Formulation of Caffeine–Hydroxypropyl-β-Cyclodextrin Complex in Hydrogel for Skin Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Solubility Studies
2.2. DPPH and ABTS Assays
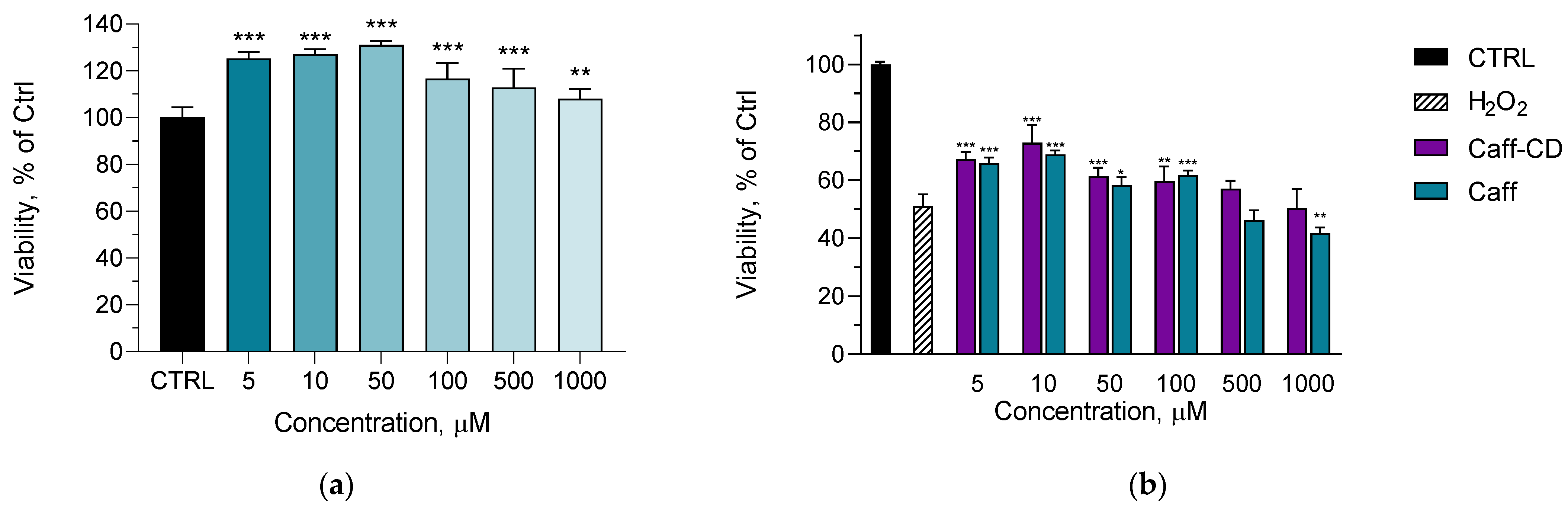
2.3. In Vitro Protective Effects Against H2O2-Induced Oxidative Stress
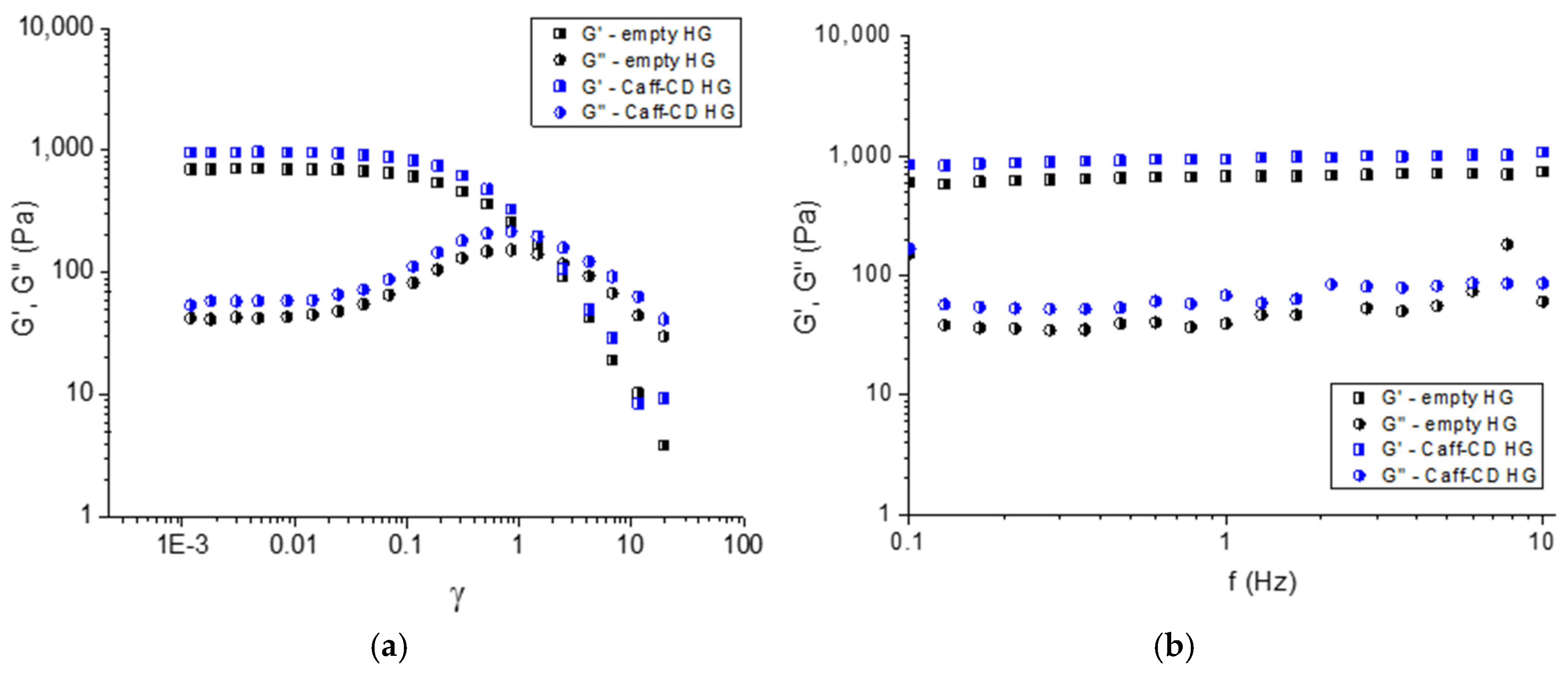
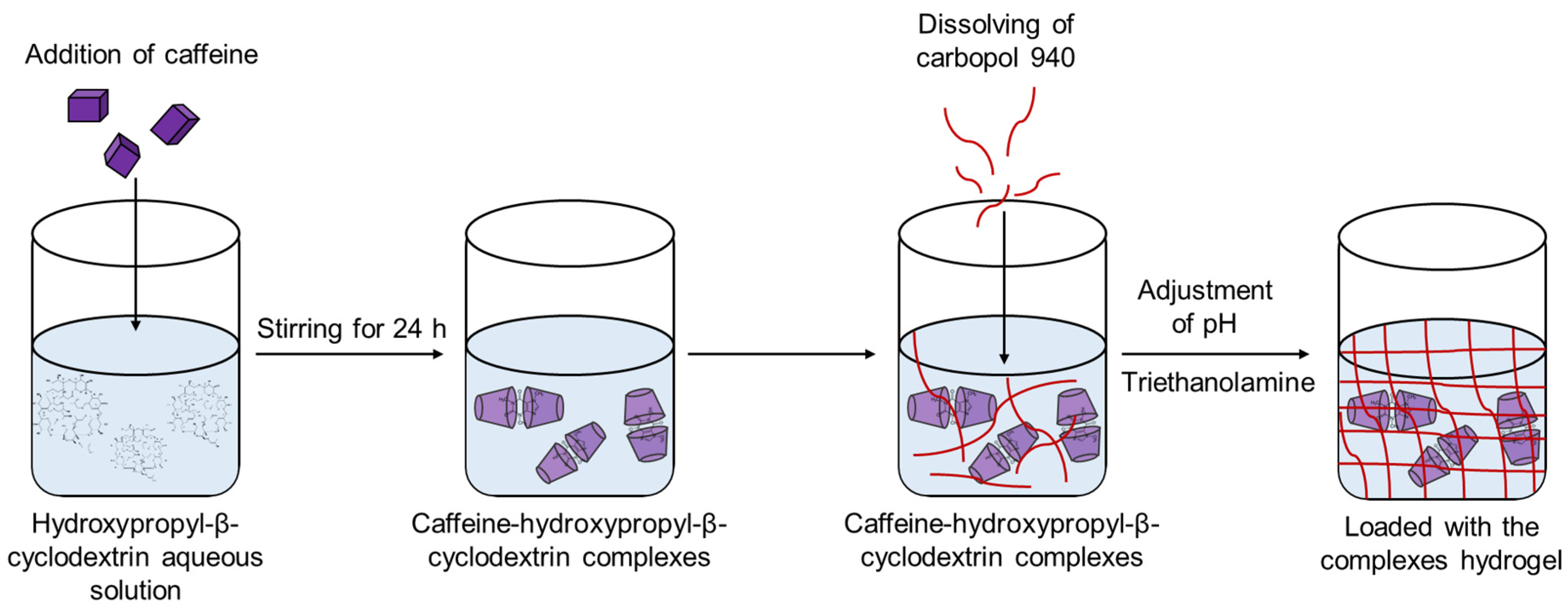
2.4. Incorporation of Caffeine Complex into Carbopol Hydrogel and Characterization of the Formulation
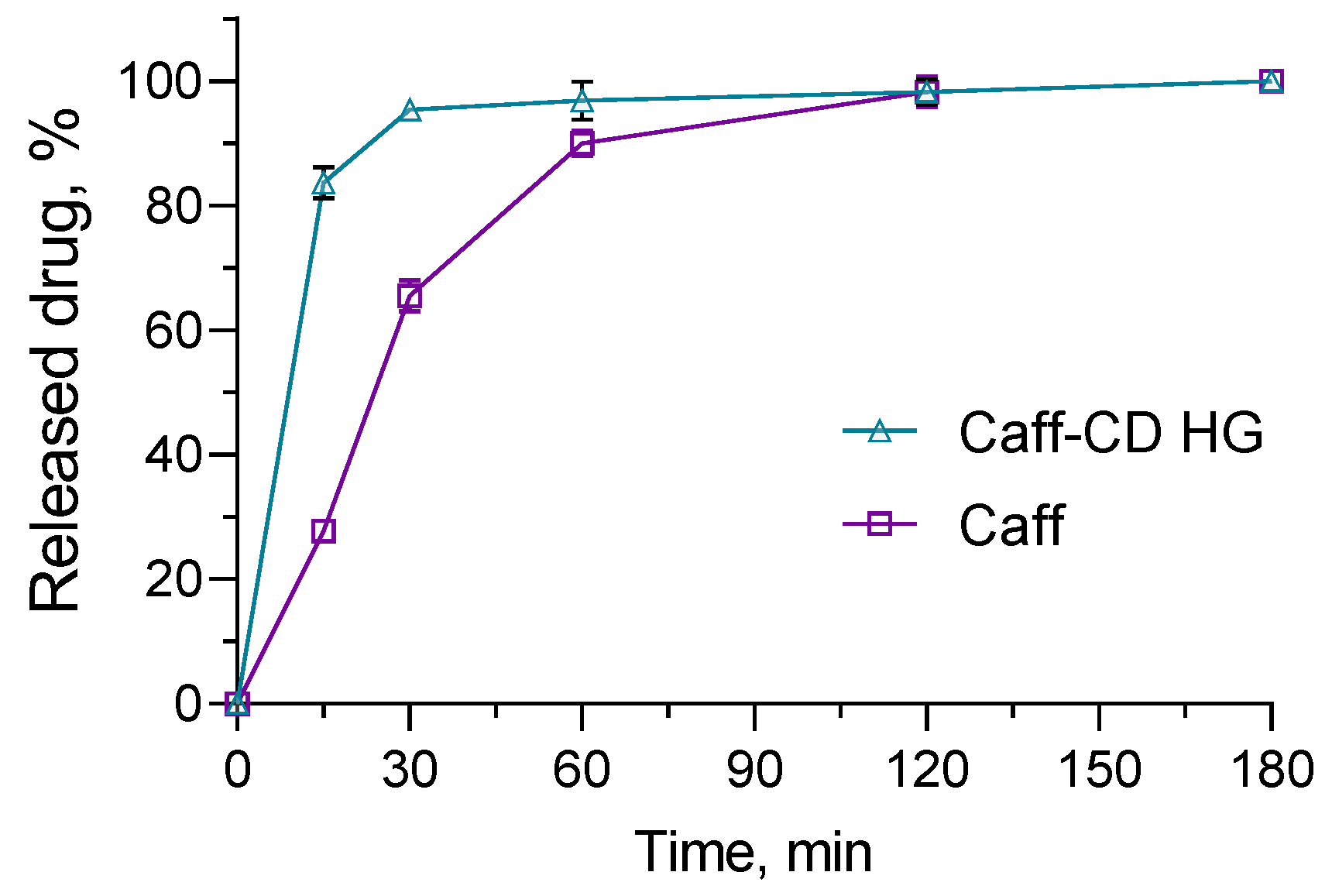
2.5. In Vitro Release Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Caffeine–Hydroxypropyl-β-Cyclodextrin Complex and Solubility Studies
4.3. DPPH and ABTS Assays
4.4. In Vitro Studies on Cells
4.5. Incorporation of Caffeine Complex in Carbopol Hydrogel
4.6. Characterization of the Hydrogel Formulation
4.7. In Vitro Dissolution Tests
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diffey, B. Climate Change, Ozone Depletion and the Impact on Ultraviolet Exposure of Human Skin. Phys. Med. Biol. 2004, 49, R1–R11. [Google Scholar] [CrossRef] [PubMed]
- Isler, M.F.; Coates, S.J.; Boos, M.D. Climate Change, the Cutaneous Microbiome and Skin Disease: Implications for a Warming World. Int. J. Dermatol. 2023, 62, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Sharma, S.; Haldar, C. The Oxidative Damages Caused by Ultraviolet Radiation Type C (UVC) to a Tropical Rodent Funambulus pennanti: Role of Melatonin. J. Photochem. Photobiol. B Biol. 2013, 125, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In Situ Forming and Reactive Oxygen Species-Scavenging Gelatin Hydrogels for Enhancing Wound Healing Efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef]
