Removal of Divalent Cations from Produced Water and Its Impact on Rheological Properties and Proppant Settling Velocity
Abstract
:1. Introduction
2. Experimental Results and Discussions
2.1. Optimization of FPW Treatment
2.2. Rheological Properties Evaluation
2.2.1. Shear Viscosity of FR Solutions
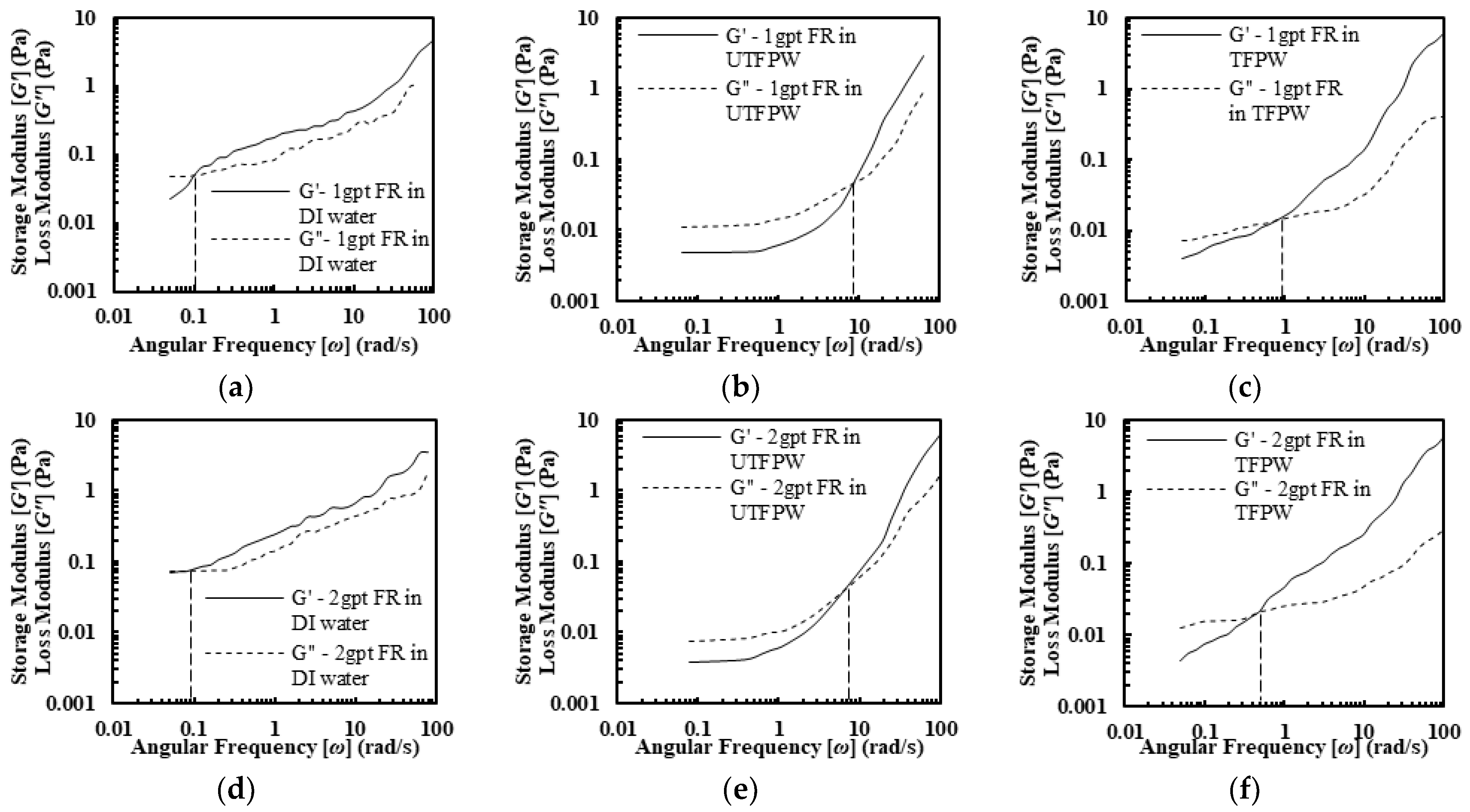
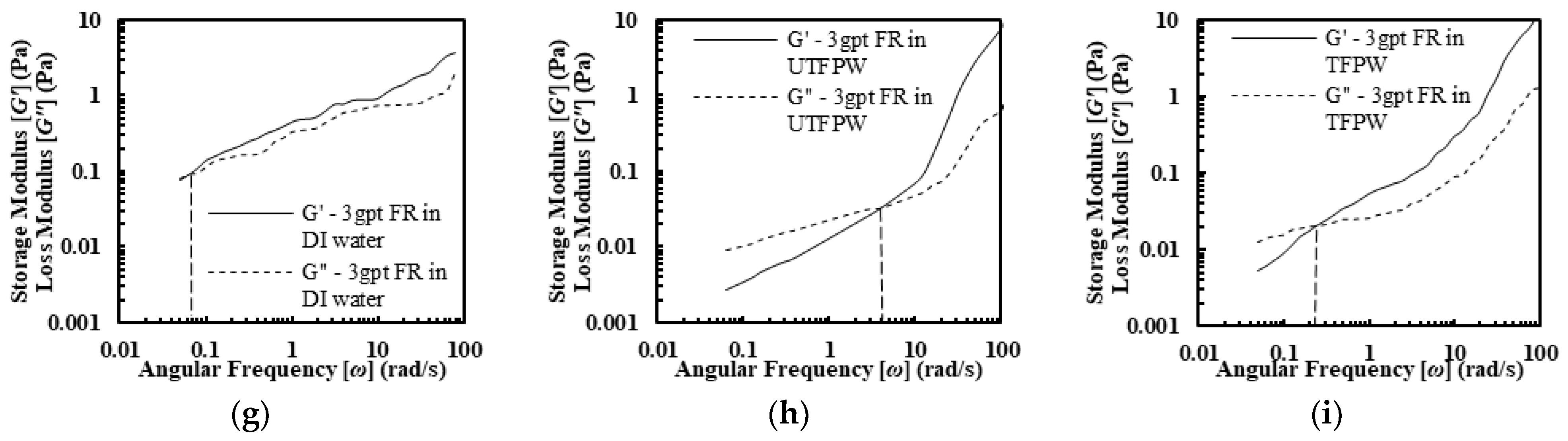
2.2.2. Viscoelasticity of FR Solutions
2.3. Settling Velocity Evaluation
2.3.1. Role of Shear Viscosity Post-Divalent Cation Removal
2.3.2. Role of Viscoelasticity Post-Divalent Cation Removal
3. Conclusions
- The FR solutions in DI water, untreated FPW (UTFPW), and treated FPW (TFPW) all displayed shear-thinning behavior. In DI water, FR solutions with different concentrations exhibited near-ideal shear-thinning with viscosity decreasing exponentially with increasing shear rates. In contrast, the power-law model was not suitable for UTFPW due to the presence of a second constant viscosity (,), and the Sisko