Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors
Abstract
1. Introduction
2. Results and Discussion
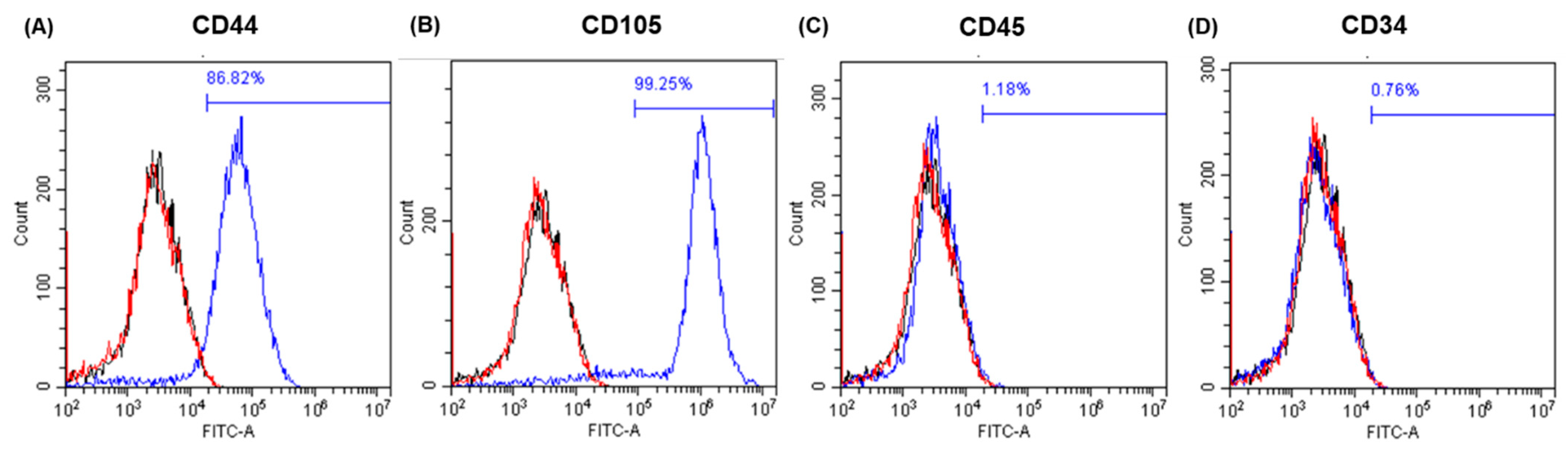
2.1. Cell Characterisation and Differentiation
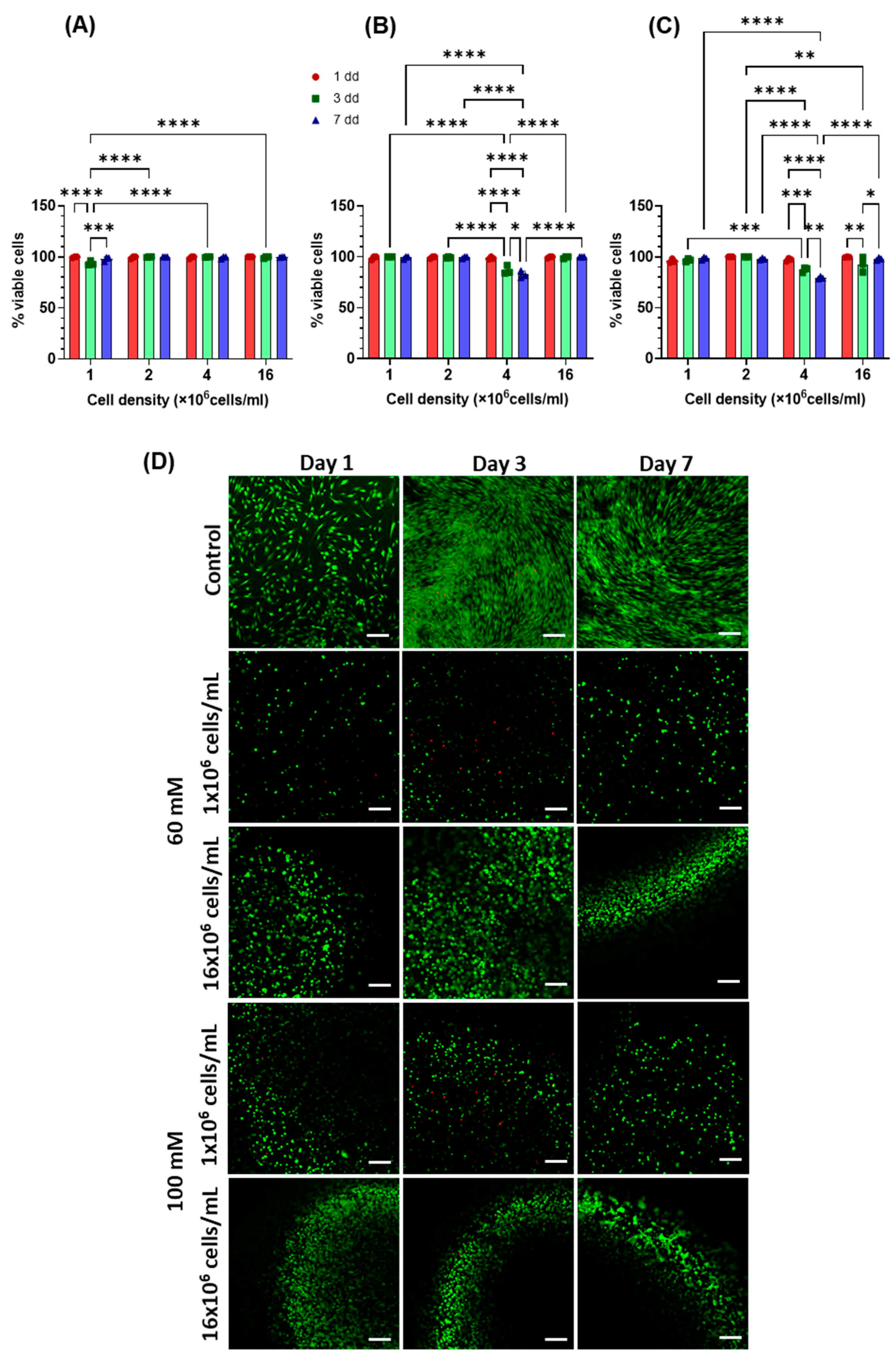
2.2. Evaluation of DNA Content
2.3. Cell Morphology
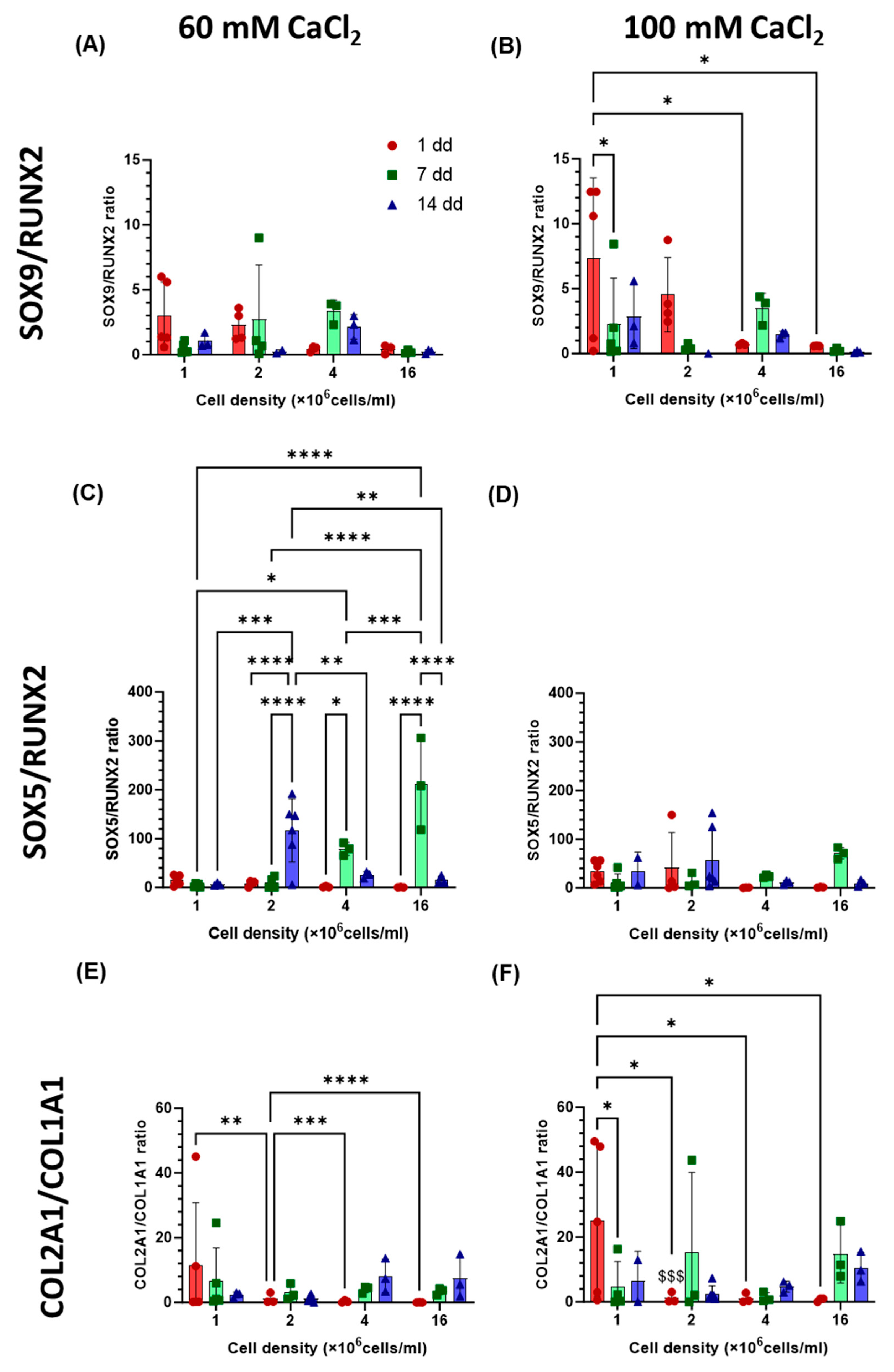
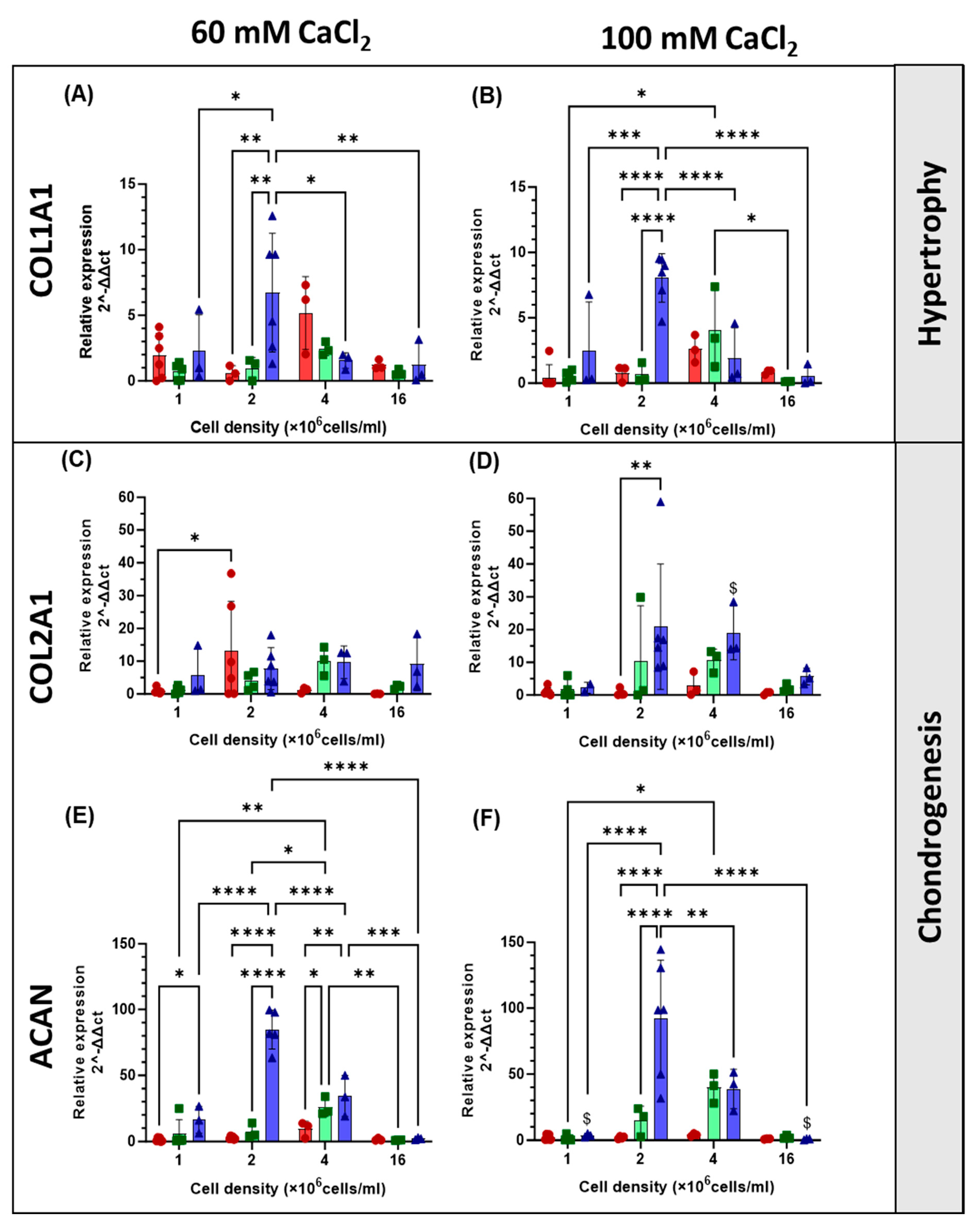
2.4. Gene Expression
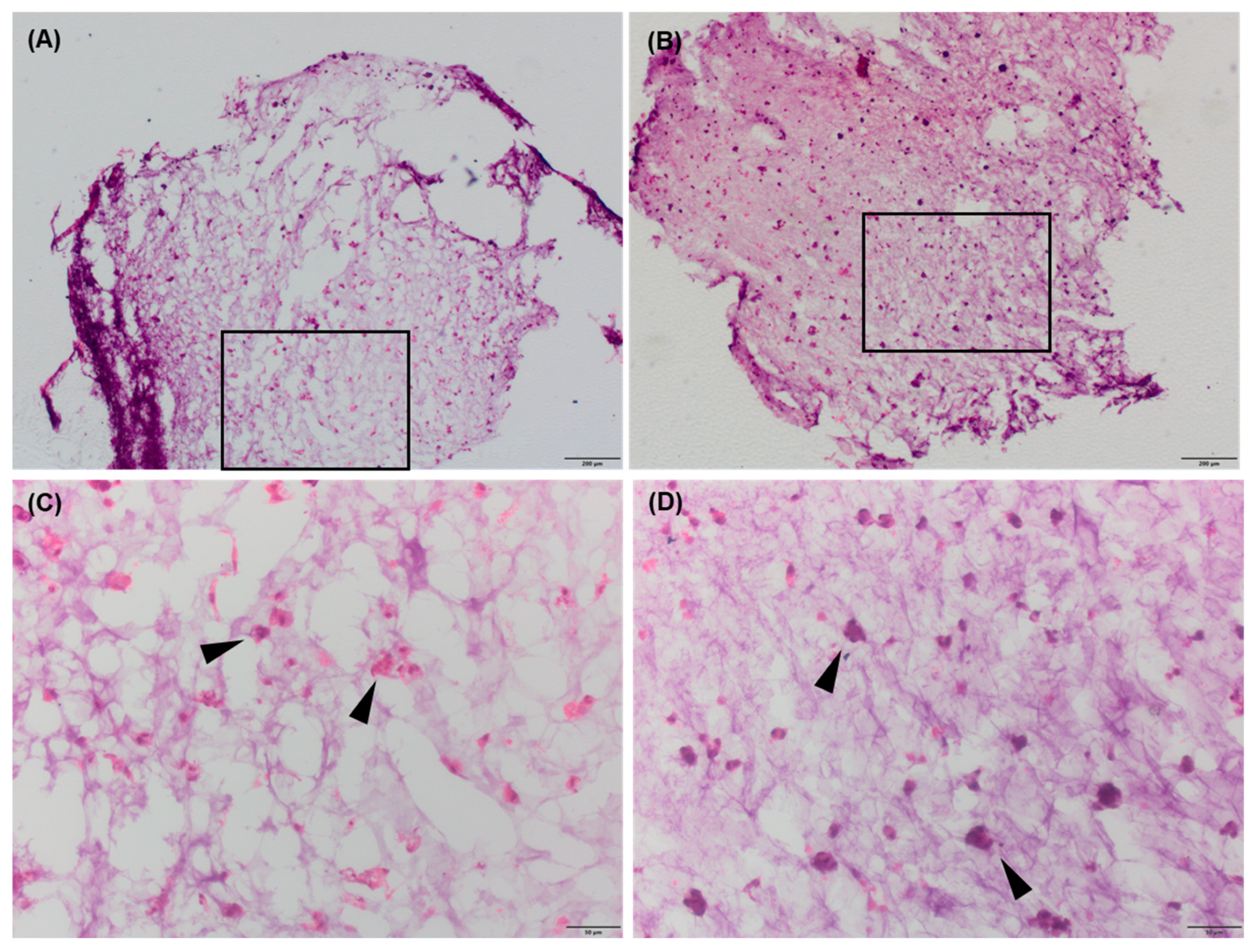
2.5. Immunohistochemistry
2.6. Three-Dimensional Hydrogel Structures Support Rounded Cell Morphology and the Formation of Cell Pellets
2.7. Cells of Different Origins Generate ECM at Different Rates
2.8. Cells from Different Species Are Sensitive to Different Substrate Stiffness Ranges
3. Conclusions
4. Materials and Methods
4.1. Cell Culture
4.2. Flow Cytometry
4.3. Multilineage Potential of hAdMSCs
4.4. Doubling Time
4.5. Hydrogel Formulation and Cell Encapsulation
4.6. Live/Dead Staining
4.7. DNA Quantification
4.8. Cell Morphology Analysis
4.9. RNA Extraction and Quantitative RT-qPCR
4.10. Immunostaining
4.11. Histology
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guillén-García, P.; Guillén-Vicente, I.; Rodríguez-Iñigo, E.; Guillén-Vicente, M.; Fernández-Jaén, T.F.; Navarro, R.; Aboli, L.; Torres, R.; Abelow, S.; López-Alcorocho, J.M. Cartilage Defect Treatment Using High-Density Autologous Chondrocyte Implantation (HD-ACI). Bioengineering 2023, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.K.; Gibson, M.A.; Elisseeff, J.H.; Trice, M.E. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res. Int. 2014, 2014, 72481. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Q.; Xia, J.; Chen, X.; Xiong, J.; Yang, L.; Liang, Y. Modification of mesenchymal stem cells for cartilage-targeted therapy. J. Transl. Med. 2022, 20, 505. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Chan, B.P.; Hui, T.Y.; Yeung, C.W.; Li, J.; Mo, I.; Chan, G.C.F. Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials 2007, 28, 4652–4666. [Google Scholar] [CrossRef]
- Djouad, F.; Plence, P.; Bony, C.; Tropel, P.; Apparailly, F.; Sany, J.; Noël, D.; Jorgensen, C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003, 102, 3837–3844. [Google Scholar] [CrossRef]
- Hui, T.Y.; Cheung, K.M.C.; Cheung, W.L.; Chan, D.; Chan, B.P. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: Influence of cell seeding density and collagen concentration. Biomaterials 2008, 29, 3201–3212. [Google Scholar] [CrossRef]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R.J. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- DeFail, A.J.; Chu, C.R.; Izzo, N.; Marra, K.G. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials 2006, 27, 1579–1585. [Google Scholar] [CrossRef]
- Roger, Y.; Sydow, S.; Burmeister, L.; Menzel, H.; Hoffmann, A. Sustained release of TGF-β3 from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells. Colloids Surf. B Biointerfaces 2020, 189, 110843. [Google Scholar] [CrossRef] [PubMed]
