Post-Stroke Recovery: A Review of Hydrogel-Based Phytochemical Delivery Systems
Abstract
1. Background
2. Current Stroke Treatment
3. Hydrogels in Stroke
3.1. Acute Applications
3.2. Long-Term Applications
4. Phytotherapy in Stroke
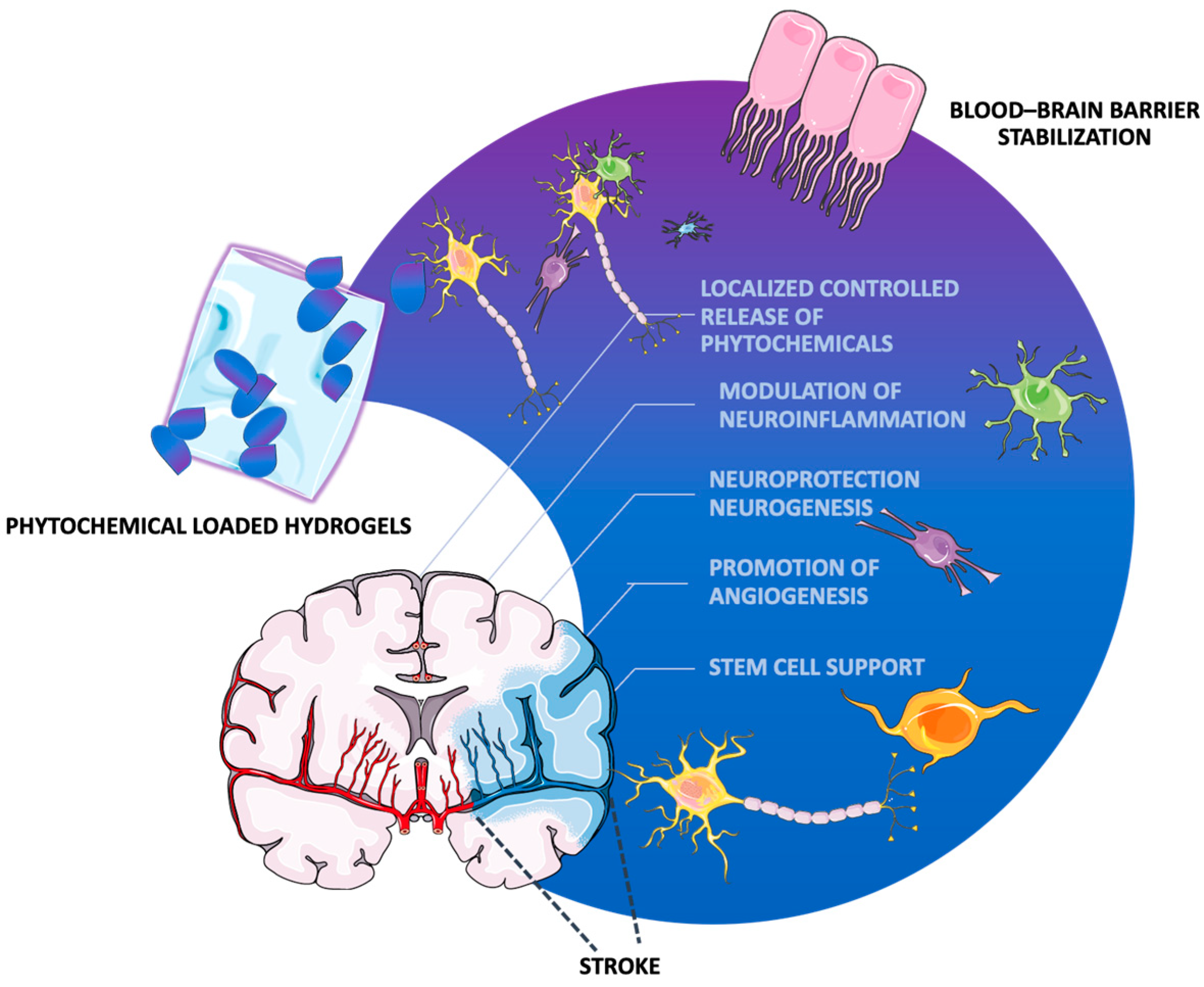
5. Hydrogel-Based Phytochemical Delivery Systems
5.1. Curcumin in Hydrogel-Based Delivery Systems
5.2. Tannic Acid in Hydrogel-Based Delivery Systems
5.3. Gallic Acid in Hydrogel-Based Delivery Systems
5.4. Ginsenosides in Hydrogel-Based Delivery Systems
5.5. Resveratrol in Hydrogel-Based Delivery Systems
5.6. Isorhamnetin in Hydrogel-Based Delivery Systems
Isorhamnetin Delivery Strategies for Stroke Recovery
6. Clinical Translatability
7. Current Trends in Hydrogel Therapy for Stroke Recovery
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- WHO EMRO. Stroke, Cerebrovascular Accident|Health Topics. Available online: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html (accessed on 21 December 2024).
