Origanum vulgare ssp. hirtum: From Plant to 3D-Printed Gummies with Antioxidant and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
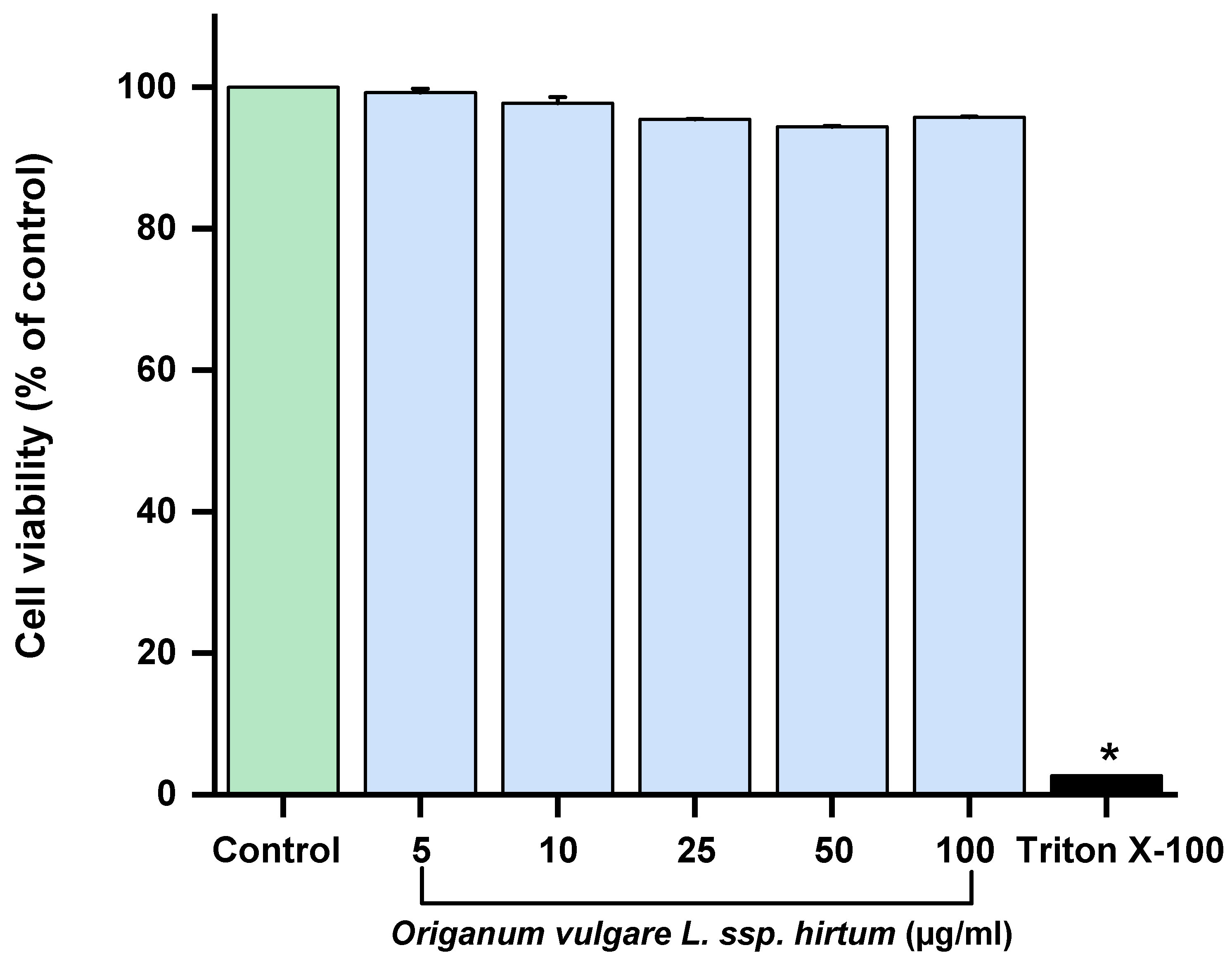
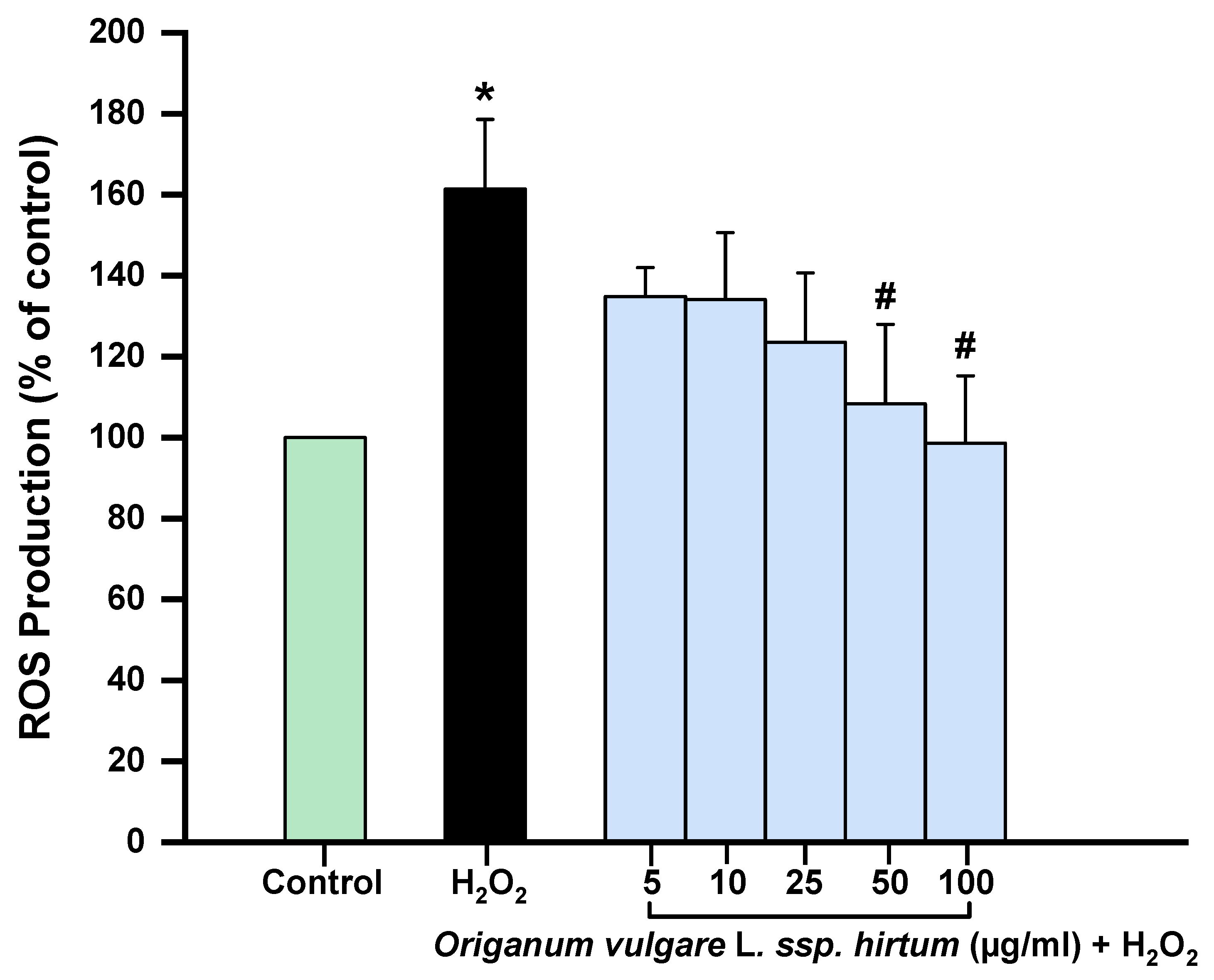
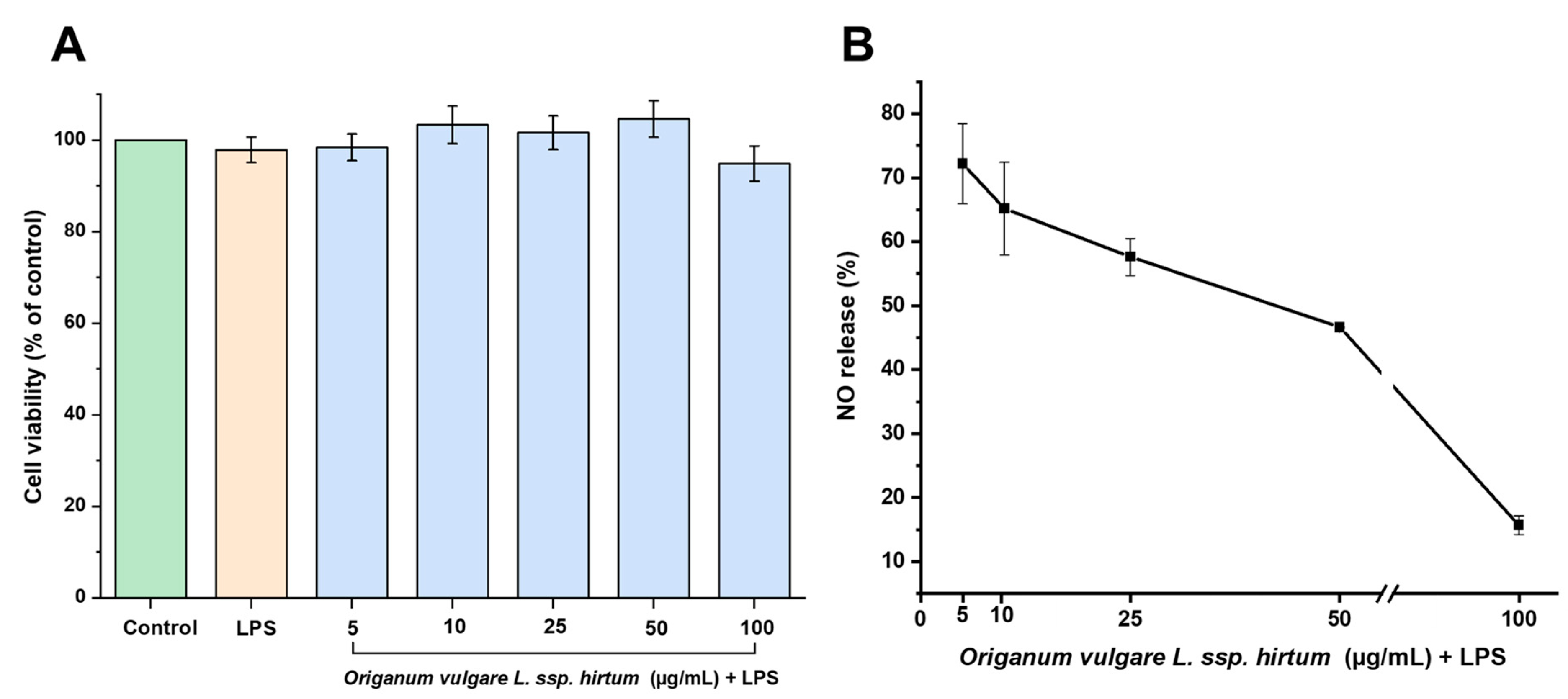
2.2. In Vitro Anti-Inflammatory and Antioxidant Activities
2.3. 3D-Printing Gummy of Origanum vulgare L. ssp. hirtum Extract and Characterization
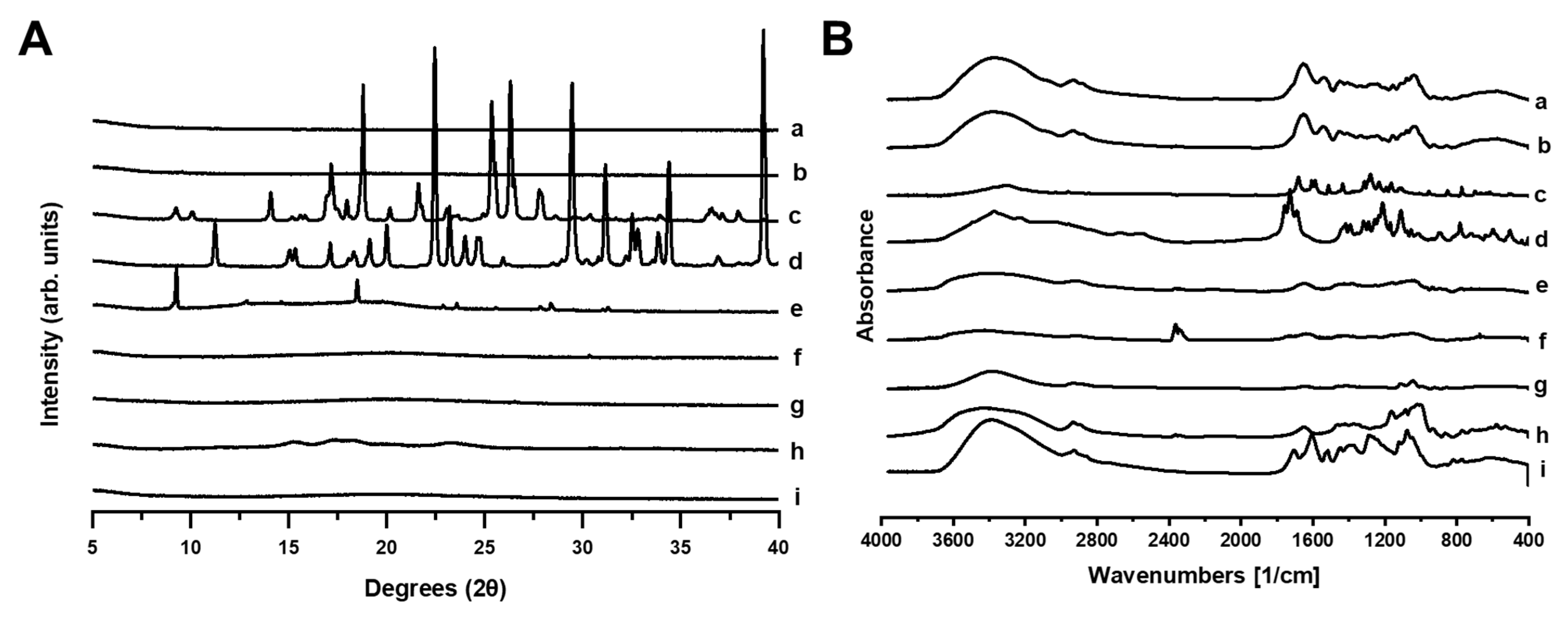
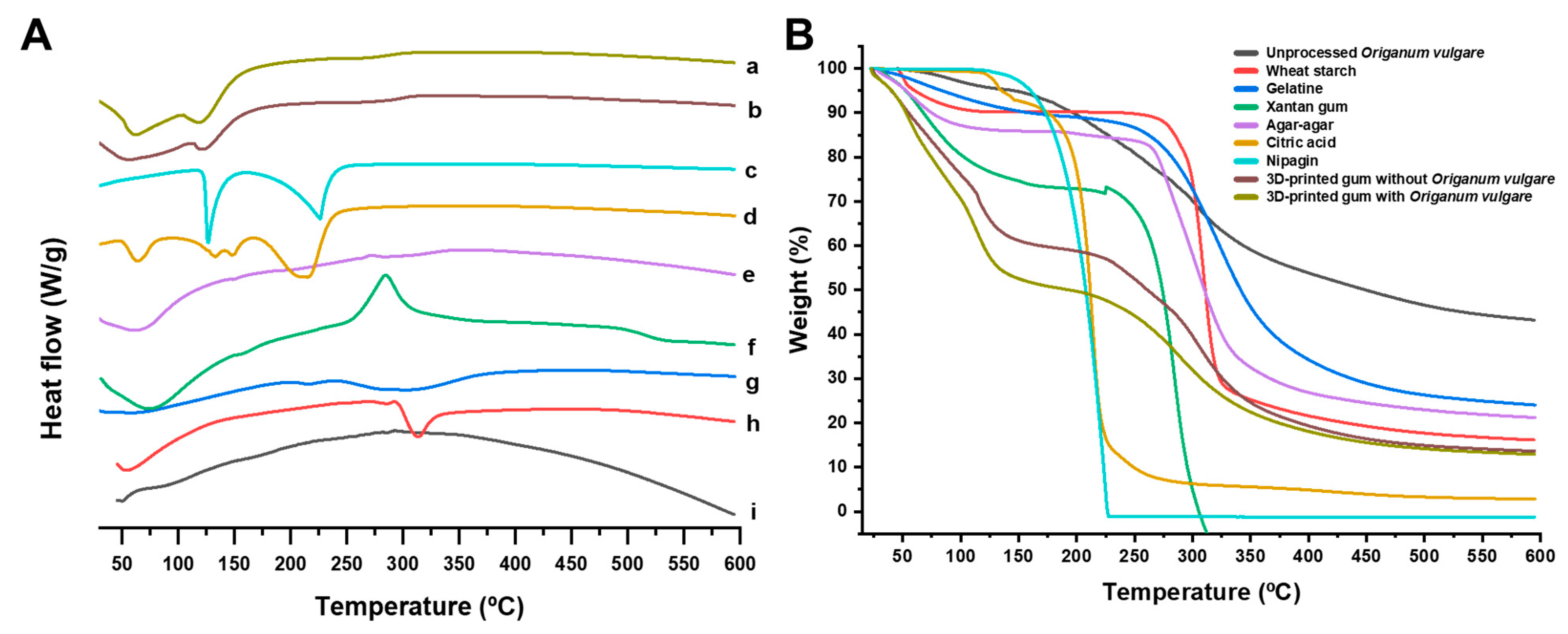
2.4. Physicochemical Characterization
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation of Plant Extracts
4.4. HPLC-PDA and HPLC-MS Analysis
4.5. In Vitro Anti-Inflammatory Activity
4.5.1. Xanthine Oxidase (XO) Inhibition
4.5.2. Lipoxygenase (LOX) Enzyme Inhibition
4.6. In Vitro Antioxidant Activity
4.6.1. Hydrogen Peroxide (H2O2) Scavenging
4.6.2. Hydroxyl Radical Scavenging
4.7. Cell Assays
4.7.1. Cell Culture
4.7.2. Cell Treatments
4.7.3. Cell Viability Assay
4.7.4. Intracellular Reactive Oxygen Species (ROS) Production
4.7.5. Nitric Oxide Determination
4.8. 3D-Printing Gummy Formulation with Origanum Vulgare
4.8.1. Composition of Gummy Formulation
4.8.2. 3D-Gummy Preparation
4.8.3. Rheology Analysis
4.8.4. Scanning Electron Microscopy
4.8.5. Mechanical Strength
4.8.6. Physicochemical Characterization
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arabacı, O.; Bayram, E.; Tan, U.; Sönmez, Ç. Determination of yield and quality properties of selected Istanbul oregano populations (Origanum vulgare subsp. hirtum (Link) Iestwaart). J. Agric. Fac. Uludag Univ. 2016, 30, 422–429. [Google Scholar]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanum vulgare) extract for food preservation and improvement in gastrointestinal health. Int. J. Nutr. 2019, 3, 43. [Google Scholar] [PubMed]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean diet: An update of the clinical trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Vanaclocha, B.V.; Folcara, S.C. Fitoterapia: Vademécum De Prescripción; Masson: Barcelona, Spain, 2003. [Google Scholar]
- Kadiasi, N.; Tako, R.; Ibraliu, A.; Stanys, V.; Gruda, N.S. Principal Component and Hierarchical Cluster Analysis of Major Compound Variation in Essential Oil among Some Red Oregano Genotypes in Albania. Agronomy 2024, 14, 1419. [Google Scholar] [CrossRef]
- Elezi, F.; Plaku, F.; Ibraliu, A.; Stefkov, G.; Karapandzova, M.; Kulevanova, S.; Aliu, S. Genetic variation of oregano (Origanum vulgare L.) for etheric oil in Albania. Agric. Sci. 2013, 4, 449–454. [Google Scholar]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar]