- Vieira, A.J.S.C.; Gaspar, E.M.; Santos, P.M.P. Mechanisms of Potential Antioxidant Activity of Caffeine. Radiat. Phys. Chem. 2020, 174, 108968. [Google Scholar] [CrossRef]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; De Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; De Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another Reason for Using Caffeine in Dermocosmetics: Sunscreen Adjuvant. Front. Physiol. 2019, 10, 519. [Google Scholar] [CrossRef]
- Vicente, S.J.V.; Ishimoto, E.Y.; Torres, E.A.F.S. Coffee Modulates Transcription Factor Nrf2 and Highly Increases the Activity of Antioxidant Enzymes in Rats. J. Agric. Food Chem. 2014, 62, 116–122. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia Sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Ashem, H.N.; Draz, A.H.; Abdel-Aziem, A.A. Caffeine Phonophoresis versus Shock Wave Therapy for Adult Women with Cellulite: A Randomized Controlled Trial. Bull. Fac. Phys. Ther. 2019, 24, 66–71. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase Inhibitors. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S252–S257. [Google Scholar] [CrossRef]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and Thermogenesis Related to the Consumption of Caffeine, Ephedrine, Capsaicin, and Green Tea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Soleymani, S.; Salimi, A.; Kalantari, H.; Sheykhi, A. Effect of Pretreatment Time with Enhancers on Caffeine Skin Permeability in Rats. Jundishapur J. Nat. Pharm. Prod. 2023, 18, e137761. [Google Scholar] [CrossRef]