- Roncada, T.; Bonithon, R.; Blunn, G.; Rodlo, M. Soft substrates direct stem cell differentiation into the chondrogenic lineage without the use of growth factors. J. Tissue Eng. 2022, 13, 20417314221122121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiu, J.; Wan, L.; Li, J. The effect of matrix stiffness on the chondrogenic differentiation of mesenchymal stem cells. J. Mol. Histol. 2022, 53, 805–816. [Google Scholar] [CrossRef]
- Ambriz, X.; De Lanerolle, P.; Ambrosio, J.R. The mechanobiology of the actin cytoskeleton in stem cells during differentiation and interaction with biomaterials. Stem Cells Int. 2018, 2018, 2891957. [Google Scholar] [CrossRef]
- Volz, M.; Wyse-Sookoo, K.R.; Travascio, F.; Huang, C.-Y.; Best, T.M. Mechanobiological Approaches for Stimulating Chondrogenesis of Stem Cells. Stem Cells Dev. 2022, 31, 460–487. [Google Scholar] [CrossRef]
- Mohammed, M.; Lai, T.S.; Lin, H.C. Substrate stiffness and sequence dependent bioactive peptide hydrogels influence the chondrogenic differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2021, 9, 1676–1685. [Google Scholar] [CrossRef]
- Aprile, P.; Kelly, D.J. Hydrostatic Pressure Regulates the Volume, Aggregation and Chondrogenic Differentiation of Bone Marrow Derived Stromal Cells. Front. Bioeng. Biotechnol. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Zhan, X. Effect of matrix stiffness and adhesion ligand density on chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2020, 108, 675–683. [Google Scholar] [CrossRef]
- Allen, J.L.; Cooke, M.E.; Alliston, T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell 2012, 23, 3731–3742. [Google Scholar] [CrossRef]