- Petrakis, E.A.; Mikropoulou, E.V.; Mitakou, S.; Halabalaki, M.; Kalpoutzakis, E. A GC–MS and LC–HRMS perspective on the chemotaxonomic investigation of the natural hybrid Origanum × lirium and its parents, O. vulgare subsp. hirtum and O. scabrum. Phytochem. Anal. 2023, 34, 289–300. [Google Scholar]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A review of the phytochemistry and antimicrobial properties of Origanum vulgare L. and subspecies. Iran. J. Pharm. Res. 2021, 20, 268. [Google Scholar]
- Capatina, L.; Napoli, E.M.; Ruberto, G.; Hritcu, L. Origanum vulgare ssp. hirtum (Lamiaceae) essential oil prevents behavioral and oxidative stress changes in the scopolamine zebrafish model. Molecules 2021, 26, 7085. [Google Scholar] [CrossRef]
- Kamenova, K.; Iliev, I.; Prancheva, A.; Tuleshkov, P.; Rusanov, K.; Atanassov, I.; Petrov, P.D. Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma. Gels 2024, 10, 627. [Google Scholar] [CrossRef]
- Grondona, E.; Gatti, G.; López, A.G.; Sánchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-efficacy of the essential oil of oregano (Origanum vulgare Lamiaceae. ssp. hirtum). Plant Foods Hum. Nutr. 2014, 69, 351–357. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart growing wild in Campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [PubMed]
- Karakaya, S.; El, S.N.; Karagözlü, N.; Şahin, S. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgare ssp. hirtum) by using different extraction methods. J. Med. Food 2011, 14, 645–652. [Google Scholar]
- Sarac, N.; Ugur, A. Antimicrobial activities of the essential oils of Origanum onites L., Origanum vulgare L. subspecies hirtum (Link) Ietswaart, Satureja thymbra L., and Thymus cilicicus Boiss. & Bal. growing wild in Turkey. J. Med. Food 2008, 11, 568–573. [Google Scholar]
- Shen, D.; Pan, M.-H.; Wu, Q.-L.; Park, C.-H.; Juliani, H.R.; Ho, C.-T.; Simon, J.E. LC-MS method for the simultaneous quantitation of the anti-inflammatory constituents in oregano (Origanum species). J. Agric. Food Chem. 2010, 58, 7119–7125. [Google Scholar] [CrossRef]
- Özer, Z.; Çarıkçı, S.; Yılmaz, H.; Kılıç, T.; Dirmenci, T.; Gören, A.C. Determination of secondary metabolites of Origanum vulgare subsp. hirtum and O. vulgare subsp. vulgare by LC-MS/MS. J. Chem. Metrol. 2020, 14, 25–34. [Google Scholar]