model provided a better fit. In UTFPW and TFPW, shear viscosity sharply declined despite increased FR concentration, which was accompanied by low flow behavior index (n) values and flow consistency index (K). Meanwhile, removing divalent cations from FPW resulted in increased K and n values. However, this treatment failed to maintain viscosity at high shear rates compared to solutions with DI water. FR solutions prepared with DI water exhibit significantly higher viscoelastic properties compared to those made with UTFPW and TFPW. Additionally, relaxation times (λ) increase with higher concentrations, further outperforming those made with UTFPW and TFPW.
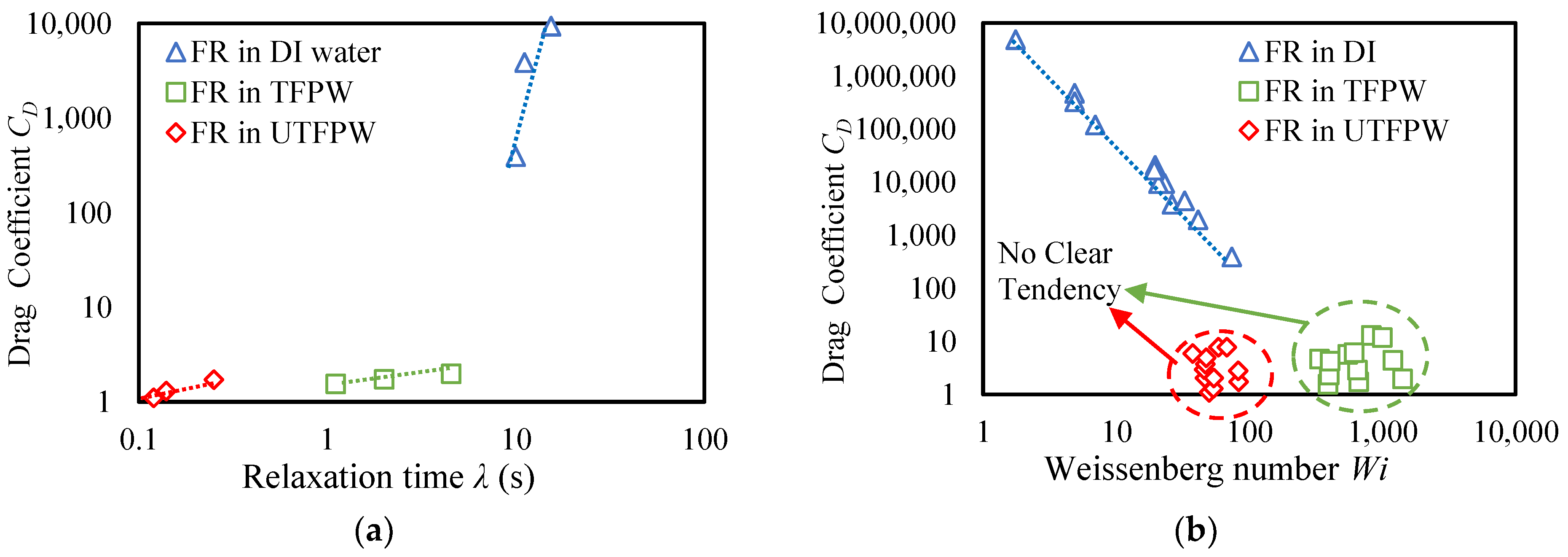
- The removal of divalent cations from FPW increases the relaxation time (λ) from 0.12 s to 1 s at a 1 gpt concentration of FR. Further increases in FR concentration raise λ to 4.5 s. However, the high NaCl content constrains these improvements, keeping them below the levels achieved with DI water.
