Synthesis and Characterization of a Superabsorbent Polymer Gel Using a Simultaneous Irradiation Technique on Corn Straw
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of the Structure and Morphology of the Sample
2.1.1. XRD Analysis
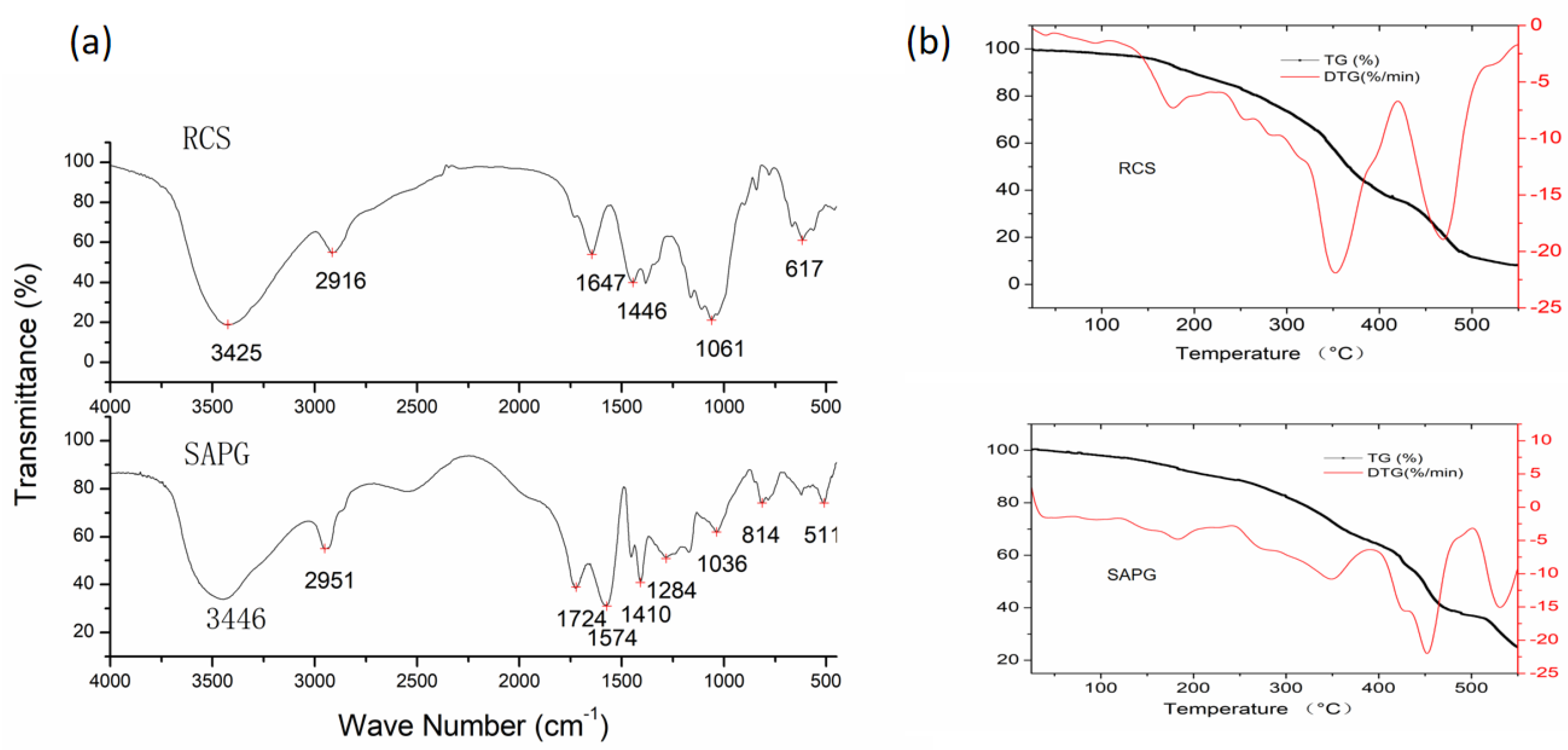
2.1.2. IR Analysis
2.1.3. Thermal Stability
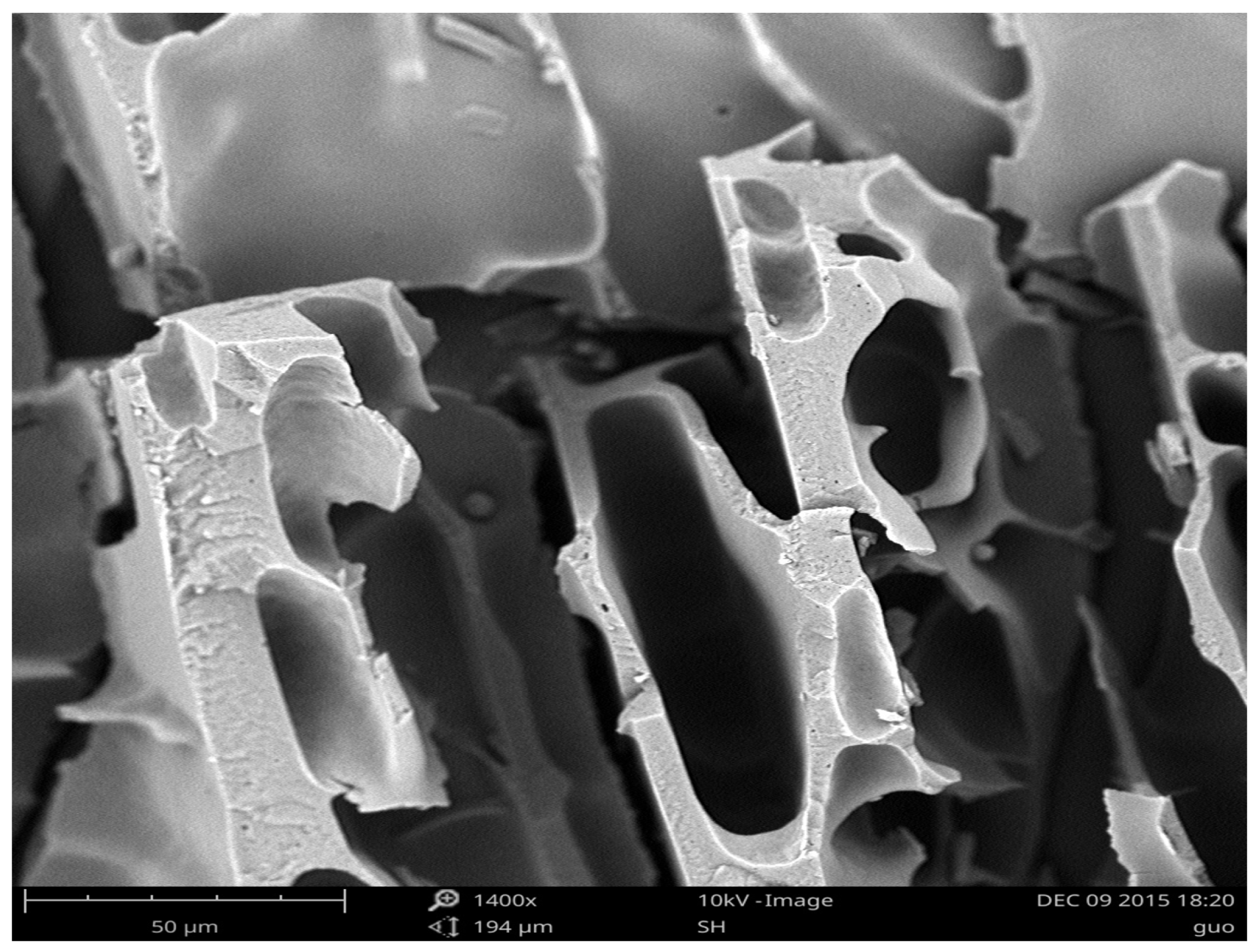
2.1.4. SEM Analysis
2.2. Single-Factor Experiment
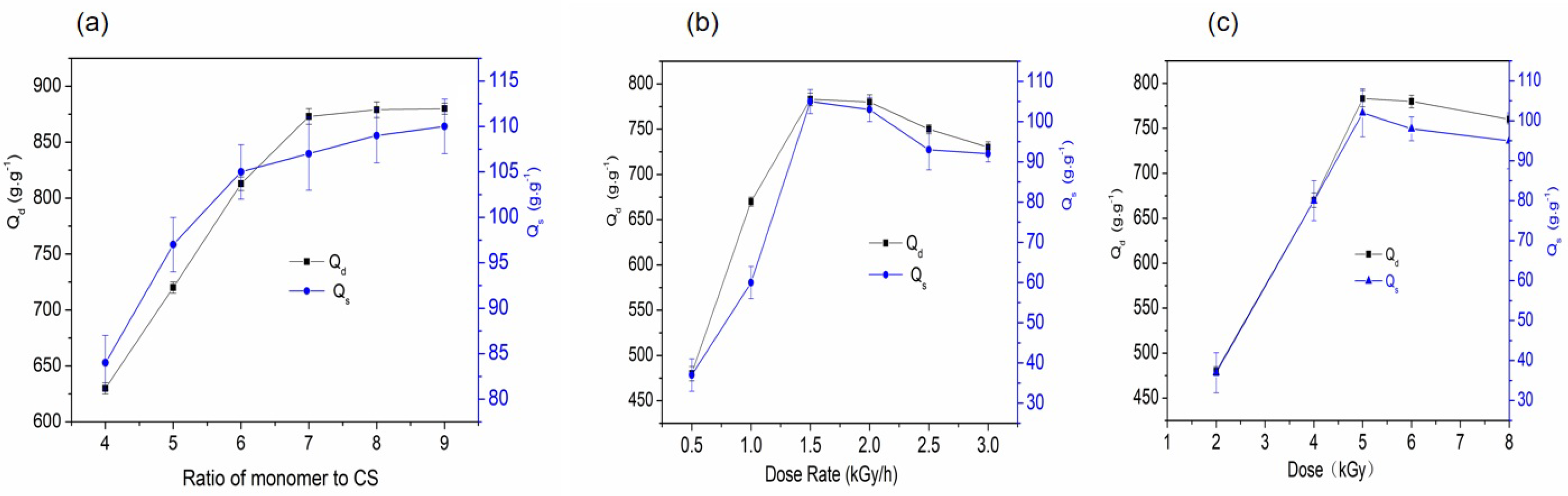
2.2.1. Effect of Monomer-to-RCS Ratio on the Water Absorbency of SAPG
2.2.2. Effect of Dose Rate on the Water Absorbency of SAPG
2.2.3. Effects of Radiation Dose on the Water Absorbency of SAPG
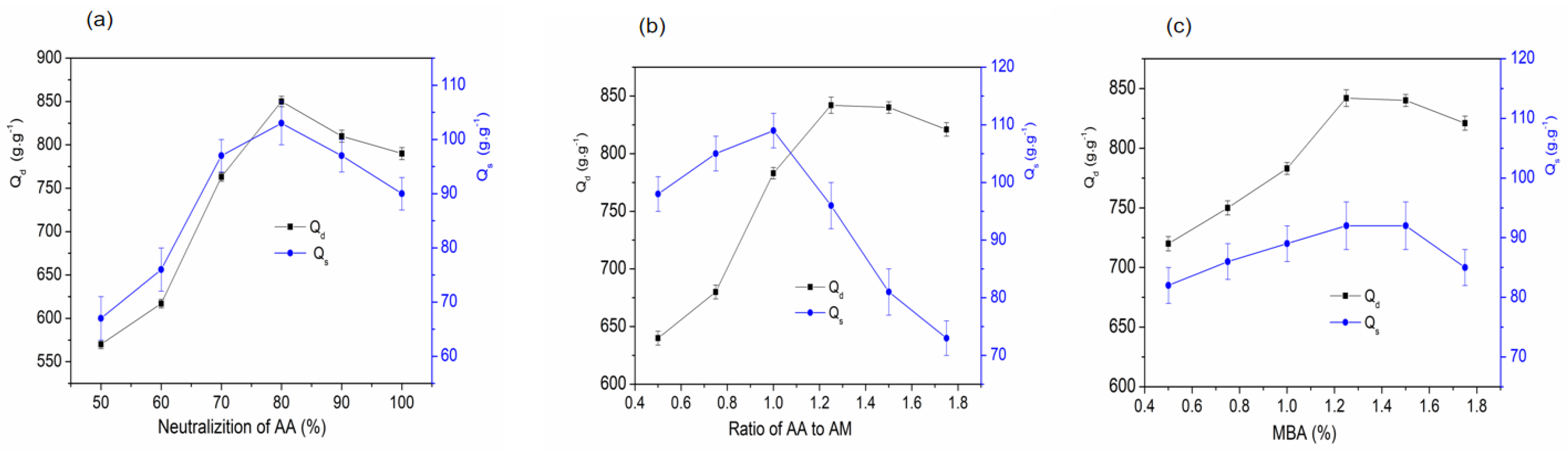
2.2.4. Effect of AA Neutralization on the Water Absorbency of SAPG
2.2.5. Effect of AA-to-Am Ratio on the Water Absorbency of SAPG
2.2.6. Effect of MBA on the Water Absorbency of SAPG
2.3. Optimization of the Reaction Parameters
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Pre-Treatment of CS
4.3. Creation of SAPG
4.4. Assessment of Water Absorption
4.5. Sample Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damiri, F.; Salave, S.; Vitore, J.; Bachra, Y.; Jadhav, R.; Kommineni, N.; Karouach, F.; Paiva-Santos, A.C.; Varma, R.S.; Berrada, M. Properties and valuable applications of superabsorbent polymers: A comprehensive review. Polym. Bull. 2024, 81, 6671–6701. [Google Scholar]
- Batara, B.; Steven, S.; Mulyana, M.; Saputra, A.S.; Hutahaean, A.C.; Yemensia, E.V.; Soekotjo, E.S.; Abidin, A.Z.; Graha, H.P.R. Recent Advances, Applications, and Challenges in Superabsorbent Polymers to Support Water Sustainability. J. Appl. Polym. Sci. 2024, 142, e56588. [Google Scholar]