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 502688. [Google Scholar] [CrossRef]
- Kim, Y.W. Update on Stroke Rehabilitation in Motor Impairment. Brain Neurorehabilit. 2022, 15, e12. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Ali, S.H.; Ahmad, S.A.; Islam, S.; Mohamad, K. Cognitive impairment and memory dysfunction after a stroke diagnosis: A post-stroke memory assessment. Neuropsychiatr. Dis. Treat. 2014, 10, 1677–1691. [Google Scholar] [CrossRef]
- Kim, J.S. Post-stroke Mood and Emotional Disturbances: Pharmacological Therapy Based on Mechanisms. J. Stroke 2016, 18, 244–255. [Google Scholar] [CrossRef]
- Towfighi, A.; Ovbiagele, B.; El Husseini, N.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, M.; Marshall, I.J.; DA Wolfe, C.; Wang, Y.; O’connell, M.D. Connell, Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Med. 2023, 20, e1004200. [Google Scholar] [CrossRef]
- Frank, D.; Gruenbaum, B.F.; Zlotnik, A.; Semyonov, M.; Frenkel, A.; Boyko, M. Pathophysiology and Current Drug Treatments for Post-Stroke Depression: A Review. Int. J. Mol. Sci. 2022, 23, 15114. [Google Scholar] [CrossRef]
- Manning, K.J.; Taylor, W.D. Poststroke Depression and Apathy: Why Should We Care? Am. J. Geriatr. Psychiatry 2020, 28, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Lillia, N.; Lax, A.; Crocamo, C.; Mantero, V.; Carrà, G.; Agostoni, E.; Clerici, M. Depression after Stroke and Risk of Mortality: A Systematic Review and Meta-Analysis. Stroke Res. Treat. 2013, 2013, 862978. [Google Scholar] [CrossRef]
- Chung, J.H.; Bin Kim, J.; Kim, J.H. Suicidal ideation and attempts in patients with stroke: A population-based study. J. Neurol. 2016, 263, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Butsing, N.; Zauszniewski, J.A.; Ruksakulpiwat, S.; Griffin, M.T.Q.; Niyomyart, A. Association between post-stroke depression and functional outcomes: A systematic review. PLoS ONE 2024, 19, e0309158. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, Z.; Xu, H.; Liu, Y.; Wang, X.; Yuan, L.; Xu, Y.; Zhu, Z.; Zhang, A.; Shao, A.; et al. Natural Products for the Treatment of Post-stroke Depression. Front. Pharmacol. 2022, 13, 918531. [Google Scholar] [CrossRef]
- Lestari, U.; Muhaimin, M.; Chaerunisaa, A.Y.; Sujarwo, W. Improved Solubility and Activity of Natural Product in Nanohydrogel. Pharmaceuticals 2023, 16, 1701. [Google Scholar] [CrossRef]
- Desai, S.M.; Jha, R.M.; Linfante, I. Collateral Circulation Augmentation and Neuroprotection as Adjuvant to Mechanical Thrombectomy in Acute Ischemic Stroke. Neurology 2021, 97, S178–S184. [Google Scholar] [CrossRef]
- Hollist, M.; Morgan, L.; Cabatbat, R.; Au, K.; Kirmani, M.F.; Kirmani, B.F. Acute Stroke Management: Overview and Recent Updates. Aging Dis. 2021, 12, 1000–1009. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, D.S.; Kim, M. Hemorrhagic Transformation After Ischemic Stroke: Mechanisms and Management. Front. Neurol. 2021, 12, 703258. [Google Scholar] [CrossRef]
- Sun, K.; Fan, J.; Han, J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm. Sin. B 2015, 5, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.O.; Rymer, M.M. Hemorrhagic Stroke: Aneurysmal Subarachnoid Hemorrhage. Mo. Med. 2011, 108, 124. [Google Scholar]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Perna, R.; Temple, J. Rehabilitation Outcomes: Ischemic versus Hemorrhagic Strokes. Behav. Neurol. 2015, 2015, 891651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Y.; Gao, S.; Fu, X.; Cao, Y.; Peng, Y.; Zhuang, J.; Hu, J.; Shao, A.; Wang, L. Potential Mechanisms and Perspectives in Ischemic Stroke Treatment Using Stem Cell Therapies. Front. Cell Dev. Biol. 2021, 9, 646927. [Google Scholar] [CrossRef]
- Tan, N.; Xin, W.; Huang, M.; Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: Novel insight into the crosstalk with immune cells. Front. Neurol. 2022, 13, 1048113. [Google Scholar] [CrossRef]
- Liu, H.; Reiter, S.; Zhou, X.; Chen, H.; Ou, Y.; Lenahan, C.; He, Y. Insight Into the Mechanisms and the Challenges on Stem Cell-Based Therapies for Cerebral Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 637210. [Google Scholar] [CrossRef]
- Savitz, S.I.; Chopp, M.; Deans, R.; Carmichael, S.T.; Phinney, D.; Wechsler, L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke 2011, 42, 825–829. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.-J.; Talifu, Z.; Liu, J.-Y.; Pan, Y.-Z.; Ke, H.; Zhang, C.-J.; Xu, X.; Yu, Y.; Du, L.-J. In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: A narrative review. Neural Regen. Res. 2023, 18, 750–755. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, T.; Jia, J.; Chen, Y.; Zhang, Y.; Fang, Z.; Zhang, C.; Bai, Y.; Li, Z.; Li, Y. Perspective insights into versatile hydrogels for stroke: From molecular mechanisms to functional applications. Biomed. Pharmacother. 2024, 173, 116309. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Alvarado-Velez, M.; Pai, S.B.; Bellamkonda, R.V. Hydrogels as Carriers for Stem Cell Transplantation. IEEE Trans. Biomed. Eng. 2014, 61, 1474–1481. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Rao, Z.; Deng, R.; Xu, Y.; Qiu, S.; Long, H.; Zhu, Q.; Liu, X.; Bai, Y.; et al. Facilitate Angiogenesis and Neurogenesis by Growth Factors Integrated Decellularized Matrix Hydrogel. Tissue Eng. Part A 2021, 27, 771–787. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Deka, D.J.; Rajak, P.; Laloo, D.; Das, T.; Chetia, P.; Saha, D.; Bharali, A.; Deka, B. Potential injectable hydrogels as biomaterials for central nervous system injury: A narrative review. Ibrain 2023, 9, 402–420. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, A.; Wu, J.; Wan, Y.; You, M.; Gu, X.; Guo, H.; Tan, S.; He, Q.; Hu, B. Nanomedicine: An Emerging Novel Therapeutic Strategy for Hemorrhagic Stroke. Int. J. Nanomed. 2022, 17, 1927–1950. [Google Scholar] [CrossRef]
- Neuron-Servier Medical Art. Available online: https://smart.servier.com/smart_image/neuron-ov/ (accessed on 13 March 2025).