- Abd, E.; Roberts, M.S.; Grice, J.E. A Comparison of the Penetration and Permeation of Caffeine into and through Human Epidermis after Application in Various Vesicle Formulations. Skin Pharmacol. Physiol. 2015, 29, 24–30. [Google Scholar] [CrossRef]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-Based Dermatological Formulations: Dermopharmaceutical and Cosmetic Applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef]
- Che, J.; Wu, Z.; Shao, W.; Guo, P.; Lin, Y.; Pan, W.; Zeng, W.; Zhang, G.; Wu, C.; Xu, Y. Synergetic Skin Targeting Effect of Hydroxypropyl-β-Cyclodextrin Combined with Microemulsion for Ketoconazole. Eur. J. Pharm. Biopharm. 2015, 93, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Sirithunyalug, J.; Khantham, C.; Leksomboon, K.; Jantrawut, P. Skin Penetration and Stability Enhancement of Celastrus Paniculatus Seed Oil by 2-Hydroxypropyl-?-Cyclodextrin Inclusion Complex for Cosmeceutical Applications. Sci. Pharm. 2018, 86, 33. [Google Scholar] [CrossRef]
- Felton, L.A.; Wiley, C.J.; Godwin, D.A. Influence of Hydroxypropyl- β-Cyclodextrin on the Transdermal Permeation and Skin Accumulation of Oxybenzone. Drug Dev. Ind. Pharm. 2002, 28, 1117–1124. [Google Scholar] [CrossRef]
