Improving Vaginal Health with a Zinc-Containing Vaginal Hydrogel
Abstract
1. Introduction
2. Results and Discussion
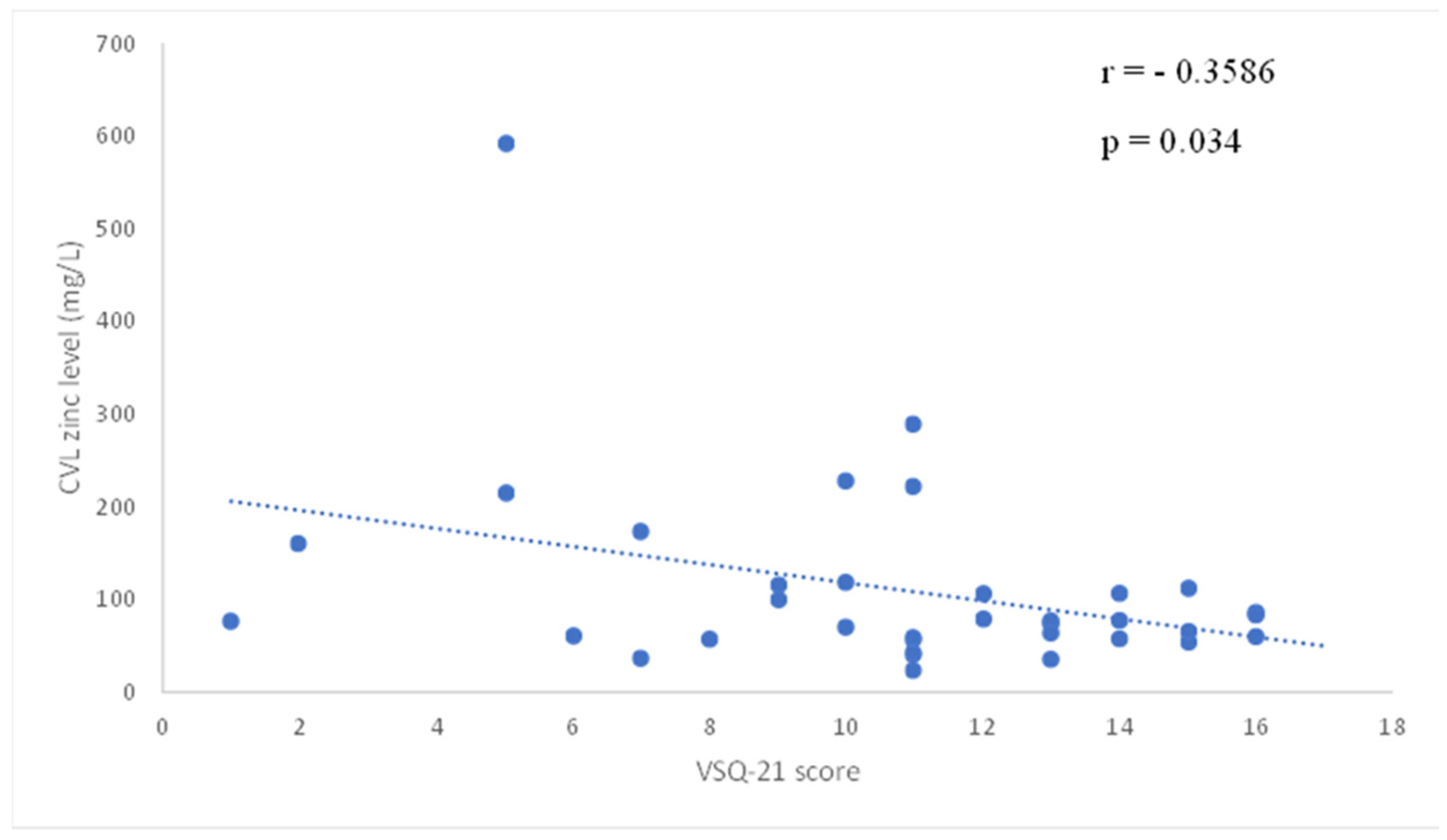
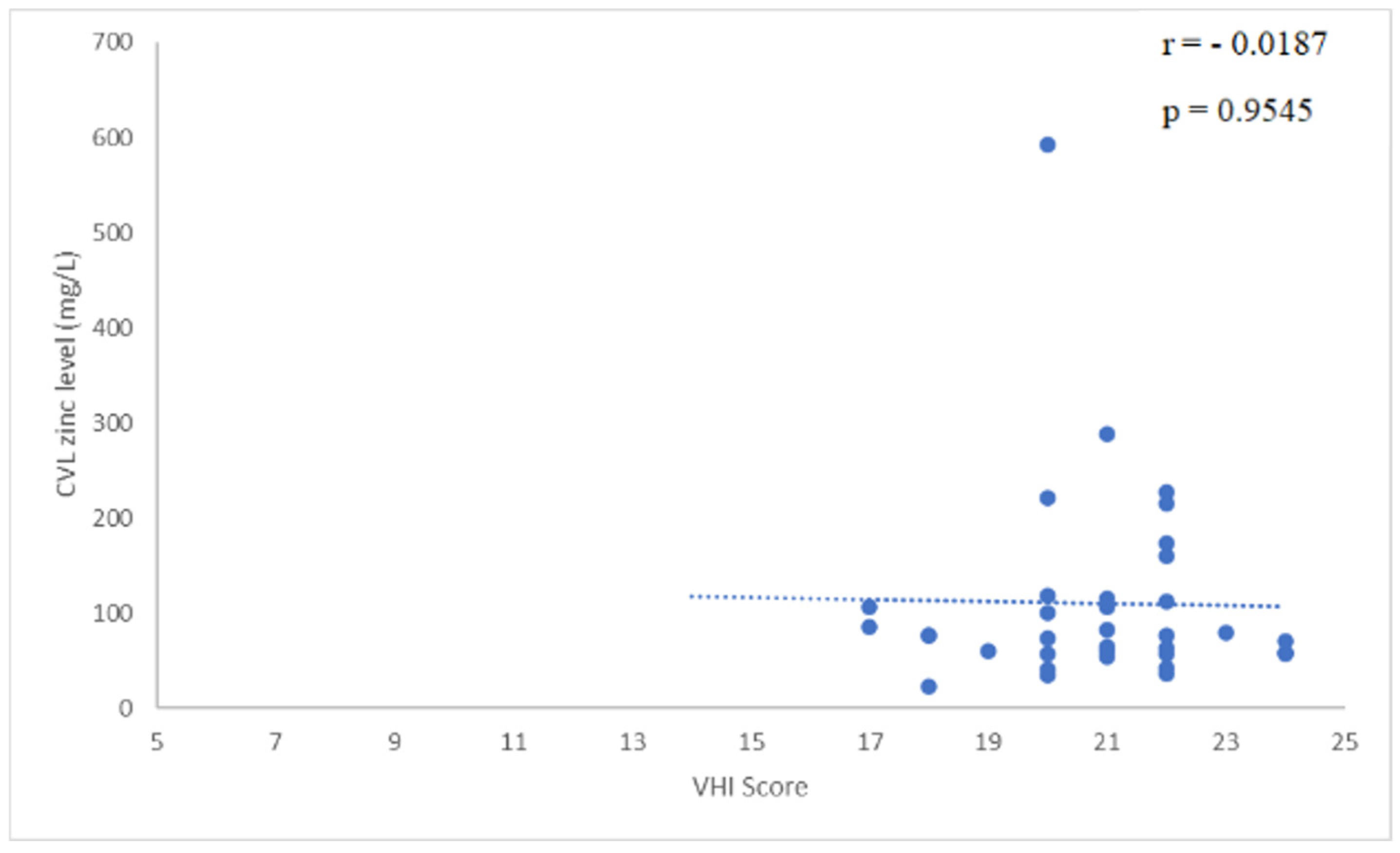
2.1. Results
2.2. Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sobel, J.D. Vaginal infections in adult women. Med. Clin. N. Am. 1990, 74, 1573–1602. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.M.; Nyirjesy, P. Recurrent vulvovaginitis. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Itriyeva, K. Evaluation of vulvovaginitis in the adolescent patient. Curr. Probl. Pediatr. Adolesc. Health Care 2020, 50, 100836. [Google Scholar] [CrossRef]
- Kingsberg, S.A.; Wysocki, S.; Magnus, L.; Krychman, M.L. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) Survey. J. Sex. Med. 2013, 10, 1790–1799. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Amsalem, H.; Gutman, Y.; Esh-Broder, E.; Daum, H. Prevalence and Characteristics of Postpartum Vulvovaginal Atrophy and Lack of Association With Postpartum Dyspareunia. J. Low. Genit. Tract Dis. 2020, 24, 411–416. [Google Scholar] [CrossRef]
- Fukazawa, E.I.; Witkin, S.S.; Robial, R.; Vinagre, J.G.; Baracat, E.C.; Linhares, I.M. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch. Gynecol. Obstet. 2019, 300, 647–650. [Google Scholar] [CrossRef]
- Graziottin, A. Maintaining vulvar, vaginal and perineal health: Clinical considerations. Womens Health 2024, 20, 17455057231223716. [Google Scholar] [CrossRef]
- Redondo-Lopez, V.; Cook, R.L.; Sobel, J.D. Emerging Role of Lactobacilli in the Control and Maintenance of the Vaginal Bacterial Microflora. Rev. Infect. Dis. 1990, 12, 856–872. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Petrova, M.I.; van den Broek, M.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Kaunitz, A.M.; Manson, J.E. ACOG Practice Bulletin No. 141: Management of menopausal symptoms. Obstet. Gynecol. 2014, 123, 202–216. [Google Scholar]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Ferris, D.; Schwebke, J.; Nyirjesy, P.; Wiesenfeld, H.C.; Peipert, J.; Soper, D.; Ohmit, S.E.; Hillier, S.L. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 2006, 194, 1283–1289. [Google Scholar] [CrossRef]