- Haddouti, E.M.; Randau, T.M.; Hilgers, C.; Masson, W.; Walgenbach, K.J.; Pflugmacher, R.; Burger, C.; Gravius, S.; Schildberg, F.A. Characterization and comparison of human and ovine mesenchymal stromal cells from three corresponding sources. Int. J. Mol. Sci. 2020, 21, 2310. [Google Scholar] [CrossRef]
- De Mori, A.; Heyraud, A.; Tallia, F.; Blunn, G.; Jones, J.R.; Roncada, T.; Cobb, J.; Al-Jabri, T. Ovine Mesenchymal Stem Cell Chondrogenesis on a Novel 3D-Printed Hybrid Scaffold In Vitro. Bioengineering 2024, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Messana, J.; Hwang, N.S.H.; Elisseeff, J.H. Reorganization of actin filaments enhances chondrogenic differentiation of cells derived from murine embryonic stem cells. Biochem. Biophys. Res. Commun. 2006, 348, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Loty, S.; Forest, N.; Boulekbache, H.; Sautier, J.M. Cytochalasin D induces changes in cell shape and promotes in vitro chondrogenesis: A morphological study. Biol. Cell 1995, 83, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Ma, L.; Wang, B.; Song, Y.; Luo, R.; Li, G.; Liao, Z.; Shi, Y.; Wang, K.; Feng, X.; et al. N-cadherin mimetic hydrogel enhances MSC chondrogenesis through cell metabolism. Acta Biomater. 2022, 150, 83–95. [Google Scholar] [CrossRef]
- Tavella, S.; Raffo, P.; Tacchetti, C.; Cancedda, R.; Castagnola, P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp. Cell Res. 1994, 215, 354–362. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A single day of TGF-β1 exposure activates chondrogenic and hypertrophic differentiation pathways in bone marrow-derived stromal cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micromass and pellet culture systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Mueller, M.; Tuan, R. Functional Characterization of hypertrophy in chondrogenesis. Arthritis Rhe. 2008, 58, 1377–1388. [Google Scholar] [CrossRef]
- Spagnuolo, F.D.; Kronemberger, G.S.; Storey, K.J.; Kelly, D.J. The maturation state and density of human cartilage microtissues influence their fusion and development into scaled-up grafts. Acta Biomater. 2025, 194, 109–121. [Google Scholar] [CrossRef]
- Zigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 2019, 25, 1369–1380. [Google Scholar] [CrossRef]
- Sarem, M.; Otto, O.; Tanaka, S.; Shastri, V.P. Cell number in mesenchymal stem cell aggregates dictates cell stiffness and chondrogenesis. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular interactions and signaling in cartilage development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; de Llano, J.J.M.; Roca, F.G.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-agarose hydrogels improve the in vitro differentiation of human dental pulp stem cells in chondrocytes. A histological study. Biomedicines 2021, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.X.; Lin, H.; Fritch, M.R.; Shen, H.; Alexander, P.G.; DeHart, M.; Tuan, R.S. Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness. Acta Biomater. 2017, 58, 302–311. [Google Scholar] [CrossRef]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling Rigidity and Degradation of Alginate Hydrogels via Molecular Weight Distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef]
