Hybrid Carrageenans Versus Kappa–Iota-Carrageenan Blends: A Comparative Study of Hydrogel Elastic Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Produced Carrageenans
2.2. Phase States in KCl and NaCl
2.3. Gel Formation During Cooling
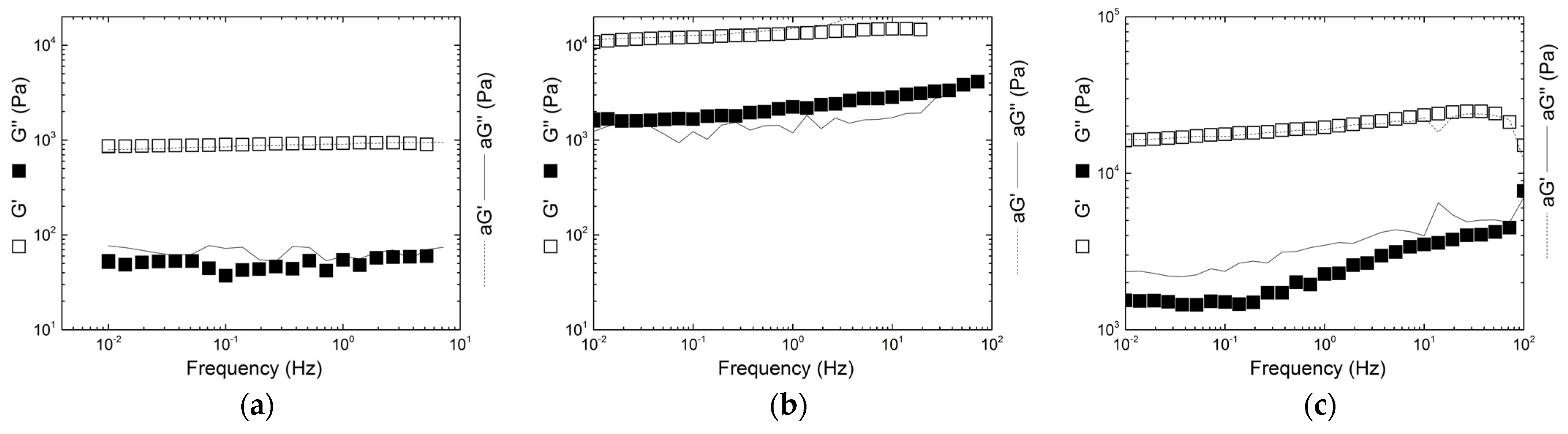
2.4. Gel Elasticity and Structure
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Carrageenan Extraction and Alkali Modification
4.3. Carrageenan Characterization
4.4. Phase States and Rheological Characterization of Gels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porse, H.; Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Mendes, M.; Cotas, J.; Pacheco, D.; Ihle, K.; Hillinger, A.; Cascais, M.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Red Seaweed (Rhodophyta) Phycocolloids: A Road from the Species to the Industry Application. Mar. Drugs 2024, 22, 432. [Google Scholar] [CrossRef]
- Zhang, J.; Langford, Z.; Waldron, S. The global carrageenan industry. In Globalisation and Livelihood Transformations in the Indonesian Seaweed Industry, 1st ed.; Langford, Z., Ed.; Routledge: New York, NY, USA, 2024; pp. 23–50. [Google Scholar]
- Pulidindi, K.; Ahuja, K. Carrageenan Market Size. Available online: https://www.gminsights.com/industry-analysis/carrageenan-market (accessed on 21 January 2025).