- Bassi, A.; Sharma, G.; Deol, P.K.; Madempudi, R.S.; Kaur, I.P. Preclinical Potential of Probiotic-Loaded Novel Gelatin–Oil Vaginal Suppositories: Efficacy, Stability, and Safety Studies. Gels 2023, 9, 244. [Google Scholar] [CrossRef]
- Mollazadeh-Narestan, Z.; Yavarikia, P.; Homayouni-Rad, A.; Samadi Kafil, H.; Mohammad-Alizadeh-Charandabi, S.; Gholizadeh, P.; Mirghafourvand, M. Comparing the Effect of Probiotic and Fluconazole on Treatment and Recurrence of Vulvovaginal Candidiasis: A Triple-Blinded Randomized Controlled Trial. Probiotics Antimicrob. Proteins 2023, 15, 1436–1446. [Google Scholar] [CrossRef]
- Takacs, P.; Kozma, B.; Erdodi, B.; Jakab, A.; Larson, K.; Poka, R. Zinc-containing Vaginal Moisturizer Gel Improves Postmenopausal Vulvovaginal Symptoms: A Pilot Study. J. Menopausal Med. 2019, 25, 63–68. [Google Scholar] [CrossRef]
- Jabbari, E. Hydrogels for Cell Delivery. Gels 2018, 4, 58. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Roselletti, E.; Pericolini, E.; Nore, A.; Takacs, P.; Kozma, B.; Sala, A.; De Seta, F.; Usher, M.C.J.; Brown, G.D.; Wilson, D. Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci. Transl. Med. 2023, 15, eadi3363. [Google Scholar] [CrossRef] [PubMed]
- Shroff, S. Infectious Vaginitis, Cervicitis, and Pelvic Inflammatory Disease. Med. Clin. N. Am. 2023, 107, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, R.; Qing, W.; Zhang, Y.; Zhou, Z.; Hou, Y.; Shi, Y.; Zhou, H.; Chen, M. Probiotics are a good choice for the treatment of bacterial vaginosis: A meta-analysis of randomized controlled trial. Reprod. Health 2022, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tao, Z.; Edupuganti, L.; Serrano, M.G.; Buck, G.A. Roles of the Microbiota of the Female Reproductive Tract in Gynecological and Reproductive Health. Microbiol. Mol. Biol. Rev. 2022, 86, 181. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagepetova, A.G.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef]