- Bianchi, S.E.; Machado, B.E.K.; da Silva, M.G.C.; da Silva, M.M.A.; Bosco, L.D.; Marques, M.S.; Horn, A.P.; Persich, L.; Geller, F.C.; Argenta, D.; et al. Coumestrol/Hydroxypropyl-β-Cyclodextrin Association Incorporated in Hydroxypropyl Methylcellulose Hydrogel Exhibits Wound Healing Effect: In Vitro and In Vivo Study. Eur. J. Pharm. Sci. 2018, 119, 179–188. [Google Scholar] [CrossRef]
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F.; et al. Alginate-Based Hydrogel Containing Minoxidil/Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Topical Alopecia Treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef]
- Garg, A.; Ahmad, J.; Hassan, M.Z. Inclusion Complex of Thymol and Hydroxypropyl-β-Cyclodextrin (HP-β-CD) in Polymeric Hydrogel for Topical Application: Physicochemical Characterization, Molecular Docking, and Stability Evaluation. J. Drug Deliv. Sci. Technol. 2021, 64, 102609. [Google Scholar] [CrossRef]
- Yao, Q.; Shi, Y.; Xia, X.; Tang, Y.; Jiang, X.; Zheng, Y.-W.; Zhang, H.; Chen, R.; Kou, L. Bioadhesive Hydrogel Comprising Bilirubin/β-Cyclodextrin Inclusion Complexes Promote Diabetic Wound Healing. Pharm. Biol. 2021, 59, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Todo, H.; Hasegawa, Y.; Okada, A.; Itakura, S.; Sugibayashi, K. Improvement of Skin Permeation of Caffeine, a Hydrophilic Drug, by the Application of Water Droplets Provided by a Novel Humidifier Device. Chem. Pharm. Bull. 2021, 69, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B. Cyclodextrin Complexes: Perspective from Drug Delivery and Formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M.; Brewster, M.E. Self-Association of Cyclodextrins and Cyclodextrin Complexes. J. Pharm. Sci. 