- Guo, J.; Huang, J.; Liu, T.; Chen, J.; Janaswamy, S. A novel superabsorbent material based on soybean straw: Synthesis and characterization. Sci. Eng. Compos. Mater. 2022, 29, 65–73. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Zhao, X. Synthesis and characterization of agricultural controllable humic acid superabsorbent. J. Environ. Sci. 2013, 25, S69–S76. [Google Scholar]
- Liu, T.; Wang, Y.; Li, B.; Deng, H.; Huang, Z.; Qian, L.; Wang, X. Urea free synthesis of chitin-based acrylate superabsorbent polymers under homogeneous conditions: Effects of the degree of deacetylation and the molecular weight. Carbohydr. Polym. 2017, 174, 464–473. [Google Scholar]
- Kiatkamjornwong, S.; Meechai, N. Enhancement of the grafting performance and of the water absorption of cassava starch graft copolymer by gamma radiation. Radiat. Phys. Chem. 1997, 49, 689–696. [Google Scholar]
- Kiatkamjornwong, S.; Chomsaksakul, W.; Sonsuk, M. Radiation modification of water absorption of cassava starch by acrylic acid/acrylamide. Radiat. Phys. Chem. 2000, 59, 413–427. [Google Scholar]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Liu, T.; Qian, L.; Li, B.; Li, J.; Zhu, K.; Deng, H.; Yang, X.; Wang, X. Homogeneous synthesis of chitin-based acrylate superabsorbents in NaOH/urea solution. Carbohydr. Polym. 2013, 94, 261–271. [Google Scholar] [CrossRef]
- Chelu, M. Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels 2024, 10, 636. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.-X.; Yu, J.-y.; Hsieh, Y.-L. Dissolution behaviour and solubility of cellulose in NaOH complex solution. Carbohydr. Polym. 2010, 81, 668–674. [Google Scholar] [CrossRef]
- Miranda, M.; Bica, C.; Nachtigall, S.; Rehman, N.; Rosa, S. Kinetical thermal degradation study of maize straw and soybean hull celluloses by simultaneous DSC–TGA and MDSC techniques. Thermochim. Acta 2013, 565, 65–71. [Google Scholar]