- Nanaki, S.; Karavas, E.; Kalantzi, L.; Bikiaris, D. Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained release carriers. Carbohydr. Polym. 2010, 79, 1157–1167. [Google Scholar] [CrossRef]
- Matas, A.; Molina-Montero, C.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Viability Study on the Use of Three Different Gels for 3D Food Printing. Gels 2023, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, L.; Xie, P.; Zhou, Q.; Chen, Y.; Karrar, E.; Qi, H.; Lin, R.; Zhu, Y.; Jin, J.; et al. Static Stability of Partially Crystalline Emulsions: Impacts of Carrageenan and Its Blends With Xanthan Gum and/or Guar Gum. Int. J. Biol. Macromol. 2022, 223, 307–315. [Google Scholar] [CrossRef]
- Piculell, L. Gelling Carrageenans. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 239–287. [Google Scholar]
- Liu, L.; Duan, G.; Yang, H. Recent advances in exploiting carrageenans as a versatile functional material for promising biomedical applications. Int. J. Biol. Macromol. 2023, 235, 123787. [Google Scholar] [CrossRef]
- Van de Velde, F. Structure and Function of Hybrid Carrageenans. Food Hydrocoll. 2008, 22, 727–734. [Google Scholar] [CrossRef]
- Bixler, H.J.; Johndro, K.; Falshaw, R. Kappa-2 carrageenan: Structure and performance of commercial extracts: II. Performance in two simulated dairy applications. Food Hydrocoll. 2001, 15, 619–630. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Mendoza, W.G.; Rodrigueza, M.R.C.; Romero, J.B.; Montaño, M.N.E. Structure and functional performance of gigartinacean kappa-iota hybrid carrageenan and solieriacean kappa-iota carrageenan blends. Food Hydrocoll. 2004, 18, 283–292. [Google Scholar] [CrossRef]
- Ward, G.M.; Faisan, J.P., Jr.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A review of reported seaweed diseases and pests in aquaculture in Asia. J. World Aquac. Soc. 2020, 51, 815–828. [Google Scholar] [CrossRef]
- Ficko-Blean, E.; Hervé, C.; Michel, G. Sweet and sour sugars from the sea: The biosynthesis and remodeling of sulfated cell wall polysaccharides from marine macroalgae. Perspect. Phycol. 2015, 2, 51–64. [Google Scholar] [CrossRef]
- Hale, J.; Gerhäuser, J.; Gaukel, V.; Wefers, D. Commercially available carrageenans show broad variation in their structure, composition, and functionality. Eur. Food. Res. Technol. 2024, 250, 2989–3003. [Google Scholar] [CrossRef]
- Hilliou, L. Structure–Elastic properties Relationships in Gelling Carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef]
- Goenzon, L.C.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation mechanism and network structure in gels of carrageenans and their mixtures viewed at different length scales—A review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Geonzon, L.C.; Hashimoto, K.; Oda, T.; Matsukawa, S.; Mayumi, K. Elaborating Spatiotemporal Hierarchical Structure of Carrageenan Gels and Their Mixtures during Sol–Gel Transition. Macromolecules 2023, 56, 8676–8687. [Google Scholar] [CrossRef]