- Karaboduk, K.; Karabacak, O.; Karaboduk, H.; Tekinay, T. Chemical analysis and antimicrobial activities of the Origanum vulgare subsp. hirtum. J. Environ. Prot. Ecol. 2014, 15, 1283–1292. [Google Scholar]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic extract of Origanum vulgare ameliorates type 1 diabetes through antioxidant, anti-inflammatory and anti-apoptotic activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef]
- Hazrati, S.; Lotfi, K.; Govahi, M.; Ebadi, M.T. A comparative study: Influence of various drying methods on essential oil components and biological properties of Stachys lavandulifolia. Food Sci. Nutr. 2021, 9, 2612–2619. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O. 3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.C.; Anaya, B.J.; Sanz-Ruiz, P.; Ribed-Sánchez, A.; González-Burgos, E.; Serrano, D.R. Engineering 3D-printed advanced healthcare materials for periprosthetic joint infections. Antibiotics 2023, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Ongoren, B.; Yuste, I.; Anaya, B.J.; Luciano, F.C.; Sánchez-Guirales, S.A.; Kara, A.; Serrano, D.R. 3D printing in personalized medicine. In Fundamentals and Future Trends of 3D Printing in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 101–126. [Google Scholar]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive manufacturing strategies for personalized drug delivery systems and medical devices: Fused filament fabrication and semi solid extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.; Sepp, J.; Jakštas, V.; Žvikas, V.; Kireyev, I.; Karpun, Y.; Odyntsova, V.; Heinämäki, J.; Raal, A. German chamomile (Matricaria chamomilla L.) flower extract, its amino acid preparations and 3D-printed dosage forms: Phytochemical, pharmacological, technological, and molecular docking study. Int. J. Mol. Sci. 2024, 25, 8292. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, D.; Vu, A.A. Ginger and garlic extracts enhance osteogenesis in 3D printed calcium phosphate bone scaffolds with bimodal pore distribution. ACS Appl. Mater. Interfaces 2022, 14, 12964–12975. [Google Scholar]
- Li, J.; Hu, S.; Feng, P.; Xia, Y.; Pei, Z.; Tian, J.; Jiang, K.; Liu, L.; Cai, X.; Wu, P. Brucine Sulfate, a Novel Bacteriostatic Agent in 3D Printed Bone Scaffold Systems. Polymers 2024, 16, 1428. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Song, A.; Wang, H.; Wu, Y.; Chang, W.; Tian, B.; Xu, J.; Dai, H.; Ma, Q. A printable hydrogel loaded with medicinal plant extract for promoting wound healing. Adv. Healthc. Mater. 2024, 13, 2303017. [Google Scholar]