- Although the removal of divalent cations increases λ of the fluids, this increase in λ does not significantly increase the settling velocity of the proppants compared to the results observed with FR in UTFPW. The drag coefficient shows a dependency on shear viscosity, remaining high when viscosity is elevated and diminishing as viscosity decreases. Furthermore, despite the improved viscoelastic properties resulting from the removal of divalent cations, these changes do not contribute to an increase in proppant carrying capacity due to the fluid’s persistently low shear resistance. This persistently low viscosity can be attributed primarily to the high NaCl concentration, which may inhibit the effective increase in viscosity even after the removal of divalent cations.
Limitation and Future Work
- 4.
- A key limitation of this study is its reliance on controlled laboratory conditions, which may not fully represent real-world applications. The removal efficiency of divalent cations and rheological measurements were conducted under standardized laboratory settings, without accounting for the variable temperature, pressure, and fluid dynamics encountered in field operations. The controlled range of FR concentrations (1–3 gpt) and the use of a confined cylinder setup for settling velocity experiments may not adequately capture the complex proppant transport behavior within actual fracture geometries, particularly in horizontal wellbores.
- 5.
- Future research should concentrate on investigating the combined effects of monovalent and divalent cations on the performance of FRs. Understanding how these cations interact can help optimize salinity levels for more efficient hydraulic fracturing operations.
- 6.
- Additionally, it is crucial to evaluate how temperature-dependent changes in viscoelastic properties influence FR behavior. This aspect of research is vital for tailoring fluid formulations to better withstand the thermal variations encountered during hydraulic fracturing.
4. Materials and Methodology
4.1. Material
4.1.1. Flowback and Produced Water (FPW)
4.1.2. Chemical Reagents
4.1.3. Friction Reducer Additive
4.1.4. Proppants
4.2. Methodology
4.2.1. Chemical Treatment of FPW
4.2.2. Water Chemistry Analysis
4.2.3. FR Solution Preparation
4.2.4. Shear Viscosity Measurements
4.2.5. Viscoelasticity Measurements
4.2.6. Settling Velocity Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Zhao, J.; Mao, J.; Tan, H.; Zhang, Y.; Song, Z. Review of Friction Reducers Used in Slickwater Fracturing Fluids for Shale Gas Reservoirs. J. Nat. Gas Sci. Eng. 2019, 62, 302–313. [Google Scholar] [CrossRef]
- Bunger, A.P.; McLennan, J.; Jeffrey, R.; Bunger, A.P.; McLennan, J.; Jeffrey, R. Effective and Sustainable Hydraulic Fracturing; IntechOpen: London, UK, 2013; ISBN 978-953-51-1137-5. [Google Scholar]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S. A Comprehensive Review of Beneficial Applications of Viscoelastic Surfactants in Wellbore Hydraulic Fracturing Fluids. Fuel 2023, 338, 127228. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Wang, G.; Wang, E.; Ju, S.; Qin, C. Experimental Study of Effect of Slickwater Fracturing on Coal Pore Structure and Methane Adsorption. Energy 2022, 239, 122421. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Kalantariasl, A.; You, Z.; Iglauer, S.; Keshavarz, A. Micro-Proppant Placement in Hydraulic and Natural Fracture Stimulation in Unconventional Reservoirs: A Review. Energy Rep. 2021, 7, 8997–9022. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.-T. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, X.; Yan, R.; Liu, S.; Song, X.; Dai, C. Synthesis and Performance Evaluation of Water-in-Water Polymer Drag-Reducing Agent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131935. [Google Scholar] [CrossRef]
