Hydration Mechanism of Solid Waste Gelling Materials Containing Semi-Dry Desulfurization Ash
Abstract
1. Introduction
2. Results and Discussion
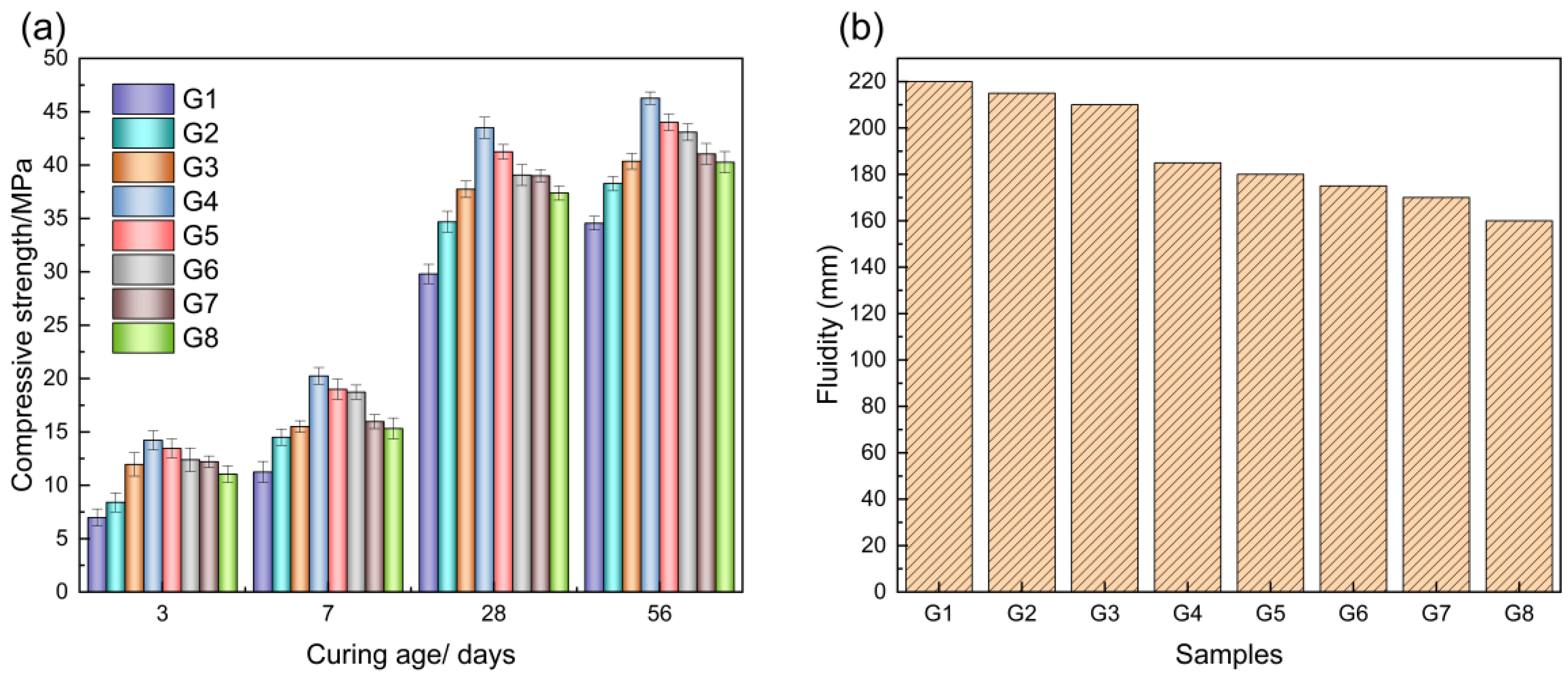
2.1. Results of Gelling Materials with Different DA Dosages
2.2. Mechanical Grinding to Enhance the Activity of Gelling Materials
2.2.1. Specific Surface Area of DA at Different Grinding Times (Individual Grinding of DA)
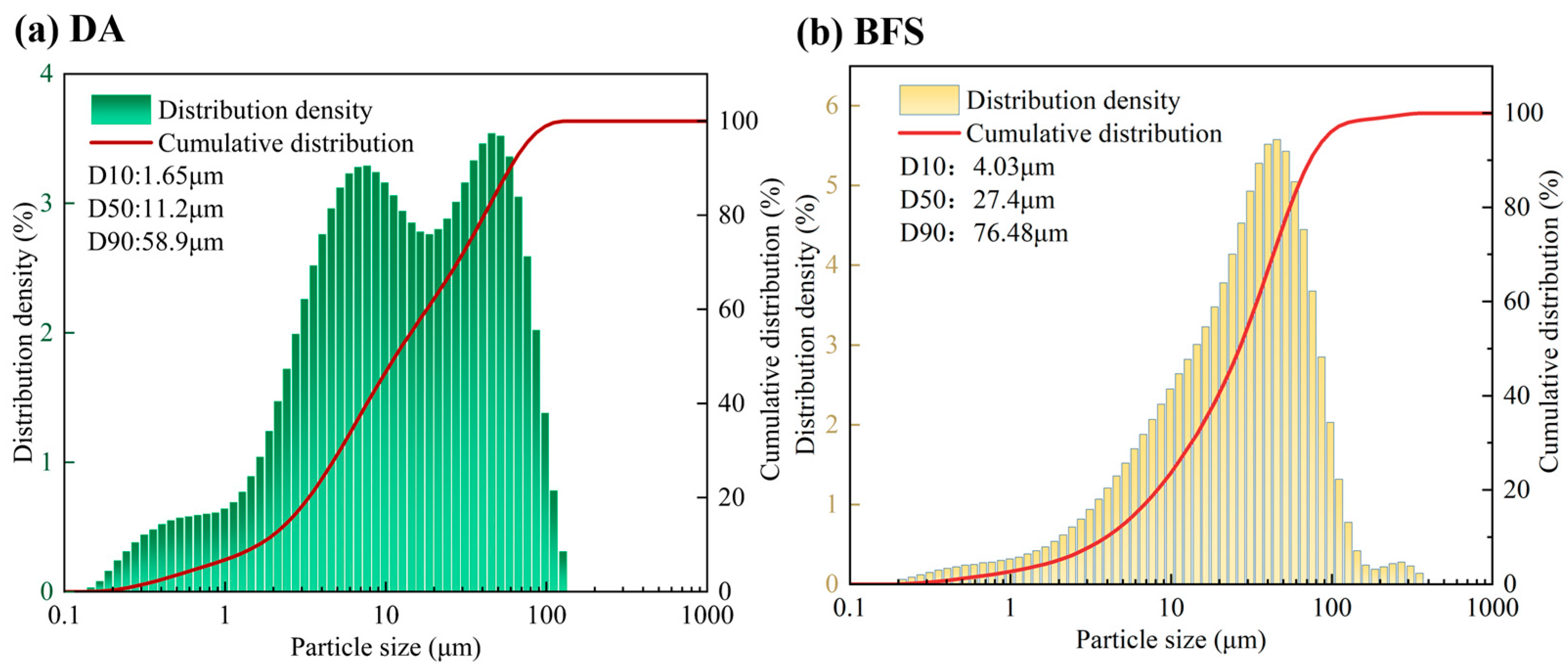
2.2.2. Particle Size Distribution of BFS-DA with Different Grinding Times (Mixed Grinding)
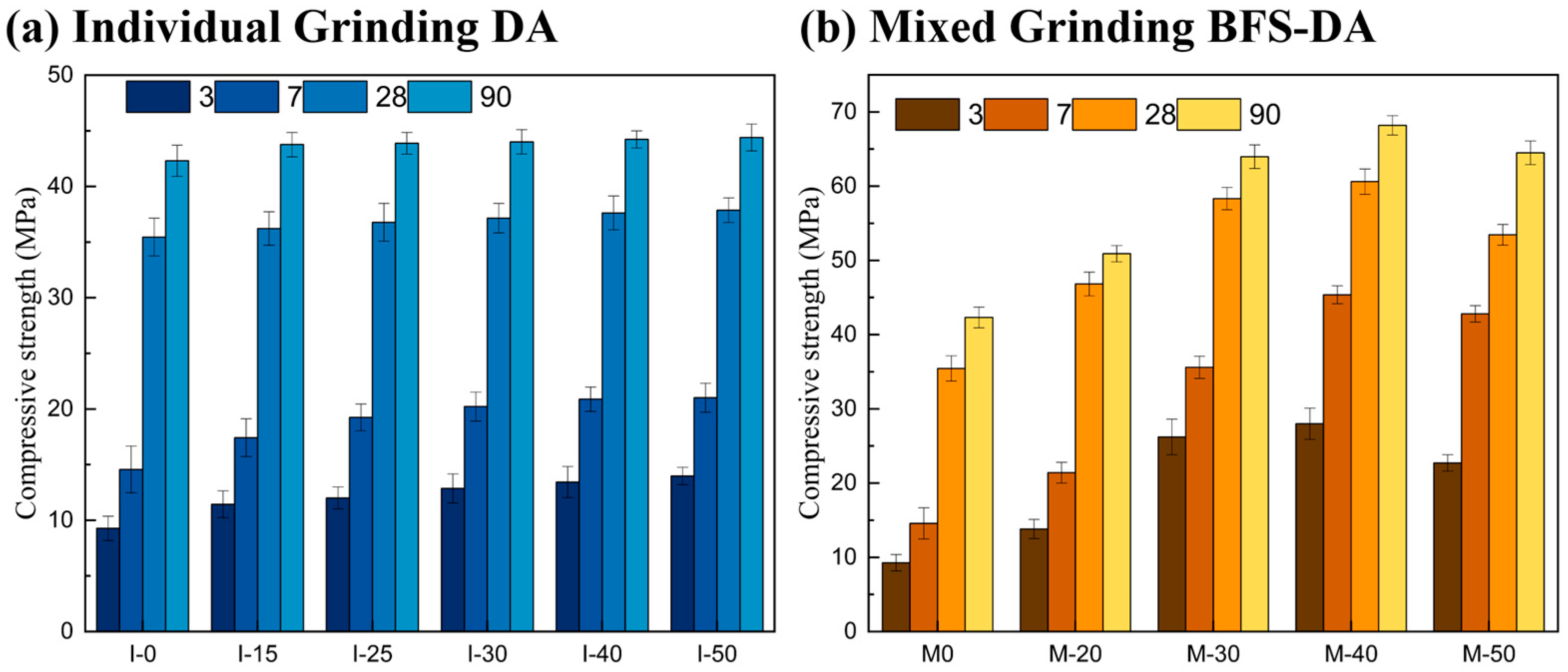
2.2.3. Compressive Strength
2.2.4. Setting Times and Stability
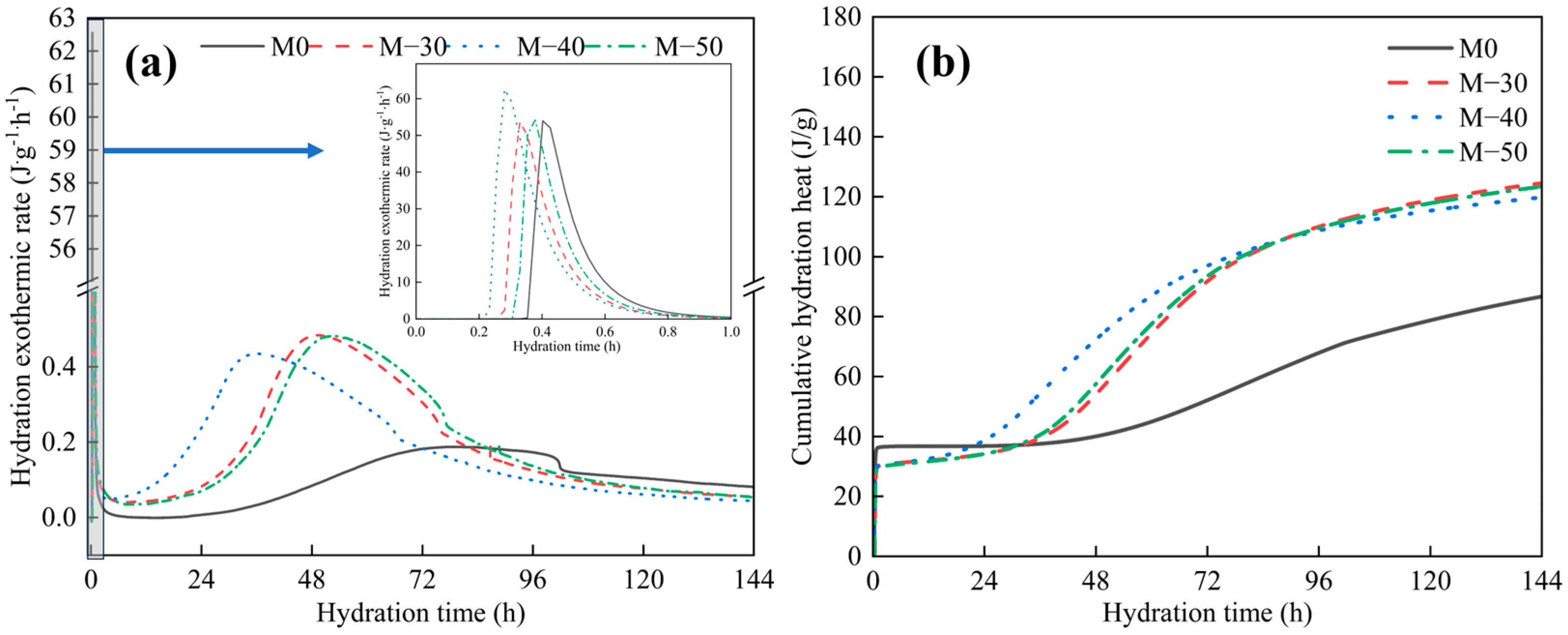
2.2.5. Hydration Heat with Different Grinding Times
2.3. Hydration Process
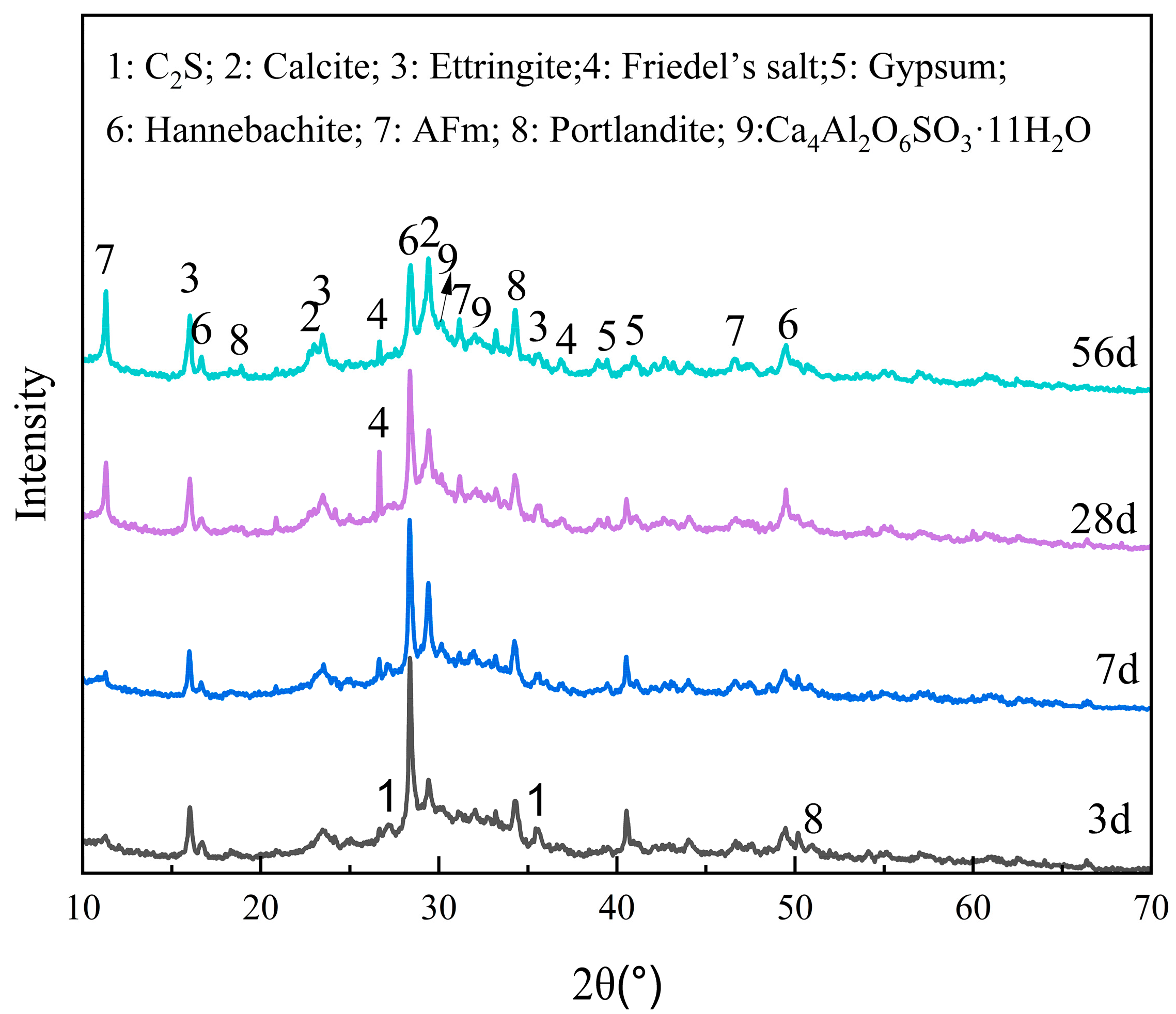
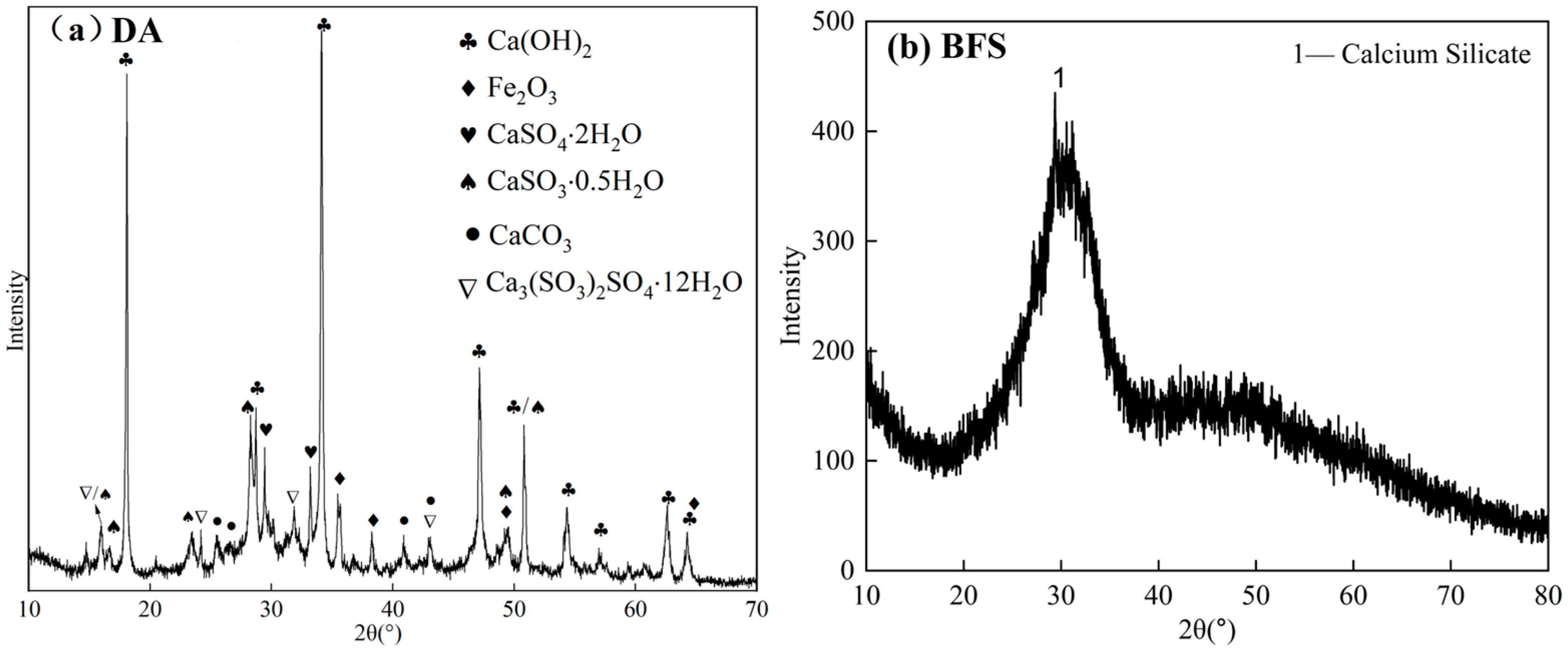
2.3.1. XRD
2.3.2. SEM-EDX
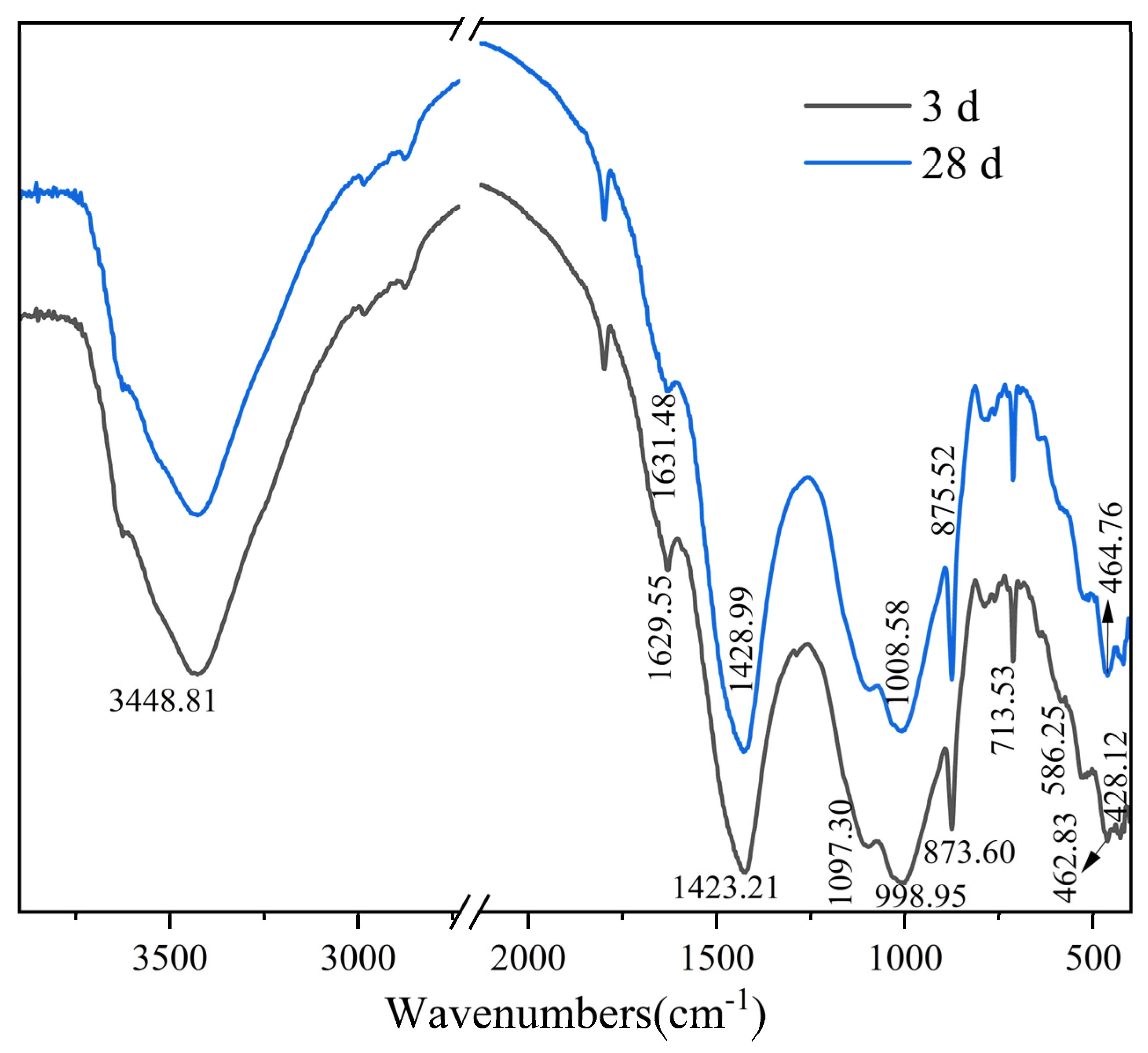
2.3.3. FTIR
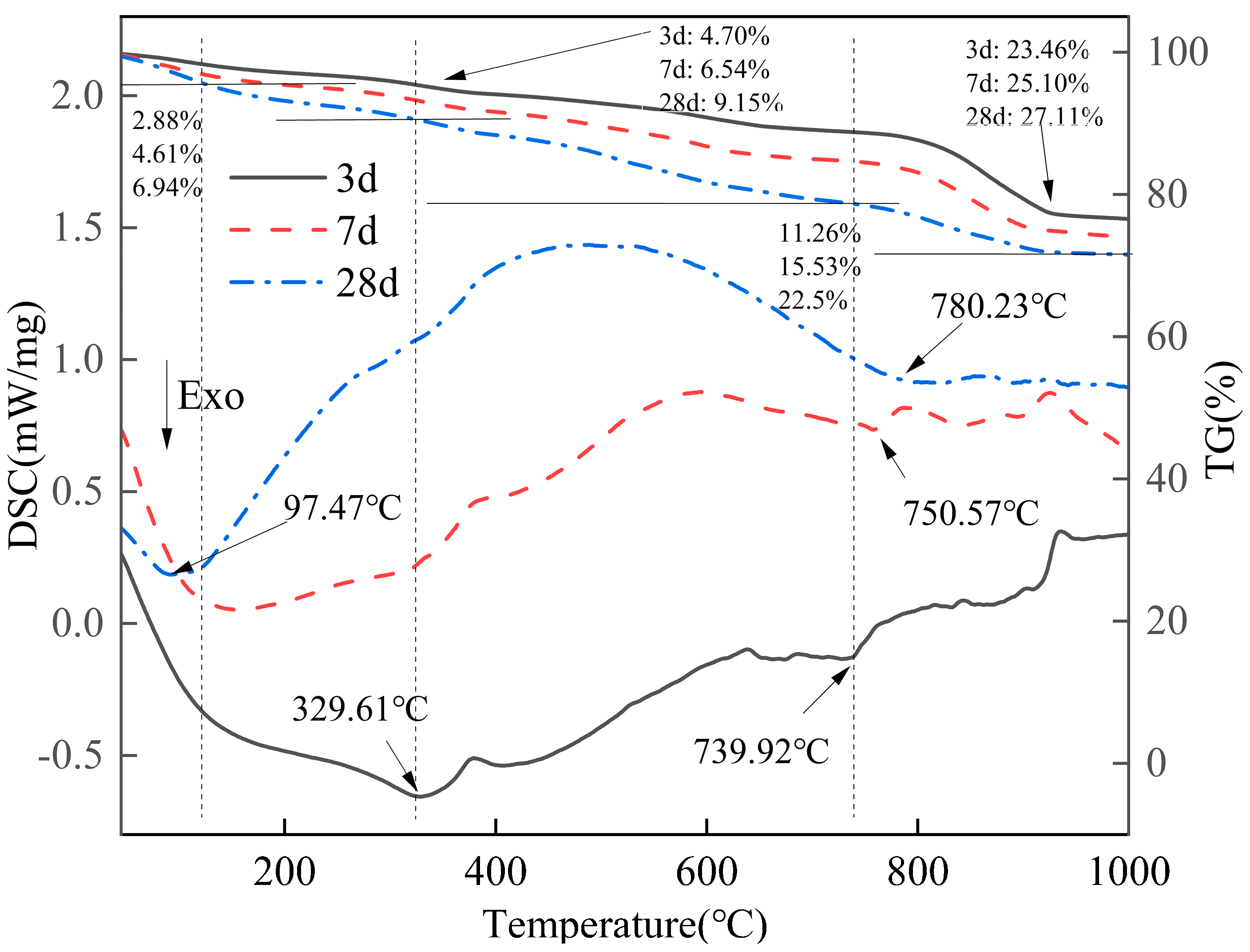
2.3.4. TG-DSC
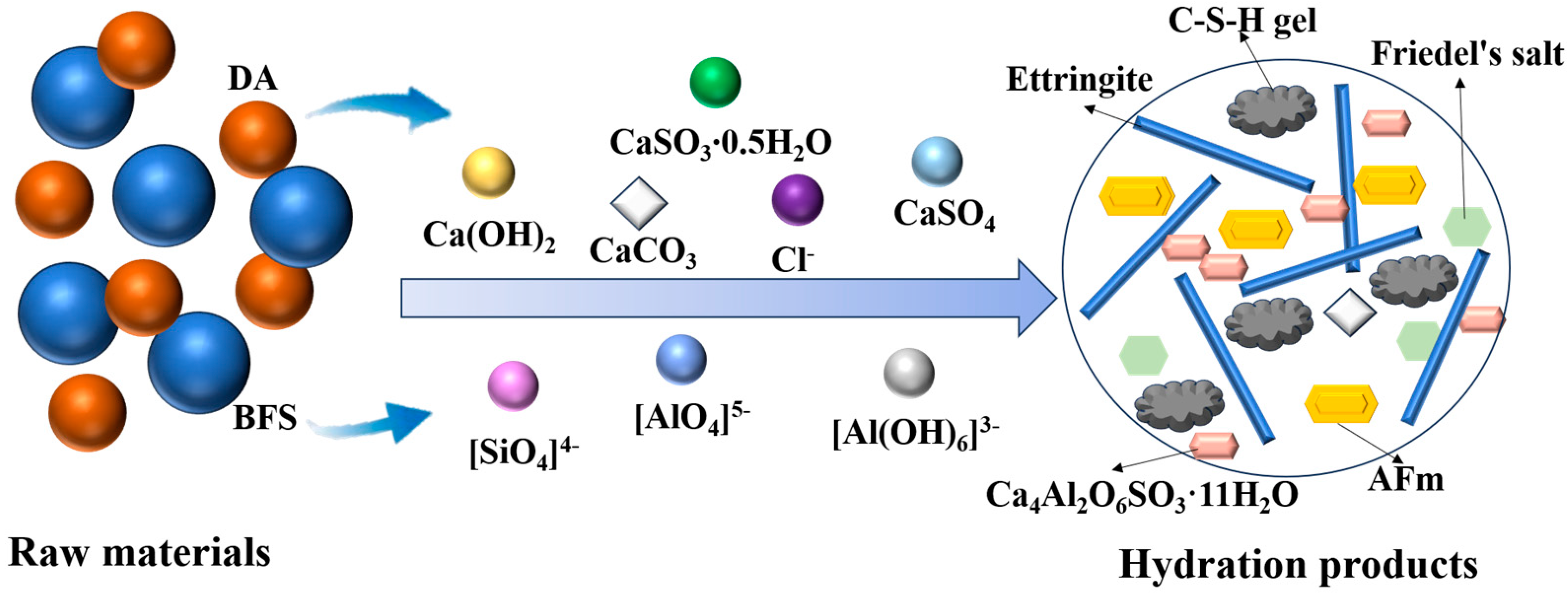
2.4. Reaction Mechanism
2.5. Economic and Environmental Benefits
3. Conclusions
4. Materials and Methods
4.1. Raw Materials
4.2. Methods
4.2.1. Preparation of Gelling Materials with Different DA Dosages
4.2.2. Different Mechanical Grinding Experiments
4.2.3. Strength Testing, Flowability, Setting Time, Stability, and Hydration Heat
4.2.4. Microanalysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Fan, X.; Zhou, Z.; Gan, M.; Gao, Z.; Sun, Z.; Ji, Z.; Wang, X.; Li, J.; Wu, Y. Resource utilization of semi-dry flue gas desulfurization ash by thermal treatment on sintering machine. J. Environ. Chem. Eng. 2024, 12, 112356. [Google Scholar] [CrossRef]