- Paczkowska-Walendowska, M.; Koumentakou, I.; Lazaridou, M.; Bikiaris, D.; Miklaszewski, A.; Plech, T.; Cielecka-Piontek, J. 3D-printed chitosan-based scaffolds with Scutellariae baicalensis extract for dental applications. Pharmaceutics 2024, 16, 359. [Google Scholar] [CrossRef]
- De Souza, A.; Amaral, G.O.; do Espirito Santo, G.; dos Santos Jorge Sousa, K.; Martignago, C.C.S.; Souza e Silva, L.C.; de Lima, L.E.; Vitor de Souza, D.; Cruz, M.A.; Ribeiro, D.A. 3D printed skin dressings manufactured with spongin-like collagen from marine sponges: Physicochemical properties and in vitro biological analysis. Biomed. Mater. 2025, 20, 025016. [Google Scholar]
- Wattanawiggan, R.; Chansakaow, S.; Jantrawut, P.; Panraksa, P.; Jiaranaikulwanitch, J.; Udomsom, S.; Worajittiphon, P.; Tipduangta, P. Design and Optimization of 3D-Printed Tablets Containing Mucuna Extracts for Erectile Dysfunction Management: A DoE-Guided Study. Plants 2024, 13, 2294. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Knez Hrnčič, M.; Cör, D.; Simonovska, J.; Knez, Ž.; Kavrakovski, Z.; Rafajlovska, V. Extraction techniques and analytical methods for characterization of active compounds in Origanum species. Molecules 2020, 25, 4735. [Google Scholar] [CrossRef] [PubMed]
- Koukoulitsa, C.; Karioti, A.; Bergonzi, M.C.; Pescitelli, G.; Di Bari, L.; Skaltsa, H. Polar constituents from the aerial parts of Origanum vulgare L. ssp. hirtum growing wild in Greece. J. Agric. Food Chem. 2006, 54, 5388–5392. [Google Scholar] [PubMed]
- Michalaki, A.; Karantonis, H.C.; Kritikou, A.S.; Thomaidis, N.S.; Dasenaki, M.E. Ultrasound-assisted extraction of total phenolic compounds and antioxidant activity evaluation from Oregano (Origanum vulgare ssp. hirtum) using response surface methodology and identification of specific phenolic compounds with HPLC-PDA and Q-TOF-MS/MS. Molecules 2023, 28, 2033. [Google Scholar]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar]
- Kühn, H.; O’Donnell, V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006, 45, 334–356. [Google Scholar] [PubMed]
- Kelley, E.E.; Khoo, N.K.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar]