- Brenner, T.; Tuvikene, R.; Parker, A.; Matsukawa, S.; Nishinari, K. Rheology and structure of mixed kappa-carrageenan/iotacarrageenan gels. Food Hydrocoll. 2014, 39, 272–279. [Google Scholar] [CrossRef]
- Parker, A.; Brigand, G.; Miniou, C.; Trespoey, A.; Vallée, P. Rheology and fracture of mixed ι- and κ-carrageenan gels: Two-step gelation. Carbohydr. Polym. 1993, 20, 253–262. [Google Scholar] [CrossRef]
- Piculell, L.; Nilsson, S.; Muhrbeck, P. Effects of small amounts of kappa-carrageenan on the rheology of aqueous iota-carrageenan. Carbohydr. Polym. 1992, 18, 199–208. [Google Scholar] [CrossRef]
- Ridout, M.J.; Garza, S.; Brownsey, G.J.; Morris, V.J. Mixed iota-kappa carrageenan gels. Int. J. Biol. Macromol. 1996, 18, 5–8. [Google Scholar] [CrossRef]
- Bui, V.T.N.T.; Nguyen, B.T.; Renou, F.; Nicolai, T. Rheology and microstructure of mixtures of iota and kappa-carrageenan. Food Hydrocoll. 2019, 89, 180–187. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Palla, C.S.; Dethe, D.H.; Joshi, Y.M. Rheological Investigation of the Network Structure in Mixed Gels of Kappa and Iota Carrageenan. Food Hydrocoll. 2024, 146, 109298. [Google Scholar] [CrossRef]
- Van de Velde, F.; Peppelman, H.A.; Rollema, H.S.; Tromp, R.H. On the structure of κ/ι-hybrid carrageenans. Carbohydr. Res. 2001, 331, 271–283. [Google Scholar] [CrossRef]
- Torres, M.D.; Azevedo, G.; Hilliou, L. Phase diagrams of hybrid carrageenans extracted from Ahnfeltiopsis devoniensis and Chondrus crispus. Carbohydr. Polym. 2016, 136, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Hilliou, L.; Moraes, I.C.F.; Almeida, P.L. From the seaweeds’ carrageenan composition to the hybrid carrageenans’ hydrogel elasticity: Identification of a relationship based on the content in iota-carrageenan. Food Hydrocoll. 2025, 62, 111007. [Google Scholar] [CrossRef]
- Moraes, I.C.F.; Hilliou, L. Viscoelastic reversibility of carrageenan hydrogels under large amplitude oscillatory shear: Hybrid-carrageenan versus blends. Gels 2024, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Kamkar, M.; Salehiyan, R.; Goudoulas, T.B.; Abbasi, M.; Saengow, C.; Erfanian, E.; Sadeghi, S.; Natale, G.; Rogers, S.A.; Giacomin, J.A.; et al. Large amplitude oscillatory shear flow: Microstructural assessment of polymeric systems. Prog. Polym. Sci. 2022, 132, 101580. [Google Scholar] [CrossRef]
- Hilliou, L.; Wilhelm, M.; Yamanoi, M.; Gonçalves, M.P. Structural and mechanical characterization of kappa/iota-hybrid carrageenan gels in potassium salt using Fourier Transform rheology. Food Hydrocoll. 2009, 23, 2322–2330. [Google Scholar] [CrossRef]