- Qin, X.; Lu, A.; Zhang, L. Effect of stirring conditions on cellulose dissolution in NaOH/urea aqueous solution at low temperature. J. Appl. Polym. Sci. 2012, 126, E470–E477. [Google Scholar] [CrossRef]
- Schuler, P.; Cormier, M.A.; Werner, R.A.; Buchmann, N.; Gessler, A.; Vitali, V.; Saurer, M.; Lehmann, M.M. A High-Temperature Water Vapor Equilibration Method to Determine Non-Exchangeable Hydrogen Isotope Ratios of Sugar, Starch and Cellulose; 0140-7791; Wiley Online Library: Hoboken, NJ, USA, 2022. [Google Scholar]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar]
- Cai, J.; Zhang, L.; Liu, S.; Liu, Y.; Xu, X.; Chen, X.; Chu, B.; Guo, X.; Xu, J.; Cheng, H. Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules 2008, 41, 9345–9351. [Google Scholar]
- Qin, X.; Lu, A.; Cai, J.; Zhang, L. Stability of inclusion complex formed by cellulose in NaOH/urea aqueous solution at low temperature. Carbohydr. Polym. 2013, 92, 1315–1320. [Google Scholar]
- Li, Q.; Ma, Z.; Yue, Q.; Gao, B.; Li, W.; Xu, X. Synthesis, characterization and swelling behavior of superabsorbent wheat straw graft copolymers. Bioresour. Technol. 2012, 118, 204–209. [Google Scholar] [CrossRef]
- Benamer, S.; Mahlous, M.; Tahtat, D.; Nacer-Khodja, A.; Arabi, M.; Lounici, H.; Mameri, N. Radiation synthesis of chitosan beads grafted with acrylic acid for metal ions sorption. Radiat. Phys. Chem. 2011, 80, 1391–1397. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Wang, Z.; Yin, G. Synthesis and characterization of a novel super-absorbent based on chemically modified pulverized wheat straw and acrylic acid. Carbohydr. Polym. 2009, 77, 131–135. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Deng, R.; Cui, Y. A new network composite material based on soy dreg modified with polyurethane prepolymer. Macromol. Mater. Eng. 2007, 292, 484–494. [Google Scholar]