- Yang, D.; Yang, B.; Ren, M.; Liu, Y.; Cao, H.; Jiang, Z.; Zhang, H. Construction of Fracturing Fluid with Excellent Proppant Transport Capacity Using Low Molecular Weight Hydrophobic Association Polymer and Surfactant. J. Mol. Liq. 2023, 377, 121546. [Google Scholar] [CrossRef]
- Ellafi, A.; Jabbari, H.; Tomomewo, O.S.; Mann, M.D.; Geri, M.B.; Tang, C. Future of Hydraulic Fracturing Application in Terms of Water Management and Environmental Issues: A Critical Review. In Proceedings of the SPE Canada Unconventional Resources Conference, Calgary, AB, Canada, 28 September–2 October 2020; OnePetro: Richardson, TX, USA, 2020. [Google Scholar] [CrossRef]
- Ellafi, A.; Jabbari, H.; Ba Geri, M.; Alkamil, E. Can HVFRs Increase the Oil Recovery in Hydraulic Fractures Applications? In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019; OnePetro: Richardson, TX, USA, 2019. [Google Scholar] [CrossRef]
- Arthur, J.D.; Bohm, B.; Cornue, D. Environmental Considerations of Modern Shale Gas Development. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; OnePetro: Richardson, TX, USA, 2009. [Google Scholar] [CrossRef]
- Aften, C.W.; Watson, W.P. Improved Friction Reducer for Hydraulic Fracturing. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 19–21 January 2009; OnePetro: Richardson, TX, USA, 2009. [Google Scholar] [CrossRef]
- Yap, N.T. Unconventional Shale Gas Development: Challenges for Environmental Policy and EA Practice. Impact Assess. Proj. Apprais. 2016, 34, 97–109. [Google Scholar] [CrossRef]
- Shih, J.-S.; Saiers, J.E.; Anisfeld, S.C.; Chu, Z.; Muehlenbachs, L.A.; Olmstead, S.M. Characterization and Analysis of Liquid Waste from Marcellus Shale Gas Development. Environ. Sci. Technol. 2015, 49, 9557–9565. [Google Scholar] [CrossRef]
- Zhang, T.; Hammack, R.W.; Vidic, R.D. Fate of Radium in Marcellus Shale Flowback Water Impoundments and Assessment of Associated Health Risks. Environ. Sci. Technol. 2015, 49, 9347–9354. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Chilkoor, G.; Wilder, J.; Gadhamshetty, V.; Stone, J.J. Potential Water Resource Impacts of Hydraulic Fracturing from Unconventional Oil Production in the Bakken Shale. Water Res. 2017, 108, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, C.; Yang, X.; Zhang, Z. Investigation on Problems of Wastewater from Hydraulic Fracturing and Their Solutions. Water Air Soil Pollut. 2018, 229, 246. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and Implemented Membrane-Based Technologies for the Treatment and Reuse of Flowback and Produced Water from Shale Gas and Oil Plays: A Review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Modern Shale Gas Development in the United States: A Primer; U.S. Department of Energy, Office of Fossil Energy: Washington, DC, USA, 2009.
- Veil, J.A.; Dusseault, M.B. Evaluation of Slurry Injection Technology for Management of Drilling Wastes; Argonne National Lab: Lemont, IL, USA, 2003. [CrossRef]
- Nadella, M.; Sharma, R.; Chellam, S. Fit-for-Purpose Treatment of Produced Water with Iron and Polymeric Coagulant for Reuse in Hydraulic Fracturing: Temperature Effects on Aggregation and High-Rate Sedimentation. Water Res. 2020, 170, 115330. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.; Friesen, D.; Sanders, A. Enhancing Friction Reducer Performance in High Salt Conditions. In Proceedings of the 6th Unconventional Resources Technology Conference, Houston, TX, USA, 23–25 July 2018; OnePetro: Richardson, TX, USA, 2018. [Google Scholar] [CrossRef]
- Mimouni, A.; Kuzmyak, N.; van Oort, E.; Sharma, M.; Katz, L. Compatibility of Hydraulic Fracturing Additives with High Salt Concentrations for Flowback Water Reuse. World Environ. Water Resour. Congr. 2015, 2015, 496–509. [Google Scholar] [CrossRef]
- Papso, J.; Blauch, M.; Grottenthaler, D. Gas Well Treated with 100% Reused Frac Fluid. Hart’s E P 2010, 83, 59–61. [Google Scholar]