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- Terekhova, I. Interactions of β-and Hydroxypropyl-β-Cyclodextrin with Some Purine Alkaloids: Thermodynamic Study. CDDT 2008, 5, 168–172. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Kumeev, R.S.; Al’per, G.A. The Interaction of Caffeine with Substituted Cyclodextrins in Water. Russ. J. Phys. Chem. 2007, 81, 1071–1075. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of Cyclodextrin Solubilization of Drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations II: Solubilization, Binding Constant, and Complexation Efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The Complexation Efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.M.; Jocic, E.; Nikolic, V.D.; Popsavin, M.M.; Rakic, S.J.; Savic-Gajic, I.M. The Effect of Complexation with Cyclodextrins on the Antioxidant and Antimicrobial Activity of Ellagic Acid. Pharm. Dev. Technol. 2019, 24, 410–418. [Google Scholar] [CrossRef]
- Veras, K.S.; Silveira Fachel, F.N.; Delagustin, M.G.; Teixeira, H.F.; Barcellos, T.; Henriques, A.T.; Bassani, V.L.; Koester, L.S. Complexation of Rosmarinic Acid with Hydroxypropyl-β-Cyclodextrin and Methyl-β-Cyclodextrin: Formation of 2:1 Complexes with Improved Antioxidant Activity. J. Mol. Struct. 2019, 1195, 582–590. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed]
- Szendzielorz, E.; Spiewak, R. