles | Antimicrobial, temperature-responsive properties, exudate absorption | Efficient exudate absorption, reduction in skin infection, superior adhesion on skin at 37 °C, non-cytotoxic, superior viscoelastic properties | Diabetic wound infections | [217] |
| Hydrogel + foam | Poly (ethylene glycol) diacrylate based | Wound moisture balance, remove exudate | Self-tuning moisture control, remove exudate fast | Exudative and dry chronic wounds | [218] |
| Foam-based antibacterial hydrogel | Carboxymethyl cellulose, polyvinyl alcohol, cerium oxide nanoparticle | Antibacterial activity, exudate absorption, biodegradability, and drug delivery | Effective antibacterial properties (E. coli and S. aureus), good swelling properties (foam gel’s swelling ratio to %1057 in just one hour), drug delivery characteristics (silver sulfadiazine up to 6 h) | Wound infection | [219] |
| Antimicrobial hydrogel foam dressing | Poly (lactic-co-glycolic acid) (PLGA) microsphere, gallium maltolate | Drug release profile, cytocompatibility, antimicrobial activity | low cytotoxicity on dermal fibroblasts, a consistent release profile throughout the desired five days, gallium maltolate prohibited bacterial growth (both drug-susceptible and resistant) | Chronic wound | [220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. https://doi.org/10.3390/gels11020123
Alberts A, Tudorache D-I, Niculescu A-G, Grumezescu AM. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels. 2025; 11(2):123. https://doi.org/10.3390/gels11020123
Chicago/Turabian StyleAlberts, Adina, Dana-Ionela Tudorache, Adelina-Gabriela Niculescu, and Alexandru Mihai Grumezescu. 2025. "Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings" Gels 11, no. 2: 123. https://doi.org/10.3390/gels11020123
APA StyleAlberts, A., Tudorache, D.-I., Niculescu, A.-G., & Grumezescu, A. M. (2025). Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels, 11(2), 123. https://doi.org/10.3390/gels11020123