- Nedunuri, A.S.S.S.; Muhammad, S. Improving the workability and workable time of sodium hydroxide-activated ground granulated blast furnace slag binder-based concrete. Cement 2024, 16, 100106. [Google Scholar] [CrossRef]
- Rathnayake, M.; Julnipitawong, P.; Tangtermsirikul, S.; Toochinda, P. Utilization of coal fly ash and bottom ash as solid sorbents for sulfur dioxide reduction from coal fired power plant: Life cycle assessment and applications. J. Clean. Prod. 2018, 202, 934–945. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, B.; Tang, C.; Xie, J.-B.; Wang, B.; Zhang, H.; Ni, H. Utilization of semi-dry sintering flue gas desulfurized ash for SO2 generation during sulfuric acid production using boiling furnace. Chem. Eng. J. 2017, 327, 914–923. [Google Scholar] [CrossRef]
- Li, X.-G.; Chen, Q.-B.; Ma, B.-G.; Huang, J.; Jian, S.-W.; Wu, B. Utilization of modified CFBC desulfurization ash as an admixture in blended cements: Physico-mechanical and hydration characteristics. Fuel 2012, 102, 674–680. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Sun, Z.; Huang, X.; Gan, M.; Ji, Z.; Chen, X.; Fan, X. Co-disposal of semi-dry desulfurization residue and red mud into high performance alkali activated material. Constr. Build. Mater. 2022, 350, 128776. [Google Scholar] [CrossRef]
- Park, J.-H.; Ahn, J.-W.; Kim, K.-H.; Son, Y.-S. Historic and futuristic review of electron beam technology for the treatment of SO2 and NOx in flue gas. Chem. Eng. J. 2019, 355, 351–366. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T.-A. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Betts, K.S. Technology solutions: Driving desulfurization technology development. Environ. Sci. Technol. 2000, 34, 142A–143A. [Google Scholar] [CrossRef]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2018, 342, 436–445. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of low-quality desulfurized ash from semi-dry flue gas desulfurization by mixing with hemihydrate gypsum. Fuel 2019, 255, 115783. [Google Scholar] [CrossRef]