- Yegane, M.M.; Hashemi, F.; Vercauteren, F.; Meulendijks, N.; Gharbi, R.; Boukany, P.; Zitha, P. Rheological Response of a Modified Polyacrylamide–Silica Nanoparticles Hybrid at High Salinity and Temperature. Soft Matter 2020, 16, 10198–10210. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Jiang, F.; Wei, B.; Tang, Y.; He, Y. Influences of Structure and Multi-Intermolecular Forces on Rheological and Oil Displacement Properties of Polymer Solutions in the Presence of Ca2+/Mg2+. RSC Adv. 2017, 7, 4430–4436. [Google Scholar] [CrossRef]
- Saito, S. Salt Effect on Polymer Solutions. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 1789–1802. [Google Scholar] [CrossRef]
- Ma, Q.; Shuler, P.J.; Aften, C.W.; Tang, Y. Theoretical Studies of Hydrolysis and Stability of Polyacrylamide Polymers. Polym. Degrad. Stab. 2015, 121, 69–77. [Google Scholar] [CrossRef]
- Shupe, R.D. Chemical Stability of Polyacrylamide Polymers. J. Pet. Technol. 1981, 33, 1513–1529. [Google Scholar] [CrossRef]
- Zaitoun, A.; Potie, B. Limiting Conditions for the Use of Hydrolyzed Polyacrylamides in Brines Containing Divalent Ions. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Denver, CO, USA, 1–3 June 1983; OnePetro: Richardson, TX, USA, 1983. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, L.; Ye, Z.; Yuan, N.; Li, X. Effect of Micro-Aggregation Behavior on the Salt Tolerance of Polymer Solutions. J. Appl. Polym. Sci. 2021, 138, 50277. [Google Scholar] [CrossRef]
- Paktinat, J.; O’Neil, B.; Aften, C.; Hurd, M. Critical Evaluation of High Brine Tolerant Additives Used in Shale Slick Water Fracs. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 27–29 March 2011; OnePetro: Richardson, TX, USA, 2011. [Google Scholar] [CrossRef]
- Muller, G.; Laine, J.P.; Fenyo, J.C. High-Molecular-Weight Hydrolyzed Polyacrylamides. I. Characterization. Effect of Salts on the Conformational Properties. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 659–672. [Google Scholar] [CrossRef]
- Malhotra, S.; Sharma, M.M. Settling of Spherical Particles in Unbounded and Confined Surfactant-Based Shear Thinning Viscoelastic Fluids: An Experimental Study. Chem. Eng. Sci. 2012, 84, 646–655. [Google Scholar] [CrossRef]
- Ba Geri, M.; Imqam, A.; Bogdan, A.; Shen, L. Investigate The Rheological Behavior of High Viscosity Friction Reducer Fracture Fluid and Its Impact on Proppant Static Settling Velocity. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium, Oklahoma City, OK, USA, 9–10 April 2019; OnePetro: Richardson, TX, USA, 2019. [Google Scholar] [CrossRef]
- Chhabra, R.P.; Uhlherr, P.H.T.; Boger, D.V. The Influence of Fluid Elasticity on the Drag Coefficient for Creeping Flow around a Sphere. J. Non Newton. Fluid Mech. 1980, 6, 187–199. [Google Scholar] [CrossRef]
- Chmielewski, C.; Nichols, K.L.; Jayaraman, K. A Comparison of the Drag Coefficients of Spheres Translating in Corn-Syrup-Based and Polybutene-Based Boger Fluids. J. Non Newton. Fluid Mech. 1990, 35, 37–49. [Google Scholar] [CrossRef]
- Peden, J.M.; Luo, Y. Settling Velocity of Variously Shaped Particles in Drilling and Fracturing Fluids. SPE Drill. Eng. 1987, 2, 337–343. [Google Scholar] [CrossRef]
- Galindo, T. Does Higher Viscosity Improve Proppant Transport? In Proceedings of the SPE Oklahoma City Oil and Gas Symposium, Oklahoma City, OK, USA, 9–10 April 2019; OnePetro: Richardson, TX, USA, 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Wang, B.; Jin, J.; Wang, B.; Cui, D. Selective Removal of Calcium Ions from Seawater or Desalination Brine Using a Modified Sodium Carbonate Method. DWT 2020, 174, 123–135. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Precipitation Behavior of Ca(OH)2, Mg(OH)2, and Mn(OH)2 from CaCl2, MgCl2, and MnCl2 in NaOH-H2O Solutions and Study of Lithium Recovery from Seawater via Two-Stage Precipitation Process. Hydrometallurgy 2014, 146, 142–148. [Google Scholar] [CrossRef]