- Babalola, O.M.; Bonassar, L.J. Effects of seeding density on proteoglycan assembly of passaged mesenchymal stem cells. Cell. Mol. Bioeng. 2010, 3, 197–206. [Google Scholar] [CrossRef]
- Trujillo, S.; Seow, M.; Lueckgen, A.; Salmeron-Sanchez, M.; Cipitria, A. Dynamic mechanical control of alginate-fibronectin hydrogels with dual crosslinking: Covalent and ionic. Polymers 2021, 13, 433. [Google Scholar] [CrossRef]
- Souza, A.; Parnell, M.; Rodriguez, B.J.; Reynaud, E.G. Role of pH and Crosslinking Ions on Cell Viability and Metabolic Activity in Alginate–Gelatin 3D Prints. Gels 2023, 9, 853. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Hussain, I.; Li, K.; Yao, F.; Fu, G. High mechanical strength and stability of alginate hydrogel induced by neodymium ions coordination. Polym. Degrad. Stab. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 45012. [Google Scholar] [CrossRef] [PubMed]
- Reppel, L.; Schiavi, J.; Charif, N.; Leger, L.; Yu, H.; Pinzano, A.; Henrionnet, C.; Stoltz, J.-F.; Bensoussan, D.; Huselstein, C. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: An alternative stem cell source for cartilage tissue engineering. Stem Cell Res. Ther. 2015, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Moulisová, V.; Poveda-Reyes, S.