- Mohammed, A.D.; Young, D.A.; Vosloo, H.C. Synthesis and study of superabsorbent properties of acryloylated starch ester grafted with acrylic acid. Starch-Stärke 2014, 66, 393–399. [Google Scholar]
- Wan, T.; Huang, R.; Zhao, Q.; Xiong, L.; Qin, L.; Tan, X.; Cai, G. Synthesis of wheat straw composite superabsorbent. J. Appl. Polym. Sci. 2013, 130, 3404–3410. [Google Scholar]
- Lv, X.; Song, W.; Ti, Y.; Qu, L.; Zhao, Z.; Zheng, H. Gamma radiation-induced grafting of acrylamide and dimethyl diallyl ammonium chloride onto starch. Carbohydr. Polym. 2013, 92, 388–393. [Google Scholar] [PubMed]
- Huacai, G.; Wan, P.; Dengke, L. Graft copolymerization of chitosan with acrylic acid under microwave irradiation and its water absorbency. Carbohydr. Polym. 2006, 66, 372–378. [Google Scholar]
- Chen, P.; Zhang, W.A.; Luo, W.; Fang, Y.E. Synthesis of superabsorbent polymers by irradiation and their applications in agriculture. J. Appl. Polym. Sci. 2004, 93, 1748–1755. [Google Scholar]
- Martínez-Barrera, G.; Martínez-López, A.; Vigueras-Santiago, E.; Martínez-López, M. Effects of gamma radiation on the physicochemical properties of polyester resin and its use in composite materials. In Textile Science and Clothing Technology (TSCT); Springer: Singapore, 2020; pp. 15–28. [Google Scholar]
- Wu, F.; Zhang, Y.; Liu, L.; Yao, J. Synthesis and characterization of a novel cellulose-g-poly (acrylic acid-co-acrylamide) superabsorbent composite based on flax yarn waste. Carbohydr. Polym. 2012, 87, 2519–2525. [Google Scholar]