- González-Gordo, S.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Soybean (Glycine max L.) lipoxygenase 1 (LOX 1) is modulated by nitric oxide and hydrogen sulfide: An in vitro approach. Int. J. Mol. Sci. 2023, 24, 8001. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase inhibition by plant extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Chai, T.-t.; Huang, Y.-n.; Ren, S.-t.; Jin, D.-l.; Fu, J.-j.; Guo, J.-y.; Chen, Y.-w. Inhibitory effects of ultrasonic and rosmarinic acid on lipid oxidation and lipoxygenase in large yellow croaker during cold storage. Ultrason. Sonochemistry 2023, 92, 106229. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Hadjipavlou–Litina, D.; Geromichalos, G.D.; Skaltsa, H. Inhibitory effect on soybean lipoxygenase and docking studies of some secondary metabolites, isolated from Origanum vulgare L. ssp. hirtum. J. Enzym. Inhib. Med. Chem. 2007, 22, 99–104. [Google Scholar] [CrossRef]
- Yuk, H.J.; Ryu, H.W.; Kim, D.-S. Potent xanthine oxidase inhibitory activity of constituents of Agastache rugosa (Fisch. and CA Mey.) Kuntze. Foods 2023, 12, 573. [Google Scholar]
- Celik, A.; Nur Herken, E.; Arslan, İ.; Zafer Özel, M.; Mercan, N. Screening of the constituents, antimicrobial and antioxidant activity of endemic Origanum hypericifolium O. Schwartz & PH Davis. Nat. Prod. Res. 2010, 24, 1568–1577. [Google Scholar] [PubMed]
- Fernando, C.D.; Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [PubMed]
- Mossa, A.; Nawwar, G. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum. Exp. Toxicol. 2011, 30, 1501–1513. [Google Scholar] [CrossRef]
- Hiebl, V.; Schachner, D.; Ladurner, A.; Heiss, E.H.; Stangl, H.; Dirsch, V.M. Caco-2 cells for measuring intestinal cholesterol transport-possibilities and limitations. Biol. Proced. Online 2020, 22, 7. [Google Scholar]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxidative Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, antibacterial, and cytotoxic activities of the ethanolic Origanum vulgare extract and its major constituents. Oxidative Med. Cell. Longev. 2016, 2016, 1404505. [Google Scholar]
- Kubiliene, A.; Munius, E.; Songailaite, G.; Kokyte, I.; Baranauskaite, J.; Liekis, A.; Sadauskiene, I. A Comparative Evaluation of Antioxidant Activity of Extract and Essential Oil of Origanum onites L. In Vivo. Molecules 2023, 28, 5302. [Google Scholar] [CrossRef] [PubMed]
- Bora, L.; Avram, S.; Pavel, I.Z.; Muntean, D.; Liga, S.; Buda, V.; Gurgus, D.; Danciu, C. An up-to-date review regarding cutaneous benefits of Origanum vulgare L. essential oil. Antibiotics 2022, 11, 549. [Google Scholar] [CrossRef]