- Sun, R.; Wang, D.; Wang, Y.; Fang, Z.; Zhang, S. The effects of four typical activators on the early hydration of sintering flue gas desulphurisation ash-steel slag-cement composite cementitious material. Cem. Concr. Compos. 2022, 131, 104588. [Google Scholar] [CrossRef]
- Dawood, E.T.; Mohammed, W.T.; Plank, J. Performance of sustainable mortar using calcined clay, fly ash, limestone powder and reinforced with hybrid fibers. Case Stud. Constr. Mater. 2022, 16, e00849. [Google Scholar] [CrossRef]
- Guo, R.; Xue, C.; Li, Q.; Guo, W.; Pan, H.; Zhao, Q. Activation of ultra-fine steel slag and ground granulated blast furnace slag in alkaline waste solutions via phosphogypsum and calcium carbide slag. Constr. Build. Mater. 2024, 457, 139333. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, J.; Zhang, C.; Li, S.; An, N. Study on optimization and mechanism of mechanical activation process of titanium-bearing blast furnace slag. J. Mater. Res. Technol. 2022, 19, 3130–3144. [Google Scholar] [CrossRef]
- Hao, S.; Luo, G.; Wang, L.; An, S.; Chai, Y.; Song, W. Tempering and modification of basic oxygen furnace slag with blast furnace slag: Mineral phase transformations and cementitious material enrichment mechanisms. Case Stud. Constr. Mater. 2024, 21, e03803. [Google Scholar] [CrossRef]