- Fernando, T.; Ariadurai, S.; Disanayaka, C.; Kulathunge, S.; Aruggoda, A. Development of radiation grafted super absorbent polymers for agricultural applications. Energy Procedia 2017, 127, 163–177. [Google Scholar]
- Piroonpan, T.; Haema, K.; Hiangrat, K.; Sriroth, K.; Pasanphan, W. Sugarcane bagasse cellulose-PAA micro-composite super-water absorbent for sandy soil amendment: Exploring a complete set of studies from one-pot electron beam processing to pot-testing. Ind. Crops Prod. 2024, 221, 119219. [Google Scholar]
- Şen, M.; Hayrabolulu, H. Radiation synthesis and characterisation of the network structure of natural/synthetic double-network superabsorbent polymers. Radiat. Phys. Chem. 2012, 81, 1378–1382. [Google Scholar]
- Zanrè, E.; Dalla Valle, E.; D’Angelo, E.; Sensi, F.; Agostini, M.; Cimetta, E. Recent Advancements in Hydrogel Biomedical Research in Italy. Gels 2024, 10, 248. [Google Scholar] [CrossRef]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar]
- Hao, Y.; Qu, J.; Tan, L.; Liu, Z.; Wang, Y.; Lin, T.; Yang, H.; Peng, J.; Zhai, M. Synthesis and property of superabsorbent polymer based on cellulose grafted 2-acrylamido-2-methyl-1-propanesulfonic acid. Int. J. Biol. Macromol. 2023, 233, 123643. [Google Scholar]
- Yang, G.; Zhang, Y.; Wei, M.; Shao, H.; Hu, X. Influence of γ-ray radiation on the structure and properties of paper grade bamboo pulp. Carbohydr. Polym. 2010, 81, 114–119. [Google Scholar]
- Wang, Y.; Deng, Y. The kinetics of cellulose dissolution in sodium hydroxide solution at low temperatures. Biotechnol. Bioeng. 2009, 102, 1398–1405. [Google Scholar] [PubMed]

| A mm:mSD (g/g) | B Neutralization of AA (%) | C Dose (kGy) | D mAA:mAm (g/g) | Qd in the Distilled Water (g/g) | Qs in the Saline Solution (g/g) | |
|---|---|---|---|---|---|---|
| 1 | 1 (6:1) | 1 (70) | 1 (4) | 1 (0.8) | 701 ± 13 | 42 ± 3 |