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Gallego Ferrer, G. Hybrid Protein-Glycosaminoglycan Hydrogels Promote Chondrogenic Stem Cell Differentiation. ACS Omega 2017, 2, 7609–7620. [Google Scholar] [CrossRef]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Seon, Y.; Chien, A.J.; Guo, J.L.; Smith, B.T.; Watson, E.; Pearce, H.A.; Koons, G.L.; Navara, A.M.; Lam, J.; Scott, D.W.; et al. Chondrogenesis of cocultures of mesenchymal stem cells and articular chondrocytes in poly (L-lysine)-loaded hydrogels ☆. J. Control. Release 2020, 328, 710–721. [Google Scholar] [CrossRef]
- Foyt, D.A.; Taheem, D.K.; Ferreira, S.A.; Norman, M.D.A.; Petzold, J.; Jell, G.; Grigoriadis, A.E.; Gentleman, E. Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater. 2019, 89, 73–83. [Google Scholar] [CrossRef]
- Angele, P.; Yoo, J.U.; Smith, C.; Mansour, J.; Jepsen, K.J.; Nerlich, M.; Johnstone, B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J. Orthop. Res. 2003, 21, 451–457. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, T.; Guo, L.; Zhang, X. An in vitro study of collagen hydrogel to induce the chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2012, 100, 2717–2725. [Google Scholar] [CrossRef]
- Stewart, A.A.; Byron, C.R.; Pondenis, H.C.; Stewart, M.C. Effect of dexamethasone supplementation on chondrogenesis of equine mesenchymal stem cells. Am. J. Vet. Res. 2008, 69, 1013–1021. [Google Scholar] [CrossRef]
- Shen, H.; Lin, H.; Sun, A.X.; Song, S.; Zhang, Z.; Dai, J.; Tuan, R.S. Chondroinductive factor-free chondrogenic differentiation of human mesenchymal stem cells in graphene oxide-incorporated hydrogels. J. Mater. Chem. B 2018, 6, 908–917. [Google Scholar] [CrossRef]