- Bai Kamara, K.B.; Ganjian, E.; Khorami, M. The effect of quarry waste dust and reclaimed asphalt filler in hydraulically bound mixtures containing plasterboard gypsum and GGBS. J. Clean. Prod. 2021, 279, 123584. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation mechanisms of three types of industrial by-product gypsums on steel slag–granulated blast furnace slag-based binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Xie, G.; Suo, Y.; Liu, L.; Zhu, M.; Xie, L.; Qu, H.; Sun, W. Mechanical grinding activation of modified magnesium slag and its use as backfilling cementitious material. Case Stud. Constr. Mater. 2023, 18, e01778. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Elmansy, A.k.; Abdellatief, M.; Elrahman, M.A. Durability and ecological assessment of low-carbon high-strength concrete with short AR-glass fibers: Effects of high-volume of solid waste materials. Constr. Build. Mater. 2024, 429, 136422. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Zhou, H.; Zhong, X.; Zhou, Z.; Li, J.; Hou, H. Preparation of environmental-friendly cementitious material from red mud and waste glass sludge by mechanical activation. Constr. Build. Mater. 2024, 423, 135861. [Google Scholar] [CrossRef]
- Hazarika, A.; Huang, L.; Erlingsson, S.; de Weerdt, K.; Löfgren, I.; Iftikhar, S.; Babaahmadi, A. Characterization, activation and reactivity—A case study of Nordic volcanic materials for application as Supplementary Cementitious Materials. Case Stud. Constr. Mater. 2025, 22, e04096. [Google Scholar] [CrossRef]
- Andersson, A.; Brander, L.; Lennartsson, A.; Roos, Å.; Engström, F. Ground granulated iron silicate slag as supplementary cementitious material: Effect of prolonged grinding and granulation temperature. Clean. Mater. 2023, 10, 100209. [Google Scholar] [CrossRef]