- Parra, C.; Muñoz, P.; Bustos, L.; Parra, F.; Simirgiotis, M.J.; Escobar, H. UHPLC-DAD characterization of Origanum vulgare L. from Atacama desert Andean region and antioxidant, antibacterial and enzyme inhibition activities. Molecules 2021, 26, 2100. [Google Scholar] [CrossRef]
- Ganatra, P.; Jyothish, L.; Mahankal, V.; Sawant, T.; Dandekar, P.; Jain, R. Drug-loaded vegan gummies for personalized dosing of simethicone: A feasibility study of semi-solid extrusion-based 3D printing of pectin-based low-calorie drug gummies. Int. J. Pharm. 2024, 651, 123777. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Peeke, L.M.; Lu, T.; Han, A.; Hickner, M.A. Silicone Rheological Properties for Material Extrusion Additive Manufacturing. Appl. Res. 2025, 4, e202400203. [Google Scholar] [CrossRef]
- Puza, F.; Lienkamp, K. 3D printing of polymer hydrogels—From basic techniques to programmable actuation. Adv. Funct. Mater. 2022, 32, 2205345. [Google Scholar] [CrossRef]
- Santamaría, K.J.; Anaya, B.J.; Lalatsa, A.; González-Barranco, P.; Cantú-Cárdenas, L.; Serrano, D.R. Engineering 3D printed gummies loaded with metformin for paediatric use. Gels 2024, 10, 620. [Google Scholar] [CrossRef]
- Rouaz-El Hajoui, K.; Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Suné-Pou, M.; Nardi-Ricart, A.; Pérez-Lozano, P.; García-Montoya, E. Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration. Int. J. Pharm. 2023, 643, 123289. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, F.-B.; Li, Y.-C.; Shi, X.-D.; Yang, Y.-W.; Wang, M. Effect of peach gum polysaccharide on the rheological and 3D printing properties of gelatin-based functional gummy candy. Int. J. Biol. Macromol. 2023, 253, 127186. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, H.; Sampath, R.R.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Polyphenolic gelatin-based bioadhesives. Acc. Mater. Res. 2023, 4, 627–640. [Google Scholar]
- Huang, Z.; Delparastan, P.; Burch, P.; Cheng, J.; Cao, Y.; Messersmith, P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 2018, 6, 2487–2495. [Google Scholar] [CrossRef]
- Giaconia, M.A.; dos Passos Ramos, S.; Pereira, C.F.; Lemes, A.C.; De Rosso, V.V.; Braga, A.R.C. Overcoming restrictions of bioactive compounds biological effects in food using nanometer-sized structures. Food Hydrocoll. 2020, 107, 105939. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Karboune, S. Combinatorial interactions of essential oils enriched with individual polyphenols, polyphenol mixes, and plant extracts: Multi-antioxidant systems. Antioxidants 2023, 12, 486. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Souza, P.R.; Vilsinski, B.H.; Winkler, M.E.; Bruschi, M.L.; Radovanovic, E.; Muniz, E.C.; Caetano, W.; Valente, A.J.; Martins, A.F. Thermo-and pH-responsive gelatin/polyphenolic tannin/graphene oxide hydrogels for efficient methylene blue delivery. Molecules 2021, 26, 4529. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Zymone, K.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V.; Janulis, V. Phenological changes in triterpenic and phenolic composition of Thymus L. species. Ind. Crops Prod. 2017, 109, 445–451. [Google Scholar] [CrossRef]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.; Mehta, R.G.; Kinghorn, A.D.; Pezzuto, J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef]
- Perera, H.D.S.M.; Samarasekera, J.K.R.R.; Handunnetti, S.M.; Weerasena, O.V.D.S.J.; Weeratunga, H.D.; Jabeen, A.; Choudhary, M.I. In vitro pro-inflammatory enzyme inhibition and anti-oxidant potential of selected Sri Lankan medicinal plants. BMC Complement. Altern. Med. 2018, 18, 271. [Google Scholar]
- Chobot, V. Simultaneous detection of pro-and antioxidative effects in the variants of the deoxyribose degradation assay. J. Agric. Food Chem. 2010, 58, 2088–2094. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar]
- Anaya, B.J.; D’Angelo, D.; Bettini, R.; Molina, G.; Sanz-Perez, A.; Dea-Ayuela, M.A.; Galiana, C.; Rodríguez, C.; Tirado, D.F.; Lalatsa, A. Heparin-azithromycin microparticles show anti-inflammatory effects and inhibit SARS-CoV-2 and bacterial pathogens associated to lung infections. Carbohydr. Polym. 2025, 348, 122930. [Google Scholar] [CrossRef] [PubMed]
- Rapti, C.; Luciano, F.C.; Anaya, B.J.; Ramirez, B.I.; Ongoren, B.; Dea-Ayuela, M.A.; Lalatsa, A.; Serrano, D.R. Amphotericin B Ocular Films for Fungal Keratitis and a Novel 3D-Printed Microfluidic Ocular Lens Infection Model. J. Fungi 2024, 10, 762. [Google Scholar] [CrossRef]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D printed personalized polypills for the treatment of the metabolic syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef]


 , red color); (A,B2) 3D-printed gummy with Origanum vulgare L. ssp. hirtum extract (
, red color); (A,B2) 3D-printed gummy with Origanum vulgare L. ssp. hirtum extract ( , black color).
, black color).
 , red color); (A,B2) 3D-printed gummy with Origanum vulgare L. ssp. hirtum extract (
, red color); (A,B2) 3D-printed gummy with Origanum vulgare L. ssp. hirtum extract ( , black color).
, black color).