- De Mori, A.; Alasa, U.J.; Mühlhölzl, A.; Blunn, G. Slipper Limpet (Crepidula fornicata) Shells Support In Vitro Osteogenesis of Human Adipose-Derived Stem Cells. Mar. Drugs 2023, 21, 248. [Google Scholar] [CrossRef]





| FORWARD | REVERSE | |
|---|---|---|
| GAPDH | CCGCATCTTCTTTGCGTCG | GCCCAATACGACAAATCCGT |
| RUNX2 | CCACCGAGACACCATGGAG | CGCCTGGGTCTCTTCACTAC |
| COL1A1 | GTTTGGATGGTGCCAAGGGA | AGCACCATCATTTCCACGAG |
| COL2A1 | CCACGCTCAAGTCCCTCAAC | AGTCACCGCTCTTCCACTCG |
| SOX9 | AGGAAGTCGGTGAAGAACGG | CGCCTTGAAGATGGCGTTG |
| SOX5 | AGCAGATGGAGAGGTAGCCA | ACAAGTCTCTTGCGTCAGCA |
| ACAN | TACACTGGCGAGCACTGTAAC | ACAGGTCCCCTTCGTAGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mori, A.; Aydin, N.; Lostia, G.; Manca, A.; Blunn, G.; Roldo, M. Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors. Gels 2025, 11, 213. https://doi.org/10.3390/gels11030213
De Mori A, Aydin N, Lostia G, Manca A, Blunn G, Roldo M. Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors. Gels. 2025; 11(3):213. https://doi.org/10.3390/gels11030213
Chicago/Turabian StyleDe Mori, Arianna, Nadide Aydin, Giada Lostia, Alessia Manca, Gordon Blunn, and Marta Roldo. 2025. "Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors" Gels 11, no. 3: 213. https://doi.org/10.3390/gels11030213
APA StyleDe Mori, A., Aydin, N., Lostia, G., Manca, A., Blunn, G., & Roldo, M. (2025). Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors. Gels, 11(3), 213. https://doi.org/10.3390/gels11030213