- Anderson, G. Thermodynamics of Natural Systems: Theory and Applications in Geochemistry and Environmental Science, 3rd ed.; Cambridge University Press: Cambridge, UK, 2017; ISBN 978-1-107-17521-1. [Google Scholar]
- Liu, W.; Sun, L.; Tao, S. Removal of Ca2+, Mg2+, Ba2+ and Sr2+ from Shale Gas Flowback Water from the Sichuan Basin in China by Chemical Softening Under the Guidance of OLI Stream Analyzer: Precipitation Behaviors and Optimization Study. Water Sci. Technol. 2020, 82, 194–206. [Google Scholar] [CrossRef]
- Hazra, S.; Madrid, V.; Luzan, T.; Van Domelen, M.; Copeland, C. Correlating the Performance of Friction Reducers with Source Water Chemistry. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium, Oklahoma City, OK, USA, 9–10 April 2019; OnePetro: Richardson, TX, USA, 2019. [Google Scholar] [CrossRef]
- McIntyre, M.; Kelly, C.; Kudrashou, V.; Kazemi, M. Review of Friction Reducers Performance Applied to the Marcellus Shale Region. In Proceedings of the SPE Eastern Regional Meeting, Farmington, PA, USA, 2–3 November 2021; OnePetro: Richardson, TX, USA, 2021. [Google Scholar] [CrossRef]
- Arnipally, S.K.; Kuru, E. Settling Velocity of Particles in Viscoelastic Fluids: A Comparison of the Shear-Viscosity and Elasticity Effects. SPE J. 2018, 23, 1689–1705. [Google Scholar] [CrossRef]
- Hu, Y.T.; Chung, H.; Maxey, J. What Is More Important for Proppant Transport, Viscosity or Elasticity? In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 3–5 February 2015; OnePetro: Richardson, TX, USA, 2015. [Google Scholar]
- Zhang, G.; Li, M.; Geng, K.; Han, R.; Xie, M.; Liao, K. New Integrated Model of the Settling Velocity of Proppants Falling in Viscoelastic Slick-Water Fracturingb Fluids. J. Nat. Gas Sci. Eng. 2016, 33, 518–526. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, H.; Guo, W.; Qu, L.; Li, C. Effect of Inorganic Cations on the Rheological Properties of Polyacrylamide/Xanthan Gum Solution. J. Nat. Gas Sci. Eng. 2016, 31, 283–292. [Google Scholar] [CrossRef]
- Yuan, R.; Li, Y.; Li, C.; Fang, H.; Wang, W. Study about How the Metal Cationic Ions Affect the Properties of Partially Hydrolyzed Hydrophobically Modified Polyacrylamide (HMHPAM) in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 16–24. [Google Scholar] [CrossRef]
- Kingston, A.; Jiang, C.; Wang, X.; Hobbs, T. Geological Survey OF Canada Open File 8974 Chemical Compositions of Flowback and Produced Water from the Duvernay Shale and Montney Tight Reservoir Developments in Western Canada: Potential for Lithium Resources from Wastewater; Geological Survey of Canada: Ottawa, Ont, USA, 2023.
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.F.; Li, L. A Comprehensive Review on Proppant Technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Krumbein, W.C.; Sloss, L.L. Stratigraphy and Sedimentation. Soil Sci. 1951, 71, 401. [Google Scholar] [CrossRef]
- Motiee, M.; Johnson, M.; Ward, B.; Gradl, C.; McKimmy, M.; Meeheib, J. High Concentration Polyacrylamide-Based Friction Reducer Used as a Direct Substitute for Guar-Based Borate Crosslinked Fluid in Fracturing Operations. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 9–11 February 2016; OnePetro: Richardson, TX, USA, 2016. [Google Scholar] [CrossRef]
- Paktinat, J.; O’Neil, B.; Tulissi, M. Case Studies: Improved Performance of High Brine Friction Reducers in Fracturing Shale Reserviors. In Proceedings of the SPE Eastern Regional Meeting, Columbus, OH, USA, 17–19 August 2011; OnePetro: Richardson, TX, USA, 2011. [Google Scholar] [CrossRef]
- Wang, J.; Elsworth, D. Fracture Penetration and Proppant Transport in Gas- and Foam-Fracturing. J. Nat. Gas Sci. Eng. 2020, 77, 103269. [Google Scholar] [CrossRef]
- Bello, A.; Ozoani, J.; Kuriashov, D. Proppant Transport in Hydraulic Fractures by Creating a Capillary Suspension. J. Pet. Sci. Eng. 2022, 208, 109508. [Google Scholar] [CrossRef]