- Das, N. Biodegradable Hydrogels for Controlled Drug Delivery. In Cellulose-Based Superabsorbent Hydrogels; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 1433–1472. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R: Rep. 2021, 146, 100641. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Rumon, M.H.; Rahman, S.; Akib, A.A.; Sohag, S.; Alam Rakib, R.; Khan, A.R.; Yesmin, F.; Shakil, S.; Khan, M.M.R. Progress in hydrogel toughening: Addressing structural and crosslinking challenges for biomedical applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Anwer, S.; Waris, A.; Gilani, S.O.; Iqbal, J.; Shaikh, N.; Pujari, A.N.; Niazi, I.K. Rehabilitation of Upper Limb Motor Impairment in Stroke: A Narrative Review on the Prevalence, Risk Factors, and Economic Statistics of Stroke and State of the Art Therapies. Healthcare 2022, 10, 190. [Google Scholar] [CrossRef]
- Gu, C.; Li, Y.; Liu, J.; Liu, S.; Long, J.; Zhang, Q.; Duan, W.; Feng, T.; Huang, J.; Qiu, Y.; et al. Neural stem cell-derived exosomes-loaded adhesive hydrogel controlled-release promotes cerebral angiogenesis and neurological function in ischemic stroke. Exp. Neurol. 2023, 370, 114547. [Google Scholar] [CrossRef]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; Llorente, I.L.; Chiu, A.S.; Machnicki, M.; I Zarembinski, T.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef]
- Makris, N.; Tsintou, M.; Dalamagkas, K.; Moore, T.L.; Rathi, Y.; Kubicki, M.; Rosene, D.L. The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: A testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches. Neural Regen. Res. 2021, 16, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Casolaro, M.; Casolaro, I. Polyelectrolyte Hydrogel Platforms for the Delivery of Antidepressant Drugs. Gels 2016, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, J.M.; Tuladhar, A.; Payne, S.L.; Ho, E.; Morshead, C.M.; Shoichet, M.S. Local Delivery of Brain-Derived Neurotrophic Factor Enables Behavioral Recovery and Tissue Repair in Stroke-Injured Rats. Tissue Eng. Part A 2019, 25, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, R.; Chen, H.; Zhang, X.; Zhang, Y.; Liu, H.; Li, C.; Chen, Y.; Zeng, Q.; Huang, G. Injectable Supramolecular Hybrid Hydrogel Delivers IL-1β-Stimulated Exosomes to Target Neuroinflammation. ACS Appl. Mater. Interfaces 2023, 15, 6486–6498. [Google Scholar] [CrossRef]
- Rotaru-Zăvăleanu, A.-D.; Dinescu, V.C.; Aldea, M.; Gresita, A. Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review. Gels 2024, 10, 476. [Google Scholar] [CrossRef]
- Saceleanu, V.M.; Toader, C.; Ples, H.; Covache-Busuioc, R.-A.; Costin, H.P.; Bratu, B.-G.; Dumitrascu, D.-I.; Bordeianu, A.; Corlatescu, A.D.; Ciurea, A.V. Integrative Approaches in Acute Ischemic Stroke: From Symptom Recognition to Future Innovations. Biomedicines 2023, 11, 2617. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; A Lane, D.; Lenarczyk, R.; Boriani, G.; Doehner, W.; A Benjamin, L.; Fisher, M.; Lowe, D.; Sacco, R.L.; Schnabel, R.; et al. Integrated care for optimizing the management of stroke and associated heart disease: A position paper of the European Society of Cardiology Council on Stroke. Eur. Hear. J. 2022, 43, 2442–2460. [Google Scholar] [CrossRef]
- Xu, H.; Wang, E.; Chen, F.; Xiao, J.; Wang, M. Neuroprotective Phytochemicals in Experimental Ischemic Stroke: Mechanisms and Potential Clinical Applications. Oxidative Med. Cell. Longev. 2021, 2021, 6687386. [Google Scholar] [CrossRef]
- Hung, W.; Ho, C.; Pan, M. Targeting the NLRP3 Inflammasome in Neuroinflammation: Health Promoting Effects of Dietary Phytochemicals in Neurological Disorders. Mol. Nutr. Food Res. 2020, 64, e1900550. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.; Li, X.; Chen, Y.; Mu, F.; Wen, A.; Liu, M.; Ding, Y. Advances of phytotherapy in ischemic stroke targeting PI3K/Akt signaling. Phytother. Res. 2023, 37, 5509–5528. [Google Scholar] [CrossRef]
- Chen, H.-S.; Qi, S.-H.; Shen, J.-G. One-Compound-Multi-Target: Combination Prospect of Natural Compounds with Thrombolytic Therapy in Acute Ischemic Stroke. Curr. Neuropharmacol. 2017, 15, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, H.; Lu, D.; Yuan, J.; Ma, R.; Li, J.; Ren, M.; Li, Y.; Chen, H.; Wang, J.; et al. Neuroprotective Effect for Cerebral Ischemia by Natural Products: A Review. Front. Pharmacol. 2021, 12, 607412. [Google Scholar] [CrossRef]
- Unalan, I.; Schruefer, S.; Schubert, D.W.; Boccaccini, A.R. 3D-Printed Multifunctional Hydrogels with Phytotherapeutic Properties: Development of Essential Oil-Incorporated ALG-XAN Hydrogels for Wound Healing Applications. ACS Biomater. Sci. Eng. 2023, 9, 4149–4167. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.-Y.; Lai, H.-Y.; Rao, N.K.; Ng, S.-F. Treatment for diabetic ulcer wounds using a fern tannin optimized hydrogel formulation with antibacterial and antioxidative properties. J. Ethnopharmacol. 2016, 189, 277–289. [Google Scholar] [CrossRef]
- Martínez-Higuera, A.; Rodríguez-Beas, C.; Villalobos-Noriega, J.M.A.; Arizmendi-Grijalva, A.; Ochoa-Sánchez, C.; Larios-Rodríguez, E.; Martínez-Soto, J.M.; Rodríguez-León, E.; Ibarra-Zazueta, C.; Mora-Monroy, R.; et al. Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci. Rep. 2021, 11, 11312. [Google Scholar] [CrossRef]
- Alesa Gyles, D.; Pereira Júnior, A.D.; Diniz Castro, L.; Santa Brigida, A.; Nobre Lamarão, M.L.; Ramos Barbosa, W.L.; Carréra Silva Júnior, J.O.; Ribeiro-Costa, R.M. Polyacrylamide-Metilcellulose Hydrogels Containing Aloe barbadensis Extract as Dressing for Treatment of Chronic Cutaneous Skin Lesions. Polymers 2020, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Saillard, J.; Thierry, M.; Lepelletier, A.; Fronteau, C.; Huon, J.-F. Anticancer agents and phytotherapy: Interactions that are often unrecognized. J. Oncol. Pharm. Pr. 2020, 27, 322–328. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Giaginis, C.; Theocharis, S. The Role of Bitter Melon in Breast and Gynecological Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 8918. [Google Scholar] [CrossRef]