- GB/T 28294; Iron and Steel Slag Composite Materials. The State Administration of Market Supervision and Administration State Standardization Administration of the People’s Republic of China: Beijing, China, 2024.
- Han, F.; Zhu, Z.; Li, Y.; Pu, S.; Zhang, Z. Effect of ultrafine limestone powder on the hydration heat and rheological properties of Portland cement paste. Powder Technol. 2025, 454, 120717. [Google Scholar] [CrossRef]
- Yao, G.; Wan, H.; Tang, G.; Gao, X.; Zhang, K.; Huang, W.; Yu, Y.; Shen, X.; Cui, S. Effect of gypsum on low-heat Portland cement early mechanical properties and hydration in the presence of DEIPA. Constr. Build. Mater. 2024, 449, 138477. [Google Scholar] [CrossRef]
- Li, B.; Sun, Q.; Chen, X.; Xu, Z.; Yang, L. Preparation and microstructure analysis of alkali-activated ground granulated blast furnace slag-steel slag grouting materials. Case Stud. Constr. Mater. 2024, 20, e03235. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Chemical activation of binary slag cement with low carbon footprint. J. Clean. Prod. 2020, 267, 121455. [Google Scholar] [CrossRef]
- Wei, C.; Li, Y.; Liu, X.; Zhang, Z.; Wu, P.; Gu, J. Large-scale application of coal gasification slag in nonburnt bricks: Hydration characteristics and mechanism analysis. Constr. Build. Mater. 2024, 421, 135674. [Google Scholar] [CrossRef]
- Zhonglin, L.; Ye, X.; Cheng, L.; Biao, P.; Yibing, L.; Weiguang, Z.; Chen, Y. Investigation on the compressive strength of desulfurization gypsum binary cementitious materials with low energy consumption: The utilization enhancement of industrial wastes. Case Stud. Constr. Mater. 2024, 21, e03582. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Lagosz, A.; Malolepszy, J. Tricalcium aluminate hydration in the presence of calcium sulfite hemihydrate. Cem. Concr. Res. 2003, 33, 333–339. [Google Scholar] [CrossRef]