- Machač, I.; Šiška, B. Calculation of Terminal Falling Velocity of Spherical Particles Moving Through Polymer Solutions Using a Power-Law Viscosity Model; University of Pardubice: Pardubice, Czechia, 2019; ISBN 978-80-7560-243-5. [Google Scholar]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, Wales, 2000; ISBN 978-0-9538032-0-0. [Google Scholar]
- Wang, J.; Zhou, F.; Bai, H.; Li, Y.; Yang, H. A Comprehensive Method to Evaluate the Viscous Slickwater as Fracturing Fluids for Hydraulic Fracturing Applications. J. Pet. Sci. Eng. 2020, 193, 107359. [Google Scholar] [CrossRef]
- Biheri, G.; Imqam, A. Settling of Spherical Particles in High Viscosity Friction Reducer Fracture Fluids. Energies 2021, 14, 2462. [Google Scholar] [CrossRef]
- Bai, H.; Zhou, F.; Zhang, M.; Gao, X.; Xu, H.; Yao, E.; Wang, J.; Li, Y. Optimization and Friction Reduction Study of a New Type of Viscoelastic Slickwater System. J. Mol. Liq. 2021, 344, 117876. [Google Scholar] [CrossRef]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Zhao, H.; Danican, S.; Torres, H.; Christanti, Y.; Nikolaev, M.; Makarychev-Mikhailov, S.; Bonnell, A. Viscous Slickwater as Enabler for Improved Hydraulic Fracturing Design in Unconventional Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018; OnePetro: Richardson, TX, USA, 2018. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Shen, Y.; Lai, X.; Guo, X. Study on Properties of Hydrophobic Associating Polymer as Drag Reduction Agent for Fracturing Fluid. J. Polym. Res. 2016, 23, 235. [Google Scholar] [CrossRef]
- Abubakar, A.; Al-Wahaibi, T.; Al-Wahaibi, Y.; Al-Hashmi, A.R.; Al-Ajmi, A. Roles of Drag Reducing Polymers in Single- and Multi-Phase Flows. Chem. Eng. Res. Des. 2014, 92, 2153–2181. [Google Scholar] [CrossRef]
- Brown, P.P.; Lawler, D.F. Sphere Drag and Settling Velocity Revisited. J. Environ. Eng. 2003, 129, 222–231. [Google Scholar] [CrossRef]
- Morrison, F.A. Data Correlation for Drag Coefficient for Sphere; Michigan Technological University: Houghton, MI, USA, 2016. [Google Scholar]
- Song, X.; Xu, Z.; Li, G.; Pang, Z.; Zhu, Z. A New Model for Predicting Drag Coefficient and Settling Velocity of Spherical and Non-Spherical Particle in Newtonian Fluid. Powder Technol. 2017, 321, 242–250. [Google Scholar] [CrossRef]
- Breakey, D.E.S.; Vaezi Ghobaeiyeh, F.; Masliyah, J.H.; Sanders, R.S. Side-View-Only Determination of Drag Coefficient and Settling Velocity for Non-Spherical Particles. Powder Technol. 2018, 339, 182–191. [Google Scholar] [CrossRef]
- Shah, S.N.; El Fadili, Y.; Chhabra, R.P. New Model for Single Spherical Particle Settling Velocity in Power Law (Visco-Inelastic) Fluids. Int. J. Multiph. Flow 2007, 33, 51–66. [Google Scholar] [CrossRef]
- Shah, S.N. Proppant Settling Correlations for Non-Newtonian Fluids Under Static and Dynamic Conditions. Soc. Pet. Eng. J. 1982, 22, 164–170. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A. A Critical Review of Hydraulic-Fracturing Fluids for Moderate- to Ultralow-Permeability Formations Over the Last Decade. SPE Prod. Oper. 2014, 29, 243–260. [Google Scholar] [CrossRef]
- Daneshy, A.A. Numerical Solution of Sand Transport in Hydraulic Fracturing. J. Pet. Technol. 1978, 30, 132–140. [Google Scholar] [CrossRef]
- Kaur, A.; Sobti, A.; Toor, A.P.; Wanchoo, R.K. Motion of Spheres and Cylinders in Viscoelastic Fluids: Asymptotic Behavior. Powder Technol. 2019, 345, 82–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Ali, W.; Jiang, C.; Dehghanpour, H. Optimal Treatment and Reuse of Flowback and Produced Water: Selective Removal of Problematic Cations for Stability of Friction Reducers. In Proceedings of the 11th Unconventional Resources Technology Conference, Denver, CO, USA, 13–15 June 2023; pp. 482–505. [Google Scholar] [CrossRef]




| Treatment Conditions | Post-Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Molar Ratio of Na2CO3 | CNaOH (mol/L) | CNa (ppm) | CCa (ppm) | ECa (%) | CMg (ppm) | EMg (%) | CFe (ppm) | EFe (%) | CSr (ppm) | ESr (%) | pH ± 0.10 |