- Rüther, L.; Voss, W. Hydrogel or ointment? Comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon 2021, 7, e06071. [Google Scholar] [CrossRef]
- Takacs, P.; Kozma, B.; Rátonyi, D.; Kozma, B.; Attila, K.; Fenyvesi, F.; Sipos, A.G. Development and Bioavailability Assessment of an Estriol-Containing Vaginal Hydrogel. Gels 2024, 10, 823. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef]
- Livne, S.; Simantov, S.; Rahmanov, A.; Jeffet, U.; Sterer, N. Hydroxyethyl Cellulose Promotes the Mucin Retention of Herbal Extracts Active against Streptococcus mutans. Materials 2022, 15, 4652. [Google Scholar] [CrossRef]
- Huynh, U.; Qiao, M.; King, J.; Trinh, B.; Valdez, J.; Haq, M.; Zastrow, M.L. Differential Effects of Transition Metals on Growth and Metal Uptake for Two Distinct Lactobacillus Species. Microbiol. Spectr. 2022, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Takacs, P.; Damjanovich, P.; Sipos, A.G.; Kozma, B. The effect of oral zinc supplementation on cervicovaginal lavage fluid zinc level. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Erekson, E.A.; Yip, S.O.; Wedderburn, T.S.; Martin, D.K.; Li, F.; Choi, J.N.; Kenton, K.S.; Fried, T.R. The Vulvovaginal Symptoms Questionnaire: A questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause 2013, 20, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Bachmann, G. Vulvovaginal complaints. Clin. Obstet. Gynecol. 2008, 51, 549–555. [Google Scholar] [CrossRef]
| (N = 37) | |
|---|---|
| Age (years, mean ± SD) | 37 ± 12 |
| Gravida (median, range) | 1 (0–4) |
| Parity (median, range) | 1 (0–3) |
| BMI (kg/m2, mean ± SD) | 26.6 ± 5.3 |
| Postmenopausal (n, %) | 5 (14) |