- Bogushevich, S.E.; Lysenko, G.N. Spectroscopic study of calcium sulfite thermolysis. Russ. J. Inorg. Chem. 2009, 54, 618–623. [Google Scholar] [CrossRef]
- Du, H.; Xu, D.; Li, X.; Li, J.; Ni, W.; Li, Y.; Fu, P. Application of molten iron desulfurization slag to replace steel slag as an alkaline component in solid waste-based cementitious materials. J. Clean. Prod. 2022, 377, 134353. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ni, W.; Li, J.; Ba, H. Deep insights into the interactions between monosulfoaluminate, ettringite, C-S-H, and antimony: Mechanisms and microstructural alterations. Process Saf. Environ. Prot. 2025, 196, 106852. [Google Scholar] [CrossRef]
- Xu, C.; Xu, D.; Zhang, W.; Ye, J.; Zhang, S. Influence of steel slag to granulated blast furnace slag ratio on the chloride binding and penetration of metallurgical slag-based binder. Constr. Build. Mater. 2024, 431, 136571. [Google Scholar] [CrossRef]
- Li, Y.; Huang, M.; Li, J.; Zhang, S.; Yang, G.; Chen, X.; Du, H.; Ni, W.; Song, X.; Hitch, M. Performance Assessment of All-Solid-Waste High-Strength Concrete Prepared from Waste Rock Aggregates. Materials 2025, 18, 624. [Google Scholar] [CrossRef]
- Song, W.; Fan, X.; Gan, M.; Ji, Z.; Sun, Z. A novel and clean utilization of flue gas desulfurization ash coupled with converter sludge to produce calcium ferrate. Chem. Eng. J. 2024, 498, 154919. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- GB/T 8077; Methods for Testing Uniformity of Concrete Admixtures. The State Administration of Market Supervision and Administration State Standardization Administration of the People’s Republic of China: Beijing, China, 2023.