- Hoveizi, E.; Hushmandi, K. Comparison of effects of P-coumaric acid and coumarin on colorectal cancer cell line by inducing apoptosis and autophagy. Avicenna J. Phytomedicine 2024, 14, 470–484. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Liu, S.; Cao, Y.; Qu, M.; Zhang, Z.; Feng, L.; Ye, Z.; Xiao, M.; Hou, S.T.; Zheng, R.; Han, Z. Curcumin protects against stroke and increases levels of Notch intracellular domain. Neurol. Res. 2016, 38, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, Y.; Sun, H.; Chen, L.; Man, X. Curcumin inhibits endoplasmic reticulum stress induced by cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2017, 14, 4047–4052. [Google Scholar] [CrossRef]
- Xie, C.; Gu, A.; Cai, J.; Wu, Y.; Chen, R. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018, 8, e00921. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Y.; Huang, S.; Wen, S.; Zhang, W.; Liu, X.; Ji, Z.; Geng, X.; Ji, X.; Du, H.; et al. Curcumin Protects against Ischemic Stroke by Titrating Microglia/Macrophage Polarization. Front. Aging Neurosci. 2017, 9, 233. [Google Scholar] [CrossRef]
- Su, W.-J.; Li, J.-M.; Zhang, T.; Cao, Z.-Y.; Hu, T.; Zhong, S.-Y.; Xu, Z.-Y.; Gong, H.; Jiang, C.-L. Microglial NLRP3 inflammasome activation mediates diabetes-induced depression-like behavior via triggering neuroinflammation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 126, 110796. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Y.; Tuolhen, Y.; Wang, Y.; Tian, G.; Xi, J.; Feng, Z.; Su, W.; Ye, L.; Liu, Z. Curcumin loaded hydrogel with double ROS-scavenging effect regulates microglia polarization to promote poststroke rehabilitation. Mater. Today Bio 2024, 28, 101177. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Wen, H.; Chen, Y.; Yu, Q.; Shen, M.; Xie, J. Curcumin-Loaded pH-Sensitive Biopolymer Hydrogels: Fabrication, Characterization, and Release Properties. ACS Food Sci. Technol. 2022, 2, 512–520. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, X.; Yu, Z.; Deng, R.; Cui, R.; Zhou, J.; Long, H.; Hu, Y.; Quan, D.; Bai, Y. Sustained delivery of NT-3 and curcumin augments microenvironment modulation effects of decellularized spinal cord matrix hydrogel for spinal cord injury repair. Regen. Biomater. 2024, 11, rbae039. [Google Scholar] [CrossRef]
- Madech, P.; Sitthichai, N.; Phetdee, C.; Prangkio, P.; Punyodom, W.; Manokruang, K. Release kinetics and binding interactions of curcumin-encapsulated nanoparticles released from chitosan-graft-(methoxy poly(ethylene glycol)-block-polycaprolactone) injectable hydrogels. Polym. Int. 2023, 72, 693–703. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Zhan, W.; Wang, Y.; Sun, X.; Zhang, Y.; Liu, X.; Han, B.; Bai, Y.; Shen, L.; et al. Sustained Release of Neuroprotective Drugs Curcumin and Edaravone from Supramolecular Hydrogel for Ischemic Stroke Treatment. Adv. Funct. Mater. 2023, 33, 2303930. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Miłek, O.; Michalska-Sionkowska, M.; Zasada, L.; Twardowska, M.; Warżyńska, O.; Kleszczyński, K.; Osyczka, A.M. Novel Eco-Friendly Tannic Acid-Enriched Hydrogels-Preparation and Characterization for Biomedical Application. Materials 2020, 13, 4572. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Warżyńska, O.; Kaczmarek-Szczepańska, B.; Łukowicz, K.; Osyczka, A.M.; Walczak, M. Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid. Polymers 2021, 13, 3412. [Google Scholar] [CrossRef] [PubMed]
- Luduvico, K.P.; Spohr, L.; de Aguiar, M.S.S.; Teixeira, F.C.; Bona, N.P.; de Mello, J.E.; Spanevello, R.M.; Stefanello, F.M. LPS-induced impairment of Na+/K+-ATPase activity: Ameliorative effect of tannic acid in mice. Metab. Brain Dis. 2022, 37, 2133–2140. [Google Scholar] [CrossRef]
- Yu, G.; Dan, N.; Dan, W.; Chen, Y. Wearable Tissue Adhesive Ternary Hydrogel of N-(2-Hydroxyl) Propyl-3-trimethyl Ammonium Chitosan, Tannic Acid, and Polyacrylamide. Ind. Eng. Chem. Res. 2022, 61, 5502–5513. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Liu, W.; Zeng, X.; Song, X.; Yang, X.; Zhang, X.; Feng, J. Metal Ion/Tannic Acid Assembly as a Versatile Photothermal Platform in Engineering Multimodal Nanotheranostics for Advanced Applications. ACS Nano 2018, 12, 3917–3927. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-Bohlouli, P.; Podstawczyk, D.; Nie, L.; Shavandi, A. Tannic acid post-treatment of enzymatically crosslinked chitosan-alginate hydrogels for biomedical applications. Carbohydr. Polym. 2022, 295, 119844. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; Ran, Y.; Geng, H.; Gao, F.; Tian, G.; Feng, Z.; Xi, J.; Ye, L.; Su, W. Nanoarchitectonics of tannic acid based injectable hydrogel regulate the microglial phenotype to enhance neuroplasticity for poststroke rehabilitation. Biomater. Res. 2023, 27, 108. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Bhuia, S.; Rahaman, M.; Islam, T.; Bappi, M.H.; Sikder, I.; Hossain, K.N.; Akter, F.; Prottay, A.A.S.; Rokonuzzman; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Farbood, Y.; Sarkaki, A.; Hashemi, S.; Mansouri, M.T.; Dianat, M. The effects of gallic acid on pain and memory following transient global ischemia/reperfusion in Wistar rats. Avicenna J. Phytomedicine 2013, 3, 329–340. [Google Scholar]
- Sun, J.; Li, Y.-Z.; Ding, Y.-H.; Wang, J.; Geng, J.; Yang, H.; Ren, J.; Tang, J.-Y.; Gao, J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014, 1589, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, L.; Mao, Y. Gallic acid attenuates cerebral ischemia/re-perfusion-induced blood–brain barrier injury by modifying polarization of microglia. J. Immunotoxicol. 2022, 19, 17–26. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Di Lorenzo, A.; Sureda, A.; Khanjani, S.; Nabavi, S.M.; Daglia, M. Post-Stroke Depression Modulation and in Vivo Antioxidant Activity of Gallic Acid and Its Synthetic Derivatives in a Murine Model System. Nutrients 2016, 8, 248. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Deshmukh, R.; Shukla, V.P.; Khunt, D.; Prajapati, B.G.; Rashid, S.; Ali, N.; Elossaily, G.M.; Suryawanshi, V.K.; Kumar, A. Recent Advancements in Gallic Acid-Based Drug Delivery: Applications, Clinical Trials, and Future Directions. Pharmaceutics 2024, 16, 1202. [Google Scholar] [CrossRef]