| Baseline | Week 4 | p-Value (Baseline vs. W4) | Week 8 | p-Value (Baseline vs. W8) | Week 12 | p-Value (Baseline vs. W12) | |
|---|---|---|---|---|---|---|---|
| Vaginal pH | 4.03 ± 0.42 | 3.91 ± 0.26 | 0.12 | 3.71 ± 0.22 | <0.01 | 3.71 ± 0.48 | <0.01 |
| Vaginal Health Index (VHI) | 20.78 ± 1.74 | 23.27 ± 2.09 | <0.01 | 23.88 ± 1.98 | <0.01 | 23.64 ± 2.59 | <0.01 |
| Vulvovaginal Symptom Questionnaire-21 (VSQ-21) | 10.78 ± 3.66 | 5.24 ± 4.78 | <0.01 | 3.00 ± 4.00 | <0.01 | 3.17 ± 4.16 | <0.01 |
| Baseline | Week 4 | p-Value (Baseline vs. W4) | Week 8 | p-Value (Baseline vs. W8) | Week 12 | p-Value (Baseline vs. W12) | |
|---|---|---|---|---|---|---|---|
| Cervicovaginal lavage zinc level (µg/L, mean ± SD) | 110 ± 102 | 62 ± 48 | <0.01 | 80 ± 55 | 0.04 | 99 ± 92 | NS |
| Parameter | Value |
|---|---|
| Pump speed (rpm) | 15 |
| Sample uptake time (sec) | 15 |
| Rinse time (sec) | 30 |
| Stabilization time (sec) | 20 |
| Nebulizer gas flow (L/min) | 0.70 |
| Number of repetitions | 3 |
| Wavelength (nm) | 213,857 |
| Observation Mode | axial |
| Reading Time (sec) | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rátonyi, D.; Kozma, B.; Sipos, A.G.; Krasznai, Z.T.; Kozma, B.; Takacs, P. Improving Vaginal Health with a Zinc-Containing Vaginal Hydrogel. Gels 2025, 11, 214. https://doi.org/10.3390/gels11030214
Rátonyi D, Kozma B, Sipos AG, Krasznai ZT, Kozma B, Takacs P. Improving Vaginal Health with a Zinc-Containing Vaginal Hydrogel. Gels. 2025; 11(3):214. https://doi.org/10.3390/gels11030214
Chicago/Turabian StyleRátonyi, Dávid, Barbara Kozma, Attila G. Sipos, Zoárd Tibor Krasznai, Bence Kozma, and Peter Takacs. 2025. "Improving Vaginal Health with a Zinc-Containing Vaginal Hydrogel" Gels 11, no. 3: 214. https://doi.org/10.3390/gels11030214
APA StyleRátonyi, D., Kozma, B., Sipos, A. G., Krasznai, Z. T., Kozma, B., & Takacs, P. (2025). Improving Vaginal Health with a Zinc-Containing Vaginal Hydrogel. Gels, 11(3), 214. https://doi.org/10.3390/gels11030214