- GB/T 1346; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. The State Administration of Market Supervision and Administration State Standardization Administration of the People’s Republic of China: Beijing, China, 2024.
- GB/T 750; Test Method for Autoclave Expansion of Cement. The State Administration of Market Supervision and Administration State Standardization Administration of the People’s Republic of China: Beijing, China, 2024.

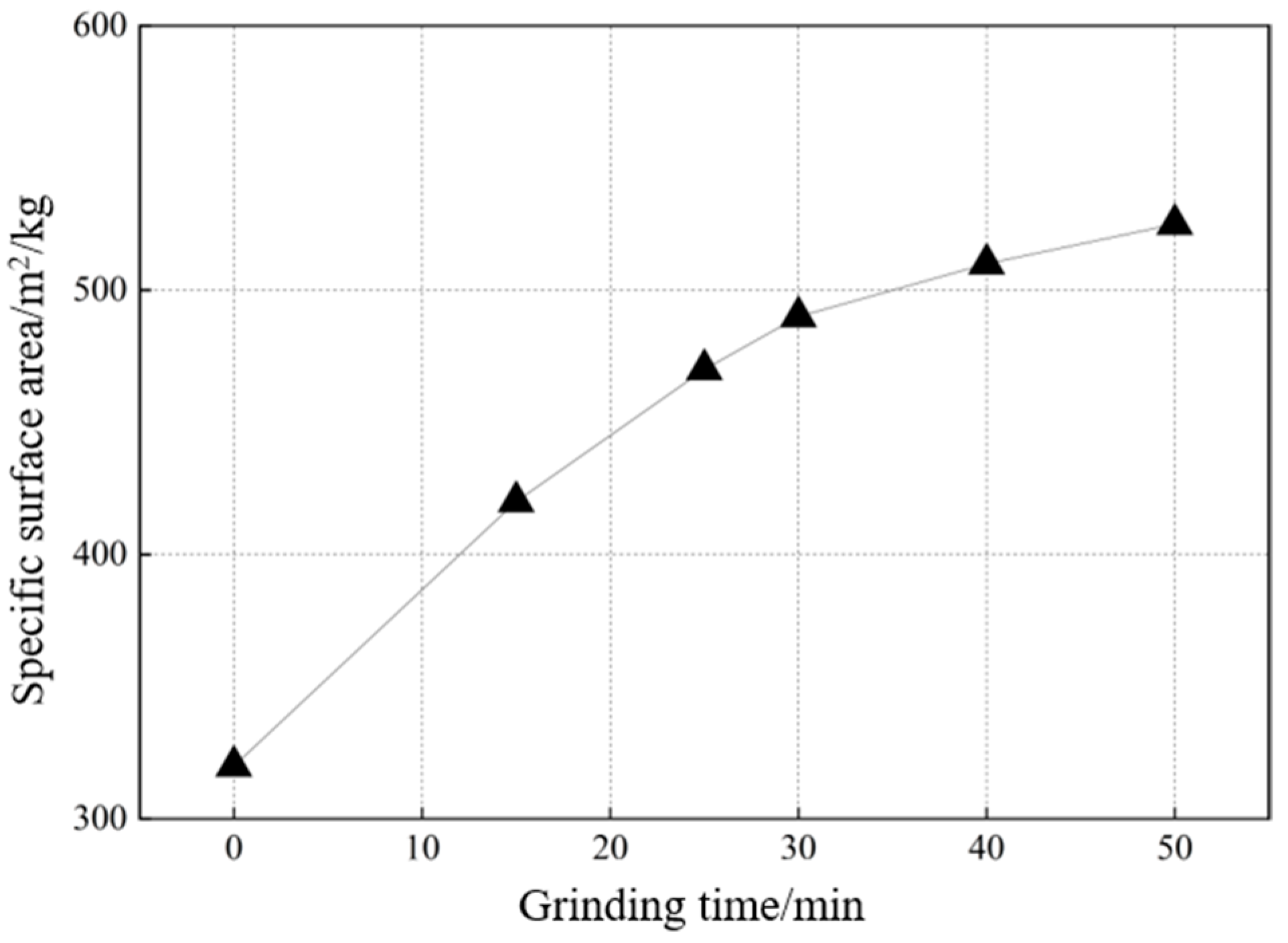
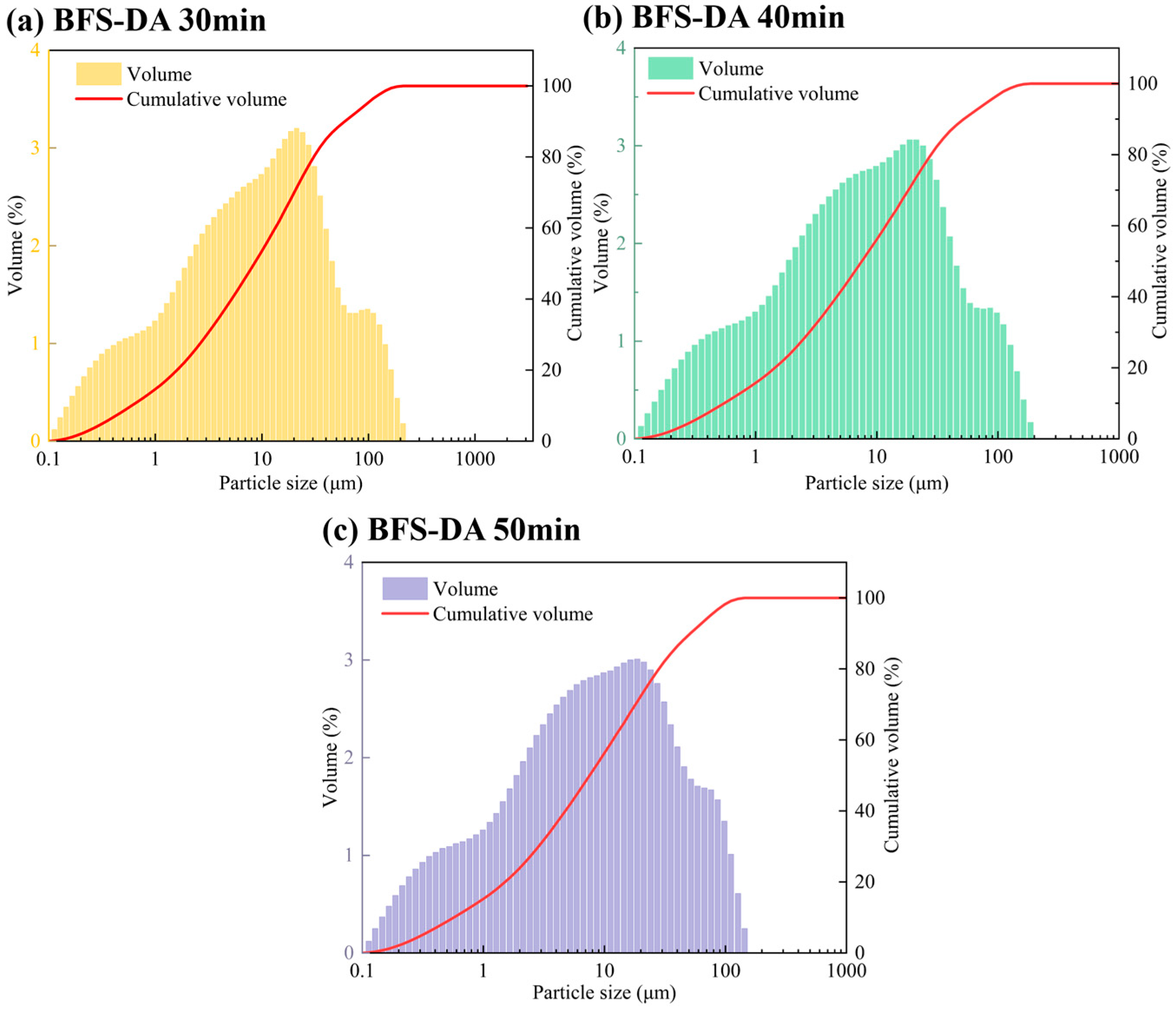

| Grinding Time (min) | Specific Surface Area (m2/kg) | Particle Size Parameters (μm) | ||
|---|---|---|---|---|
| D10 | D50 | D90 | ||
| 0 | 395 | 2.34 | 17.19 | 74.60 |
| 30 | 580 | 0.65 | 9.64 | 68.00 |
| 40 | 591 | 0.62 | 8.55 | 58.30 |
| 50 | 578 | 0.63 | 8.56 | 59.30 |
| Sample | Initial Setting Time (min) | GB/T 28294 | Final Setting Time (min) | GB/T 28294 | Autoclave Expansion (%) | GB/T 28294 | Soundness |
|---|---|---|---|---|---|---|---|
| M0 | 495 | ≥45 | 570 | ≤600 | 0.35 | ≤0.50% | compliant with the standard |
| M-40 | 451 | ≥45 | 563 | ≤600 | 0.25 | ≤0.50% | compliant with the standard |
| Compounds | Ca | O | Al | Si | S | Fe | |
|---|---|---|---|---|---|---|---|
| Point 1 | Portlandite | 45.07 | 54.93 | - | - | - | |
| Point 2 | Ettringite | 63.81 | 7.71 | 5.88 | 14.05 | 6.25 | 0.47 |
| Point 3 | C-S-H gel | 18.78 | 43.35 | 3.76 | 33.33 | 0.58 | 0.60 |
| Point 4 | AFm | 26.97 | 56.30 | 10.44 | - | 5.67 | - |