- Shukla, S.; Singh, B.; Singh, A.; Singh, C. Emerging and advanced drug delivery systems for improved biopharmaceutical attributes of gallic acid: A review. Phytomedicine Plus 2022, 2, 100369. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Zhu, Z.; Sun, Y. Improved Neuroprotective Effects of Gallic Acid-Loaded Chitosan Nanoparticles Against Ischemic Stroke. Rejuvenation Res. 2020, 23, 284–292. [Google Scholar] [CrossRef]
- Weian, W.; Yunxin, Y.; Ziyan, W.; Qianzhou, J.; Lvhua, G. Gallic acid: Design of a pyrogallol-containing hydrogel and its biomedical applications. Biomater. Sci. 2024, 12, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Sun, M.; Wang, X.; Pei, D.; Lei, B.; Li, A. Engineering antioxidant poly (citrate-gallic acid)-Exosome hybrid hydrogel with microglia immunoregulation for Traumatic Brain Injury-post neuro-restoration. Compos. Part B Eng. 2022, 242, 110034. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, Y.; He, Y.; Chang, R.; Guo, S.; Ma, S.; Guan, F.; Yao, M. In situ forming and biocompatible hyaluronic acid hydrogel with reactive oxygen species-scavenging activity to improve traumatic brain injury repair by suppressing oxidative stress and neuroinflammation. Mater. Today Bio 2022, 15, 100278. [Google Scholar] [CrossRef]
- Wan, J.; Wu, L.; Liu, H.; Zhao, J.; Xie, T.; Li, X.; Huang, S.; Yu, F. Incorporation of Zinc–Strontium Phosphate into Gallic Acid–Gelatin Composite Hydrogel with Multiple Biological Functions for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 5057–5067. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Guo, Y.; Wang, X.; Song, J.; Yang, G.; Shen, L.; Wang, Y.; Zhao, X.; Guo, B.; Wang, W. Multiple crosslinked, self-healing, and shape-adaptable hydrogel laden with pain-relieving chitosan@borneol nanoparticles for infected burn wound healing. Theranostics 2025, 15, 1439–1455. [Google Scholar] [CrossRef]
- Kim, N.; Jayakodi, M.; Lee, S.; Choi, B.; Jang, W.; Lee, J.; Kim, H.H.; Waminal, N.E.; Lakshmanan, M.; van Nguyen, B.; et al. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol. J. 2018, 16, 1904–1917. [Google Scholar] [CrossRef]
- Sarhene, M.; Ni, J.Y.; Duncan, E.S.; Liu, Z.; Li, S.; Zhang, J.; Guo, R.; Gao, S.; Gao, X.; Fan, G. Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol. Res. 2021, 166, 105481. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H. Ginsenosides for the treatment of insulin resistance and diabetes: Therapeutic perspectives and mechanistic insights. J. Ginseng Res. 2024, 48, 276–285. [Google Scholar] [CrossRef]
- Yao, W.; Guan, Y. Ginsenosides in cancer: A focus on the regulation of cell metabolism. Biomed. Pharmacother. 2022, 156, 113756. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, N.; Yao, M.; Zhang, Y.; Yao, Z.; Feng, Y.; Liu, J.; Zhou, G. A Review of Neuroprotective Effects and Mechanisms of Ginsenosides From Panax Ginseng in Treating Ischemic Stroke. Front. Pharmacol. 2022, 13, 946752. [Google Scholar] [CrossRef]
- Xie, C.-L.; Wang, W.-W.; Xue, X.-D.; Zhang, S.-F.; Gan, J.; Liu, Z.-G. A systematic review and meta-analysis of Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci. Rep. 2015, 5, 7790. [Google Scholar] [CrossRef]
- Shang, W.; Zhao, X.; Yang, F.; Wang, D.; Lu, L.; Xu, Z.; Zhao, Z.; Cai, H.; Shen, J. Ginsenoside Rg1 Nanoparticles Induce Demethylation of H3K27me3 in VEGF-A and Jagged 1 Promoter Regions to Activate Angiogenesis After Ischemic Stroke. Int. J. Nanomed. 2022, 17, 5447–5468. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Li, Y.; Wang, Y.; Xu, Z.; Fu, H.; Zheng, G.-Q. Ginsenoside-Rb1 for Ischemic Stroke: A Systematic Review and Meta-analysis of Preclinical Evidence and Possible Mechanisms. Front. Pharmacol. 2020, 11, 285. [Google Scholar] [CrossRef]
- Zhou, A.-F.; Zhu, K.; Pu, P.-M.; Li, Z.-Y.; Zhang, Y.-Y.; Shu, B.; Cui, X.-J.; Yao, M.; Wang, Y.-J. Neuroprotective Effect and Possible Mechanisms of Ginsenoside-Rd for Cerebral Ischemia/Reperfusion Damage in Experimental Animal: A Meta-Analysis and Systematic Review. Oxidative Med. Cell. Longev. 2022, 2022, 7650438. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, S.-H. The effect of ginsenosides on depression in preclinical studies: A systematic review and meta-analysis. J. Ginseng Res. 2021, 45, 420–432. [Google Scholar] [CrossRef]
- Pan, W.; Xue, B.; Yang, C.; Miao, L.; Zhou, L.; Chen, Q.; Cai, Q.; Liu, Y.; Liu, D.; He, H.; et al. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 2018, 129, 272–282. [Google Scholar] [CrossRef]
- Sana, S.S.; Chandel, A.K.S.; Raorane, C.J.; Aly, M.A.S.; Kim, S.-C.; Raj, V.; Lee, S. Recent advances in nano and micro formulations of Ginsenoside to enhance their therapeutic efficacy. Phytomedicine 2024, 134, 156007. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Alam, J.; Kim, B.; Kang, C.-W.; Kim, J.-H. Ginsenoside-Rb1 prevents bone cartilage destruction through down-regulation of p-Akt, p-P38, and p-P65 signaling in rabbit. Phytomedicine 2022, 100, 154039. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Zhang, Y.-F.; Xie, L.; Rong, F.; Zhu, X.-Y.; Xie, J.; Zhou, H.; Xu, T. Progress in the treatment of drug-induced liver injury with natural products. Pharmacol. Res. 2022, 183, 106361. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, M.; Xu, Y.; Xiao, Q.; Wang, H.; Wu, C.; Li, F.; Peng, L. All-in-one smart dressing for simultaneous angiogenesis and neural regeneration. J. Nanobiotechnology 2023, 21, 38. [Google Scholar] [CrossRef]
- Peng, X.; Ding, C.; Zhao, Y.; Hao, M.; Liu, W.; Yang, M.; Xiao, F.; Zheng, Y. Poloxamer 407 and Hyaluronic Acid Thermosensitive Hydrogel-Encapsulated Ginsenoside Rg3 to Promote Skin Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 831007. [Google Scholar] [CrossRef]
- Xue, Y.; Fu, W.; Liu, Y.; Yu, P.; Sun, M.; Li, X.; Yu, X.; Sui, D. Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J. Food Sci. 2020, 85, 4039–4049. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, G.; Luo, L.; Li, L.; Gao, Y.; Tan, X.; Wang, S.; Chang, H.; Liu, Y.; Wei, Y.; et al. Ginsenoside Rg3 nanoparticles with permeation enhancing based chitosan derivatives were encapsulated with doxorubicin by thermosensitive hydrogel and anti-cancer evaluation of peritumoral hydrogel injection combined with PD-L1 antibody. Biomater. Res. 2022, 26, 77. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Huang, Y.; Hu, Z. Resveratrol has an Overall Neuroprotective Role in Ischemic Stroke: A Meta-Analysis in Rodents. Front. Pharmacol. 2021, 12, 795409. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. Resveratrol Neuroprotection in Stroke and Traumatic CNS injury. Neurochem. Int. 2015, 89, 75–82. [Google Scholar] [CrossRef]