| Peak No. | Tentative Identity | Rt (min) | λ max | (M-H)− (m/z) | Other Ions− (m/z) | Amount, µg/g |
|---|---|---|---|---|---|---|
| 1 | Gallocatechin * | 10.29 | 280 | 305 | 289 | 20,544.5 ± 1027.2 |
| 2 | Procyanidin B1 * | 11.89 | 280 | 577 | 425–407–289 | 1348.5 ± 67.4 |
| 3 | Protocatechuic acid * | 14.03 | 219–259–293 | 153 | 108 | 1767.5 ± 88.4 |
| 4 | Chlorogenic acid * | 15.37 | 330 | 353 | 191 | 22.5 ± 1.1 |
| 5 | Caffeic acid * | 22.14 | 323 | 179 | 135 | 341.5 ± 17.1 |
| 6 | Luteolin 3,7-diglucoside * | 22.71 | 253–347 | 609 | 447–285–248 | 2546.5 ± 127.3 |
| 7 | Luteolin 7-rutinoside * | 23.42 | 254–347 | 593 | 285 | 167.0 ± 8.3 |
| 8 | Luteolin 7-glucoside * | 28.26 | 254–347 | 447 | 285 | 16,789.5 ± 439.5 |
| 9 | Luteolin 7-glucuronide * | 29.29 | 253–346 | 461 | 285–248 | 5811.0 ± 290.6 |
| 10 | Luteolin glycoside I ** | 29.63 | 253–346 | 579 | 285–417–248 | 1839.0 ± 91.9 |
| 11 | Apigenin 7-glucoside * | 33.79 | 266–332 | 431 | 269 | 826.0 ± 41.3 |
| 12 | Luteolin glycoside II ** | 34.84 | 253–346 | 417 | 285–248 | 6711.5 ± 335.6 |
| 13 | Luteolin glycoside III ** | 36.52 | 251–346 | 475 | 285–248 | 1774.5 ± 88.7 |
| 14 | Rosmarinic acid * | 38.72 | 329 | 359 | 161 | 60,241.0 ± 3012.1 |
| 15 | Apigenin glycoside ** | 40.05 | 266–337 | 635 | 269–117 | 3199.5 ± 159.9 |
| 16 | Luteolin * | 45.58 | 250–346 | 285 | 248 | 2133.5 ± 106.6 |
| 17 | Apigenin * | 49.76 | 266–335 | 269 | 149–117 | 71.5 ± 3.6 |
| Antioxidant Properties (SC50 µg/mL) | Anti-Inflammatory Properties (IC50 µg/mL) | ||
|---|---|---|---|
| Hydroxy Peroxide Scavenging | Xanthine Oxidase Inhibition | Lipoxygenase Inhibition | |
| Oregano extract | 99.2 ± 6.8 | >500 | 32.0 ± 2.8 |
| Reference compounds | |||
| Gallic acid | 5.6 ±0.46 | - | - |
| Allopurinol | - | 17.2 ± 0.8 | - |
| Naproxen | - | - | 3.4 ± 0.4 |
| Component | Concentration (% w/w) | Amount (mg) |
| Origanum vulgare ssp. hirtum extract | 20.4 | 250 |
| Gelatin | 49.0 | 600 |
| Starch | 16.3 | 200 |
| Agar-agar | 8.2 | 100 |
| Xanthan gum | 4.1 | 50 |
| Citric acid | 1.6 | 20 |
| Nipagin | 0.4 | 5 |
| Additional components | ||
| Lemon essential oil * | 3 drops | |
| Glycerin | 3 drops | |
| Sweetening agent * | 2 drops | |
| Purified water | 4 mL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya, B.J.; Raudone, L.; Ureña-Vacas, I.; Sanz-Perez, A.; Marksa, M.; Vilkickyte, G.; García-Rodríguez, J.J.; Serrano, D.R.; González-Burgos, E. Origanum vulgare ssp. hirtum: From Plant to 3D-Printed Gummies with Antioxidant and Anti-Inflammatory Properties. Gels 2025, 11, 246. https://doi.org/10.3390/gels11040246
Anaya BJ, Raudone L, Ureña-Vacas I, Sanz-Perez A, Marksa M, Vilkickyte G, García-Rodríguez JJ, Serrano DR, González-Burgos E. Origanum vulgare ssp. hirtum: From Plant to 3D-Printed Gummies with Antioxidant and Anti-Inflammatory Properties. Gels. 2025; 11(4):246. https://doi.org/10.3390/gels11040246
Chicago/Turabian StyleAnaya, Brayan J., Lina Raudone, Isabel Ureña-Vacas, Amadeo Sanz-Perez, Mindaugas Marksa, Gabriele Vilkickyte, Juan José García-Rodríguez, Dolores R. Serrano, and Elena González-Burgos. 2025. "Origanum vulgare ssp. hirtum: From Plant to 3D-Printed Gummies with Antioxidant and Anti-Inflammatory Properties" Gels 11, no. 4: 246. https://doi.org/10.3390/gels11040246
APA StyleAnaya, B. J., Raudone, L., Ureña-Vacas, I., Sanz-Perez, A., Marksa, M., Vilkickyte, G., García-Rodríguez, J. J., Serrano, D. R., & González-Burgos, E. (2025). Origanum vulgare ssp. hirtum: From Plant to 3D-Printed Gummies with Antioxidant and Anti-Inflammatory Properties. Gels, 11(4), 246. https://doi.org/10.3390/gels11040246