| 2 | 1 | 2 (80) | 3 (6) | 2 (1.0) | 829 ± 10 | 81 ± 4 |
| 3 | 1 | 3 (90) | 2 (5) | 3 (1.2) | 977 ± 8 | 78 ± 2 |
| 4 | 2 (7:1) | 1 | 3 | 3 | 869 ± 6 | 62 ± 2 |
| 5 | 2 | 2 | 2 | 1 | 752 ± 13 | 82 ± 4 |
| 6 | 2 | 3 | 1 | 2 | 986 ± 19 | 71 ± 3 |
| 7 | 3 (8:1) | 1 | 2 | 2 | 934 ± 10 | 68 ± 2 |
| 8 | 3 | 2 | 1 | 3 | 814 ± 6 | 65 ± 6 |
| 9 | 3 | 3 | 3 | 1 | 736 ± 8 | 61 ± 2 |
| a | 834 ± 10 | 835 ± 10 | 834 ± 13 | 730 ± 11 | ||
| 869 ± 13 | 798 ± 10 | 888 ± 10 | 916 ± 11 | |||
| 828 ± 8 | 900 ± 12 | 811 ± 8 | 887 ± 7 | |||
| R b | 41 | 112 | 77 | 186 | ||
| c | 67 ± 3 | 57 ± 2 | 59 ± 5 | 62 ± 3 | ||
| 72 ± 3 | 76 ± 5 | 76 ± 3 | 73 ± 3 | |||
| 65 ± 3 | 70 ± 2 | 68 ± 3 | 68 ± 3 | |||
| d | 7 | 19 | 17 | 11 | ||
| Q | A2 | B3 | C2 | D2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Guo, J.; Wang, A.; Wang, Q.; Yang, Y.; Xu, M. Synthesis and Characterization of a Superabsorbent Polymer Gel Using a Simultaneous Irradiation Technique on Corn Straw. Gels 2025, 11, 244. https://doi.org/10.3390/gels11040244
Tao X, Guo J, Wang A, Wang Q, Yang Y, Xu M. Synthesis and Characterization of a Superabsorbent Polymer Gel Using a Simultaneous Irradiation Technique on Corn Straw. Gels. 2025; 11(4):244. https://doi.org/10.3390/gels11040244
Chicago/Turabian StyleTao, Xingkui, Jun Guo, Aihua Wang, Qiang Wang, Yang Yang, and Minwei Xu. 2025. "Synthesis and Characterization of a Superabsorbent Polymer Gel Using a Simultaneous Irradiation Technique on Corn Straw" Gels 11, no. 4: 244. https://doi.org/10.3390/gels11040244
APA StyleTao, X., Guo, J., Wang, A., Wang, Q., Yang, Y., & Xu, M. (2025). Synthesis and Characterization of a Superabsorbent Polymer Gel Using a Simultaneous Irradiation Technique on Corn Straw. Gels, 11(4), 244. https://doi.org/10.3390/gels11040244