- Ghazavi, H.; Shirzad, S.; Forouzanfar, F.; Negah, S.S.; Rad, M.R.; Vafaee, F. The role of resveratrol as a natural modulator in glia activation in experimental models of stroke. Avicenna J. Phytomedicine 2020, 10, 557–573. [Google Scholar]
- Owjfard, M.; Rahimian, Z.; Karimi, F.; Borhani-Haghighi, A.; Mallahzadeh, A. A comprehensive review on the neuroprotective potential of resveratrol in ischemic stroke. Heliyon 2024, 10, e34121. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Bariya, A.; Allam, A.; Othman, S.; Butani, S.B. In situ nanostructured hydrogel of resveratrol for brain targeting: In vitro-in vivo characterization. Drug Deliv. Transl. Res. 2018, 8, 1460–1470. [Google Scholar] [CrossRef]
- Conte, R.; De Luca, I.; Valentino, A.; Cerruti, P.; Pedram, P.; Cabrera-Barjas, G.; Moeini, A.; Calarco, A. Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. J. Funct. Biomater. 2023, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Wu, T.; Shi, K.; Bei, Z.; Wang, M.; Chu, B.; Xu, K.; Pan, M.; Li, Y.; et al. An Injectable Thermosensitive Hydrogel Containing Resveratrol and Dexamethasone-Loaded Carbonated Hydroxyapatite Microspheres for the Regeneration of Osteoporotic Bone Defects. Small Methods 2023, 8, e2300843. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Song, J.-Q.; Pan, S.-Y.; Wang, K. Treatment with Isorhamnetin Protects the Brain Against Ischemic Injury in Mice. Neurochem. Res. 2016, 41, 1939–1948. [Google Scholar] [CrossRef]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: Role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef]
- Ekici, M.; Güngör, H.; Mert, D. Kaempferol and Isorhamnetin alleviate Lipopolysaccharide-Induced Anxiety and Depression-Like Behavioral in Balb/C Mice. J. Hell. Veter-Med Soc. 2023, 74, 5749–5760. [Google Scholar] [CrossRef]
- Gammoh, O.; Qnais, E.Y.; Athamneh, R.Y.; Al-Jaidi, B.; Al-Tawalbeh, D.; Altaber, S.; Alqudah, A.; Aljabali, A.A.A.; Tambuwala, M.M. Unraveling the Potential of Isorhamnetin as an Adjuvant in Depression Treatment with Escitalopram. Curr. Issues Mol. Biol. 2023, 45, 7668–7679. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Yan, M.; He, P.; Chen, Z.; Dai, H. The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen–glucose deprivation. J. Neurochem. 2016, 136, 651–659. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, L.; Wang, Y.; Ding, J.; Wang, R. Isorhamnetin Alleviates High Glucose-Aggravated Inflammatory Response and Apoptosis in Oxygen-Glucose Deprivation and Reoxygenation-Induced HT22 Hippocampal Neurons Through Akt/SIRT1/Nrf2/HO-1 Signaling Pathway. Inflammation 2021, 44, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, C.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.J.; Song, J.K.; Shi, F.; Zhang, W.; Du, H.G.; Guo, C.C. Isorhamnetin prevents pc12 cell from rotenone-induced iiyury via pdk/akt/gsk-3p/creb signaling pathway. Chin. Pharmacol. Bull. 2020, 36, 272–276. [Google Scholar] [CrossRef]
- Xu, S.L.; Choi, R.C.Y.; Zhu, K.Y.; Leung, K.-W.; Guo, A.J.Y.; Bi, D.; Xu, H.; Lau, D.T.W.; Dong, T.T.X.; Tsim, K.W.K. Isorhamnetin, A Flavonol Aglycone from Ginkgo biloba L., Induces Neuronal Differentiation of Cultured PC12 Cells: Potentiating the Effect of Nerve Growth Factor. Evid. Based Complement. Altern. Med. 2012, 2012, 278273. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Rodríguez-Rodríguez, C.; Gutiérrez-Uribe, J.A.; Cepeda-Cañedo, E.; Serna-Saldívar, S.O. Bioaccessibility, Intestinal Permeability and Plasma Stability of Isorhamnetin Glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Y.; Zhang, Y.; Li, Y.; Ye, H.; Guo, D.; Lu, Q.; Cai, X. System to screen and purify active ingredients from herbal medicines using hydrogel-modified human umbilical vein endothelial cell membrane chromatography coupled with semi-preparative high-performance liquid chromatography-offline-high-performance liquid chromatography-mass spectrometry. J. Sep. Sci. 2023, 46, e2201010. [Google Scholar] [CrossRef]
- Doneda, E.; Bianchi, S.E.; Pittol, V.; Kreutz, T.; Scholl, J.N.; Ibañez, I.L.; Bracalente, C.; Durán, H.; Figueiró, F.; Klamt, F.; et al. 3-O-Methylquercetin from Achyrocline satureioides—Cytotoxic activity against A375-derived human melanoma cell lines and its incorporation into cyclodextrins-hydrogels for topical administration. Drug Deliv. Transl. Res. 2021, 11, 2151–2168. [Google Scholar] [CrossRef] [PubMed]
- Schwingel, L.C.; Bianchi, S.E.; Zorzi, G.K.; Gonçalves, P.; Teixeira, H.F.; Bassani, V.L. Quercetin and 3-O-methylquercetin in vitro skin layers permeation/retention from hydrogels: Why only a methoxy group difference determines different behaviors? J. Pharm. Pharmacol. 2019, 71, 733–745. [Google Scholar] [CrossRef]
- García-Valderrama, E.J.; Mamidi, N.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Del Angel-Sanchez, K.; Elías-Zúñiga, A. Engineering and Evaluation of Forcespun Gelatin Nanofibers as an Isorhamnetin Glycosides Delivery System. Pharmaceutics 2022, 14, 1116. [Google Scholar] [CrossRef]
- Mancera-Andrade, E.I.; Parsaeimehr, A.; Ruiz-Ruiz, F.; Rorrer, G.L.; González-Valdez, J.; Iqbal, H.M.; Parra-Saldivar, R. Isorhamnetin encapsulation into biogenic silica from Cyclotella sp. using a microfluidic device for drug delivery applications. Biocatal. Agric. Biotechnol. 2019, 19, 101175. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, J.; George, J.; Ye, H.; Cui, Z.; Li, Z.; Liu, Q.; Zhang, Y.; Ge, D.; Liu, Y. Study of neuroprotective function of Ginkgo biloba extract (EGb761) derived-flavonoid monomers using a three-dimensional stem cell-derived neural model. Biotechnol. Prog. 2016, 32, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Duan, J.; Xie, Y.; Lin, G.; Luo, H.; Li, G.; Yuan, X. Effects of solid dispersion and self-emulsifying formulations on the solubility, dissolution, permeability and pharmacokinetics of isorhamnetin, quercetin and kaempferol in total flavones of Hippophae rhamnoides L. Drug Dev. Ind. Pharm. 2013, 39, 1037–1045. [Google Scholar] [CrossRef]