- Carrillo, J.-M.Y.; MacKintosh, F.C.; Dobrynin, A.V. Nonlinear elasticity: From single chain to networks and gels. Macromolecules 2013, 46, 3679–3692. [Google Scholar] [CrossRef]
- Meng, F.; Terentjev, E.M. Nonlinear elasticity of semiflexible filament networks. Soft Matter 2016, 12, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, G.; Hilliou, L.; Bernardo, G.; Sousa-Pinto, I.; Adams, R.W.; Nilsson, M.; Villanueva, R. Tailoring kappa/iota-hybrid carrageenan from Mastocarpus stellatus with desired gel quality through pre-extraction alkali treatment. Food Hydrocoll. 2013, 31, 94–102. [Google Scholar] [CrossRef]
- Bahari, A.; Moelants, K.; Wallecan, J.; Mangiante, G.; Mazoyer, J.; Hendrickx, M.; Grauwet, T. Understanding the effect of time, temperature and salts on carrageenan extraction from Chondrus crispus. Algal Res. 2021, 58, 102371. [Google Scholar] [CrossRef]
- Gonçalves, G.; Faria, B.; Moraes, I.C.F.; Hilliou, L. Role of the Molecular Mass on the Elastic Properties of Hybrid Carrageenan Hydrogels. Gels 2025, 11, 77. [Google Scholar] [CrossRef]
- Michel, A.-S.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties of carrageenan gels in presence of various cations. Int. J. Biol. Macromol. 1997, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; Antipova, A.S.; Rollema, H.S.; Burova, T.V.; Grinberg, N.V.; Pereira, L.; Gilseman, P.M.; Tromp, R.H.; Rudolph, B.; Grinberg, V.Y. The structure of κ/ι-hybrid carrageenans II. Coil–helix transition as a function of chain composition. Carbohydr. Res. 2005, 340, 1113–1129. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Gelling characteristics and rheology of kappa/iota-hybrid carrageenans extracted from Mastocarpus stellatus dried at different temperatures. J. Appl. Phycol. 2016, 28, 3635–3644. [Google Scholar] [CrossRef]
- Torres, M.D.; Chenlo, F.; Moreira, R. Rheology of κ/ι-hybrid carrageenan from Mastocarpus stellatus: Critical parameters for the gel formation. Int. J. Biol. Macromol. 2016, 86, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Chenlo, F.; Moreira, R. Thermal reversibility of kappa/iota-hybrid carrageenan gels extracted from Mastocarpus stellatus at different ionic strengths. J. Taiwan Inst. Chem. Eng. 2017, 71, 414–420. [Google Scholar] [CrossRef]
- Du, L.; Brenner, T.; Xie, J.; Matsukawa, S. A study on phase separation behavior in kappa/iota carrageenan mixtures by micro DSC, rheological measurements and simulating water and cations migration between phases. Food Hydrocoll. 2016, 55, 81–88. [Google Scholar] [CrossRef]