| Sample | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | Na2O | MnO | Fe2O3 | K2O | Cl | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | 47.61 | 5.5 | 0.52 | 1.06 | 24.76 | - | 1.33 | 1.06 | 8.16 | 6.36 | 1.58 | 2.05 |
| BFS | 48.70 | 30.26 | 9.97 | 5.70 | 2.39 | 0.39 | 0.26 | 0.18 | 0.49 | 0.42 | 0.03 | 1.27 |
| DA | BFS | W/S | |
|---|---|---|---|
| G1 | 25 | 75 | 0.32 |
| G2 | 30 | 70 | 0.32 |
| G3 | 35 | 65 | 0.32 |
| G4 | 40 | 60 | 0.32 |
| G5 | 45 | 55 | 0.32 |
| G6 | 50 | 50 | 0.32 |
| G7 | 55 | 45 | 0.32 |
| G8 | 60 | 40 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, S.; Huang, M.; Yang, G.; Li, J.; Ma, M.; Hu, W.; Ni, W. Hydration Mechanism of Solid Waste Gelling Materials Containing Semi-Dry Desulfurization Ash. Gels 2025, 11, 193. https://doi.org/10.3390/gels11030193
Li Y, Zhang S, Huang M, Yang G, Li J, Ma M, Hu W, Ni W. Hydration Mechanism of Solid Waste Gelling Materials Containing Semi-Dry Desulfurization Ash. Gels. 2025; 11(3):193. https://doi.org/10.3390/gels11030193
Chicago/Turabian StyleLi, Yunyun, Siqi Zhang, Meixiang Huang, Guodong Yang, Jiajie Li, Mengqi Ma, Wentao Hu, and Wen Ni. 2025. "Hydration Mechanism of Solid Waste Gelling Materials Containing Semi-Dry Desulfurization Ash" Gels 11, no. 3: 193. https://doi.org/10.3390/gels11030193
APA StyleLi, Y., Zhang, S., Huang, M., Yang, G., Li, J., Ma, M., Hu, W., & Ni, W. (2025). Hydration Mechanism of Solid Waste Gelling Materials Containing Semi-Dry Desulfurization Ash. Gels, 11(3), 193. https://doi.org/10.3390/gels11030193