- Rassu, G.; Vlčková, H.K.; Giunchedi, P.; Dias, P.; Cossu, M.; Pourová, J.; Harčárová, P.; Lomozová, Z.; Nováková, L.; Gavini, E.; et al. A water-soluble preparation for intravenous administration of isorhamnetin and its pharmacokinetics in rats. Chem. Interact. 2024, 396, 111064. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Silva, E.; Ali, O.; Mooney, D.; Bell, W.; Yu, S.J.; Kaneko, Y.; Borlongan, C. Injectable VEGF Hydrogels Produce Near Complete Neurological and Anatomical Protection following Cerebral Ischemia in Rats. Cell Transplant. 2010, 19, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Lv, W.; Chen, Y.; Liu, L.; Yin, J.; Li, S.; Min, Z.; Zhang, Q.; He, W.; Ma, K.; et al. Enhanced the treatment of ischemic stroke through intranasal temperature-sensitive hydrogels of edaravone and borneol inclusion complex. Int. J. Pharm. 2024, 651, 123748. [Google Scholar] [CrossRef]
- Ghuman, H.; Mauney, C.; Donnelly, J.; Massensini, A.R.; Badylak, S.F.; Modo, M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018, 80, 66–84. [Google Scholar] [CrossRef]
- Nih, L.R.; Sideris, E.; Carmichael, S.T.; Segura, T. Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater. 2017, 29, 1606471. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Shankarappa, S.A.; Rajanikant, G.K. Hydrogel Scaffolds: Towards Restitution of Ischemic Stroke-Injured Brain. Transl. Stroke Res. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nakaguchi, K.; Jinnou, H.; Kaneko, N.; Sawada, M.; Hikita, T.; Saitoh, S.; Tabata, Y.; Sawamoto, K. Growth Factors Released from Gelatin Hydrogel Microspheres Increase New Neurons in the Adult Mouse Brain. Stem Cells Int. 2012, 2012, 915160. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)–Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; Pereira, M.d.L.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef]
- Mușat, M.I.; Mitran, S.I.; Udriștoiu, I.; Albu, C.V.; Cătălin, B. The impact of stress on the behavior of C57BL/6 mice with liver injury: A comparative study. Front. Behav. Neurosci. 2024, 18, 1358964. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Das Pramanik, D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Cheng, L.; Lv, S.; Wei, C.; Li, S.; Liu, H.; Chen, Y.; Luo, Z.; Cui, H. Nature’s magic: How natural products work hand in hand with mitochondria to treat stroke. Front. Pharmacol. 2025, 15, 1434948. [Google Scholar] [CrossRef]
- Marín-Medina, D.S.; Arenas-Vargas, P.A.; Arias-Botero, J.C.; Gómez-Vásquez, M.; Jaramillo-López, M.F.; Gaspar-Toro, J.M. New approaches to recovery after stroke. Neurol. Sci. 2024, 45, 55–63. [Google Scholar] [CrossRef]
- Janas-Naze, A.; Zhang, W. Perioperative anaphylaxis to fibrin sealants in children with Noonan Syndrome. Ann. Allergy Asthma Immunol. 2022, 129, 95–100. [Google Scholar] [CrossRef]
- Rana, M.; Siegler, H.D.l.H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]

| Name of the Hydrogel | Method of Preparation | Summary of the Research Work (Novelty) | References |
|---|---|---|---|
| Curcumin-loaded hydrogel with ROS scavenging | Double ROS-scavenging hydrogel; likely involves dual-crosslinking mechanisms for sustained release and antioxidant activity. | Regulates microglia activity and enhances stroke rehabilitation through a sustained curcumin release mechanism. | Zhang et al., 2024 [80] |
| Curcumin-loaded pH-sensitive biopolymer hydrogels | pH-sensitive biopolymer hydrogel, responds to acidic microenvironments; likely ionic crosslinking for controlled release. | Designed to release curcumin in response to pH variations, ensuring targeted delivery in ischemic stroke models. | Yang et al., 2022 [81] |
| NT-3 and curcumin delivery via spinal cord matrix hydrogel | Decellularized spinal cord matrix hydrogel, mimicking ECM structure; physical crosslinking with embedded growth factors. | Supports neural regeneration and neuroprotection through a biomimetic scaffold incorporating NT-3 and curcumin. | Chen et al., 2024 [83] |
| Curcumin-encapsulated nanoparticles in injected hydrogels | Chitosan-based injectable hydrogels with PEG–PCL grafting; hydrophobic interactions for nanoparticle retention. | Enhances bioavailability and retention of curcumin nanoparticles within ischemic stroke regions. | Madech et al., 2023 [84] |
| Supramolecular hydrogel for curcumin and edaravone release in ischemic stroke | Supramolecular hydrogel, non-covalent interactions, providing dynamic, self-healing properties. | Facilitates controlled, long-term release of neuroprotective agents in ischemic stroke therapy. | Jia et al., 2023 [85] |
| Name of the Hydrogel | Method of Preparation | Summary of the Research Work (Novelty) | References |
|---|---|---|---|
| Tannic acid as a crosslinker for functional polymeric networks | Tannic acid used as a crosslinking agent; forms hydrogen bonds with polymers, enhancing mechanical strength. | Investigates the role of tannic acid in forming robust polymeric hydrogel networks with improved stability. | Chen et al., 2022 [87] |
| Tannic acid-enriched eco-friendly hydrogels | Tannic acid-crosslinked hydrogels; biocompatible and biodegradable for biomedical applications. | Develops sustainable, eco-friendly hydrogels for potential biomedical and wound-healing applications. | Kaczmarek et al., 2020 [88] |
| Collagen/beta-glucan hydrogels crosslinked with tannic acid | Hydrogen bonding and physical crosslinking between tannic acid and collagen, enhancing mechanical properties. | Enhances the bioactivity and structural integrity of collagen-based hydrogels for tissue engineering. | Michalska-Sionkowska et al., 2021 [89] |
| Wearable tissue adhesive hydrogel with tannic acid | Ternary hydrogel composed of tannic acid, chitosan, and polyacrylamide; provides adhesive and flexible properties. | Designed for wearable biomedical applications with strong adhesion and tissue compatibility. | Yu et al., 2022 [91] |