- Chanvrier, H.; Durand, S.; Garnier, C.; Sworn, G.; Bourriot, S.; Doublier, J.L. Gelation behaviour and rheological properties of κ/ι hybrid carrageenans. In Gums and Stabilisers for the Food Industry; Williams, P.A., Phillips, G.O., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2004; Volume 12, pp. 139–145. [Google Scholar]
- Hu, B.; Du, L.; Matsukawa, S. NMR study on the network structure of a mixed gel of kappa and iota carrageenans. Carbohydr. Polym. 2016, 150, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Geonzon, L.C.; Santoya, A.M.; Jung, H.; Yuson, H.; Bacabac, R.G.; Matsukawa, S. Study on the heterogeneity in mixture carrageenan gels viewed by long time particle tracking. Food Hydrocoll. 2022, 123, 107095. [Google Scholar] [CrossRef]
- Broedersz, C.P.; MacKintosh, F.C. Modelling semiflexible polymer networks. Rev. Mod. Phys. 2014, 86, 995–1036. [Google Scholar] [CrossRef]
- van de Velde, F.; Pereira, L.; Rollema, H.S. The Revised NMR Chemical Shift Data of Carrageenans. Carbohydr. Res. 2004, 339, 2309–2313. [Google Scholar] [CrossRef] [PubMed]

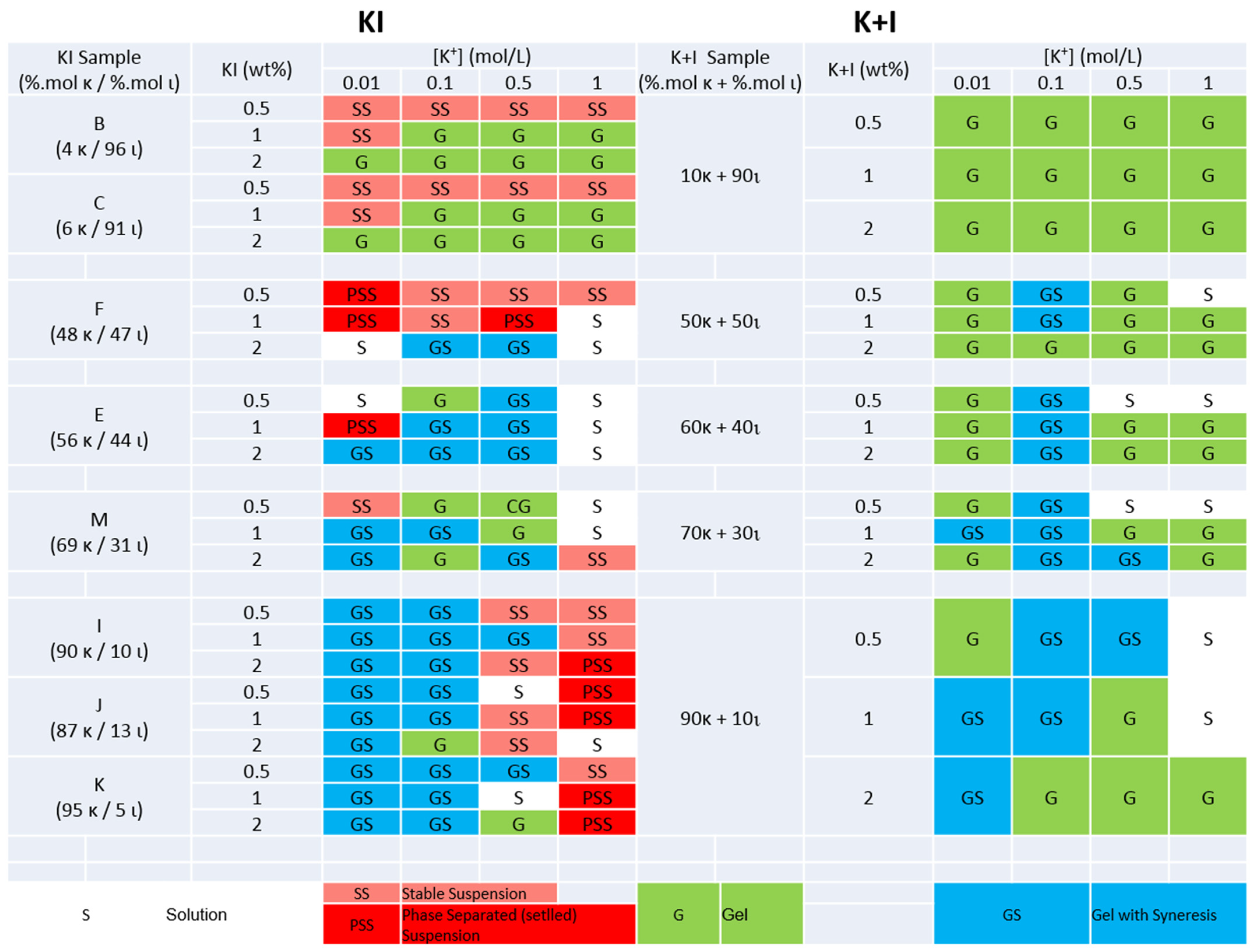



| Sample | KOH | Sample | NaOH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ν | μ | ι | κ | Mw | PDI | ν | μ | ι | κ | Mw | PDI | ||
| B | 0 | 0 | 96 ± 5 | 4 ± 2 | 5.4 ± 0.1 | 4.1 | B′ | 1 ± 1 | 4 ± 4 | 90 ± 2 | 5 ± 1 | 6.1 ± 1.5 | 4.7 |
| C | 3 ± 1 | 0 | 91 ± 1 | 6 ± 2 | 5.8 ± 0.1 | 1.6 | C′ | 0 ± 5 | 0 | 95 ± 2 | 5 ± 4 | 8.9 ± 1.2 | 6.2 |
| E | 0 | 0 | 44 ± 6 | 56 ± 4 | 3.1 ± 0.1 | 2.1 | E′ | 1 ± 5 | 0 | 42 ± 6 | 57 ± 3 | 4.4 ± 0.1 | 2.8 |
| F | 5 ± 5 | 0 | 47 ± 1 | 48 ± 1 | 2.5 ± 0.1 | 4.6 | F′ | 1 ± 5 | 0 | 45 ± 5 | 54 ± 3 | 2.9 ± 0.1 | 2.4 |
| I | 0 | 0 | 10 ± 5 | 90 ± 4 | 9.6 ± 0.3 | 3.4 | I′ | 0 | 0 | 10 ± 5 | 90 ± 5 | 7.9 ± 0.3 | 4.5 |
| J | 0 | 0 | 13 ± 3 | 87 ± 5 | 8.4 ± 0.1 | 2.5 | J′ | 0 | 0 | 12 ± 6 | 88 ± 4 | 10.6 ± 0.1 | 2.6 |
| K | 0 | 0 | 5 ± 5 | 95 ± 5 | 5.8 ± 0.1 | 2.9 | K′ | 0 | 0 | 7 ± 5 | 93 ± 4 | 9.9 ± 0.1 | 2.7 |
| M | 0 | 0 | 31 ± 2 | 69 ± 1 | 6.5 ± 0.1 | 2.8 | M′ | 0 | 4 ± 5 | 29 ± 1 | 67 ± 3 | 5.3 ± 0.1 | 2.9 |
| KAPPA | 0 | 0 | 0 | 100 ± 1 | 11.3 ± 0.5 | 2.7 | D′ | 2 ± 2 | 0 | 88 ± 1 | 10 ± 6 | 14.7 ± 0.2 | 3.9 |
| IOTA | 0 | 0 | 92 ± 1 | 8 ± 5 | 9.0 ± 0.2 | 4.7 | H′ | 0 ± 5 | 1 ± 5 | 37 ± 6 | 62 ± 1 | 3.1 ± 0.1 | 2.6 |
| Gelling Conditions | Samples | Tg (°C) | T2 (°C) | G0 (Pa) | γF (%) | LAOS |
|---|---|---|---|---|---|---|
| 2 wt.%–0.01 M KCl | C | 43.6 ± 0.2 | 31.7 ± 0.1 | 2180 ± 17 | 377 ± 30 | SOFT |
| 10K + 90I | 47.6 ± 0.3 | 34 ± 2 | 1022 ± 20 | 118 ± 13 | SOFT/HARD | |
| 1 wt.%–1 M KCl | C | >85 | 60 ± 2 | 48.7 ± 0.3 | 331 ± 14 | SOFT |
| 10K + 90I | 79.4 ± 0.8 | 46 ± 3 | 237 ± 2 | 556 ± 34 | SOFT/HARD | |
| 2 wt.%–1 M KCl | C | 85.4 ± 4.3 | 74.3 ± 0.5 | 985 ± 6 | 404 ± 30 | SOFT/HARD |
| 10K + 90I | >85 | - | 11,281 ± 238 | 388 ± 30 | SOFT | |