| 0.8 | 0 | 80985 | 1836.7 | 85.97 | 623.1 | 27.71 | 0.35 | 99.1 | 752.1 | 57.32 | 7.24 |
| 1 | 0 | 84532 | 20.9 | 99.81 | 351.6 | 59.25 | 0 | 100 | 15.26 | 98.83 | 8.89 |
| 1.2 | 0 | 87884 | 0.6 | 100 | 169.2 | 80.38 | 0 | 100 | 0 | 100 | 10.02 |
| 1 | 0.0167 | 84358 | 3.8 | 99.97 | 31.6 | 96.33 | 0 | 100 | 0 | 100 | 9.57 |
| 1 | 0.0467 | 85414 | 3.0 | 99.97 | 1.05 | 99.88 | 0 | 100 | 0 | 100 | 10.01 |
| 1 | 0.0667 | 86011 | 1.2 | 99.99 | 0.5 | 99.94 | 0 | 100 | 0 | 100 | 10.81 |
| Samples | R2 | |||
|---|---|---|---|---|
| 1 gpt FR in DI water | 0.2239 | 0.4403 | N/A | 0.998 |
| 2 gpt FR in DI water | 0.3964 | 0.4279 | N/A | 0.998 |
| 3 gpt FR in DI water | 0.6031 | 0.4371 | N/A | 0.999 |
| 1 gpt FR in UTFPW | 0.0223 | 0.0001 | 2.5 | 0.999 |
| 2 gpt FR in UTFPW | 0.0248 | 0.0008 | 2.9 | 0.999 |
| 3 gpt FR in UTFPW | 0.0402 | 0.0155 | 3.2 | 0.999 |
| 1 gpt FR in TFPW | 0.0272 | 0.2533 | 3.1 | 0.999 |
| 2 gpt FR in TFPW | 0.0472 | 0.1152 | 3.7 | 0.999 |
| 3 gpt FR in TFPW | 0.0664 | 0.0753 | 4.1 | 0.999 |
| Samples | FR conc. (gpt) | (s) | Samples | FR conc. (gpt) | (s) | Samples | FR conc. (gpt) | (s) |
|---|---|---|---|---|---|---|---|---|
| DI water | 1 | 10 | UTFPW | 1 | 0.12 | TFPW | 1 | 1.11 |
| DI water | 2 | 11.1 | UTFPW | 2 | 0.14 | TFPW | 2 | 2 |
| DI water | 3 | 15.4 | UTFPW | 3 | 0.25 | TFPW | 3 | 4.55 |
| Settling Velocity (cm/s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Proppants | Size (16–30) | STD | Size (20–40) | STD | Size (30–50) | STD | Size (40–70) | STD | |
| Samples | |||||||||
| DI 1gpt | 0.680 | 0.207 | 0.244 | 0.020 | 0.145 | 0.003 | 0.065 | 0.031 | |
| DI 2gpt | 0.216 | 0.048 | 0.111 | 0.037 | 0.028 | 0.009 | 0.015 | 0.005 | |
| DI 3gpt | 0.139 | 0.045 | 0.075 | 0.024 | 0.014 | 0.002 | 0.004 | 0.000 | |
| UTFPW 1gpt | 12.701 | 1.818 | 7.823 | 0.603 | 5.648 | 0.322 | 4.354 | 0.348 | |
| UTFPW 2gpt | 11.728 | 1.065 | 7.601 | 0.631 | 3.982 | 0.397 | 3.790 | 0.355 | |
| UTFPW 3gpt | 10.177 | 0.832 | 6.504 | 0.502 | 3.470 | 0.490 | 3.022 | 0.139 | |
| TFPW 1gpt | 10.747 | 1.501 | 7.106 | 0.392 | 4.479 | 0.216 | 4.073 | 0.391 | |
| TFPW 2gpt | 10.122 | 1.471 | 6.408 | 0.452 | 4.060 | 0.301 | 3.409 | 0.315 | |
| TFPW 3gpt | 9.497 | 1.502 | 5.193 | 0.499 | 2.685 | 0.341 | 2.453 | 0.153 | |
| Cations | Anions | ||
|---|---|---|---|
| Ion | mg/L | Ion | mg/L |
| Na+ | 67,162 | Cl− | 145,700 |
| K+ | 1949.9 | Br− | 1570 |
| Ca2+ | 13,088 | I− | 120 |
| Mg2+ | 861.9 | HCO3− | 40.7 |
| Ba2+ | 17 | SO42− | 276 |
| Sr2+ | 1312 | CO32− | Nil |
| Fe2+ | 38.6 | OH− | Nil |
| Proppants | 16/30 | 20/40 | 30/50 | 40/70 |
|---|---|---|---|---|
| Mean Particle Diameter (mm) | 0.92 | 0.60 | 0.45 | 0.34 |
| Bulk Density (g/cm3) | 1.50 | 1.51 | 1.51 | 1.51 |
| Specific Density (g/cm3) | 2.52 | 2.56 | 2.65 | 2.61 |
| Sphericity (Krumbein) | 0.7 | 0.7 | 0.7 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ali, W.; Dehghanpour, H. Removal of Divalent Cations from Produced Water and Its Impact on Rheological Properties and Proppant Settling Velocity. Gels 2025, 11, 158. https://doi.org/10.3390/gels11030158
Zhang Y, Ali W, Dehghanpour H. Removal of Divalent Cations from Produced Water and Its Impact on Rheological Properties and Proppant Settling Velocity. Gels. 2025; 11(3):158. https://doi.org/10.3390/gels11030158
Chicago/Turabian StyleZhang, Yanze, Wajid Ali, and Hassan Dehghanpour. 2025. "Removal of Divalent Cations from Produced Water and Its Impact on Rheological Properties and Proppant Settling Velocity" Gels 11, no. 3: 158. https://doi.org/10.3390/gels11030158
APA StyleZhang, Y., Ali, W., & Dehghanpour, H. (2025). Removal of Divalent Cations from Produced Water and Its Impact on Rheological Properties and Proppant Settling Velocity. Gels, 11(3), 158. https://doi.org/10.3390/gels11030158