| pH-responsive tannic acid–agarose composite hydrogels | pH-responsive hydrogel; ionic crosslinking enhances antibacterial and anti-inflammatory effects. | Aims to develop responsive hydrogels for controlled drug delivery and antimicrobial applications. | Ninan et al., 2016 [92] |
| Tannic acid-based injectable hydrogel regulating microglial phenotype | Injectable hydrogel with nanoarchitectonic design; promotes neuroplasticity for post-stroke rehabilitation. | Investigates the neuroprotective effects of tannic acid-based injectable hydrogels in stroke recovery. | Liu et al., 2023 [95] |
| Name of the Hydrogel | Method of Preparation | Summary of the Research Work (Novelty) | References |
|---|---|---|---|
| Gallic acid-loaded chitosan nanoparticles for ischemic stroke | Chitosan nanoparticle hydrogel; promotes sustained release; electrostatic interactions for improved bioavailability. | Investigates the neuroprotective effects of gallic acid in a chitosan-based hydrogel system designed for ischemic stroke therapy. | Zhao et al., 2020 [104] |
| Pyrogallol-containing hydrogel with gallic acid | Pyrogallol-functionalized hydrogel; likely uses covalent bonding for structural integrity. | Enhances antioxidant activity and structural stability for biomedical applications. | Weian et al., 2024 [105] |
| Poly (citrate–gallic acid)–exosome hybrid hydrogel | Hybrid hydrogel combining polymer–exosome structures for enhanced neuro-restoration. | Develops a regenerative hydrogel system combining gallic acid derivatives with exosomes to improve neuroprotection and functional recovery. | Li et al., 2022 [106] |
| Name of the Hydrogel | Method of Preparation | Summary of the Research Work (Novelty) | References |
|---|---|---|---|
| Ginsenoside Rg1 nanoparticles activating angiogenesis | Nanoparticle-loaded hydrogel promoting epigenetic modifications and angiogenesis. | Investigates the role of Ginsenoside Rg1 in enhancing neurovascular remodeling and angiogenesis post-ischemic stroke. | Shang et al., 2022 [116] |
| Ginsenoside Rg3 in hyaluronic acid thermosensitive hydrogel | Thermosensitive hydrogel based on Poloxamer 407 and hyaluronic acid for skin wound healing. | Utilizes a biocompatible hydrogel for controlled release of Ginsenoside Rg3, accelerating wound-healing processes. | Peng et al., 2022 [125] |
| Ginsenoside Rg3 nanoparticles in thermosensitive hydrogel for cancer therapy | Chitosan derivative-based hydrogel with thermosensitive properties for localized drug delivery. | Develops a targeted drug delivery system for cancer therapy, ensuring sustained release of Ginsenoside Rg3 at tumor sites. | Wu et al., 2022 [127] |
| Name of the Hydrogel | Method of Preparation | Summary of the Research Work (Novelty) | References |
|---|---|---|---|
| Resveratrol nanostructured hydrogel for brain targeting | In situ nanostructured hydrogel, promotes brain-targeted delivery via nanoparticle encapsulation. | Enhances bioavailability and targeted delivery of resveratrol to the brain for neuroprotection and therapeutic effects. | Rajput et al., 2018 [135] |
| Hyaluronic acid hydrogel with resveratrol-loaded chitosan nanoparticles | Hyaluronic acid hydrogel for skin applications, combined with chitosan nanoparticles for enhanced permeability. | Develops a skin-friendly hydrogel system incorporating resveratrol for improved dermal penetration and antioxidant effects. | Conte et al., 2023 [136] |
| Composite hydrogel with resveratrol-laden nanoparticles for wound healing | Composite hydrogel integrating platelet-derived extracellular vesicles for regenerative purposes. | Combines bioactive extracellular vesicles with resveratrol for enhanced tissue regeneration and wound healing. | Zhu et al., 2022 [137] |
| Name of the Hydrogel | Method of Preparation | Summary of the Research Work (Novelty) | References |
|---|---|---|---|
| Hydrogel-modified chromatography for isolating active ingredients | Hydrogel-modified chromatography system, facilitates selective purification of bioactive compounds. | Enhances the efficiency of active ingredient isolation by integrating hydrogel-based modifications in chromatographic techniques. | Huang et al., 2023 [150] |
| Isorhamnetin glycoside (IRGs)-loaded gelatin (GL) nanofiber mats | Fabrication of gelatin nanofibers loaded with Opuntia ficus-indica flour (12% w/v) by forcespinning, using 25% and 30% (w/v) GL solutions, followed by glutaraldehyde crosslinking. | Development and characterization of IRG-loaded gelatin nanofibers with controlled release and cytocompatibility, providing a novel delivery system for phytochemicals with potential biomedical applications. | García-Valderrama et al., 2022 [153] |
| Isorhamnetin-loaded biogenic silica (frustules) from Cyclotella sp. | Encapsulation of isorhamnetin into diatom-derived silica frustules using a novel microfluidic device fabricated by ESCARGOT technology, optimizing drug concentration and residence time. | Development of a reproducible microfluidic-based method for loading hydrophobic drugs into biogenic silica carriers, demonstrating controlled isorhamnetin release and potential for standardized drug delivery systems. | Marcia-Andrade et al., 2019 [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, I.; Rotaru-Zavaleanu, A.D.; Sfredel, V.; Aldea, M.; Gresita, A.; Glavan, D.G. Post-Stroke Recovery: A Review of Hydrogel-Based Phytochemical Delivery Systems. Gels 2025, 11, 260. https://doi.org/10.3390/gels11040260
Musa I, Rotaru-Zavaleanu AD, Sfredel V, Aldea M, Gresita A, Glavan DG. Post-Stroke Recovery: A Review of Hydrogel-Based Phytochemical Delivery Systems. Gels. 2025; 11(4):260. https://doi.org/10.3390/gels11040260
Chicago/Turabian StyleMusa, Irina, Alexandra Daniela Rotaru-Zavaleanu, Veronica Sfredel, Madalina Aldea, Andrei Gresita, and Daniela Gabriela Glavan. 2025. "Post-Stroke Recovery: A Review of Hydrogel-Based Phytochemical Delivery Systems" Gels 11, no. 4: 260. https://doi.org/10.3390/gels11040260
APA StyleMusa, I., Rotaru-Zavaleanu, A. D., Sfredel, V., Aldea, M., Gresita, A., & Glavan, D. G. (2025). Post-Stroke Recovery: A Review of Hydrogel-Based Phytochemical Delivery Systems. Gels, 11(4), 260. https://doi.org/10.3390/gels11040260