| 2 wt.%–0.01 M KCl | E | 66.1± 0.2 | 54 ± 1 | 7831 ± 23 | 37 ± 5 | SOFT |
| 60K + 40I | 47.5 ± 0.4 | 39.5 ± 0.5 | 15,351 ± 46 | 60 ± 7 | SOFT/HARD | |
| 1 wt.%–0.5 M KCl | E | >85 | - | 2391 ± 14 | 35 ± 4 | SOFT |
| 60K + 40I | >85 | - | 157 ± 1 | 104 ± 10 | SOFT | |
| 2 wt.%–0.5 M KCl | E | >85 | - | 14,860 ± 134 | 11 ± 1 | SOFT |
| 60K + 40I | >85 | - | 357 ± 2 | 92 ± 4 | HARD | |
| 0.5 wt.%–0.01 M KCl | K | 43.5 ± 1.5 * | - | 490 ± 4 | 123 ± 6 | HARD |
| 90K + 10I | 32 ± 1 * | 27 ± 1 | 535 ± 5 | 136 ± 7 | HARD | |
| 0.5 wt.%–0.5 M KCl | K | >85 | - | 257 ± 2 | 100 ± 9 | SOFT |
| 90K + 10I | >85 | - | 1650 ± 61 | 25 ± 2 | SOFT | |
| 2 wt.%–0.5 M KCl | K | >85 | - | 20,476 ± 71 | 18 ± 2 | SOFT |
| 90K + 10I | >85 | - | 81,921 ± 4012 | 3.7 ± 0.6 | SOFT |
| Gelling Conditions | Samples | Tg (°C) | T2 (°C) | G0 (Pa) | γF (%) | LAOS |
|---|---|---|---|---|---|---|
| 2 wt.%–0.01 M NaCl | C′ | 30.76 ± 0.5 | 29.3 ± 0.1 | 738 ± 3 | 181 ± 5 | SOFT |
| 10K + 90I | 52.0 ± 1.5 | 30 ± 1 | 1400 ± 128 | 155 ± 13 | SOFT/HARD | |
| 1 wt.%–1 M NaCl | C′ | 83.9 ± 3.4 | - | 100 ± 60 | 391 ± 47 | SOFT |
| 10K + 90I | 55.6 ± 0.3 | - | 800 ± 400 | 25 ± 3 | SOFT | |
| 2 wt.%–1 M NaCl | C′ | <25 | - | 80 ± 20 | 355 ± 30 | SOFT |
| 10K + 90I | >85 | 62 ± 3 | 410 ± 50 | 315 ± 38 | SOFT/HARD | |
| 2 wt.%–0.01 M NaCl | K′ | 39.4 ± 0.4 | 40.5 ± 0.5 | 18,226 ± 111 | 36 ± 5 | SOFT |
| 90K + 10I | 44.5 ± 0.5 * | - | 3832 ± 140 | 100 ± 5 | HARD | |
| 1 wt.%–0.5 M NaCl | K′ | 53 ± 1 * | - | 3384 ± 82 | 41 ± 2 | HARD |
| 90K + 10I | 46.0 ± 0.3 * | - | 6797 ± 45 | 23 ± 1 | SOFT | |
| 2 wt.%–0.5 M NaCl | K′ | 50 ± 1 * | - | 3118 ± 96 | 11 ± 4 | HARD |
| 90K + 10I | 54.7 ± 0.3 * | - | 25,535 ± 311 | 49 ± 7 | HARD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.A.F.; Faria, B.; Moraes, I.C.F.; Hilliou, L. Hybrid Carrageenans Versus Kappa–Iota-Carrageenan Blends: A Comparative Study of Hydrogel Elastic Properties. Gels 2025, 11, 157. https://doi.org/10.3390/gels11030157
Monteiro MAF, Faria B, Moraes ICF, Hilliou L. Hybrid Carrageenans Versus Kappa–Iota-Carrageenan Blends: A Comparative Study of Hydrogel Elastic Properties. Gels. 2025; 11(3):157. https://doi.org/10.3390/gels11030157
Chicago/Turabian StyleMonteiro, Maria Alice Freitas, Bruno Faria, Izabel Cristina Freitas Moraes, and Loic Hilliou. 2025. "Hybrid Carrageenans Versus Kappa–Iota-Carrageenan Blends: A Comparative Study of Hydrogel Elastic Properties" Gels 11, no. 3: 157. https://doi.org/10.3390/gels11030157
APA StyleMonteiro, M. A. F., Faria, B., Moraes, I. C. F., & Hilliou, L. (2025). Hybrid Carrageenans Versus Kappa–Iota-Carrageenan Blends: A Comparative Study of Hydrogel Elastic Properties. Gels, 11(3), 157. https://doi.org/10.3390/gels11030157






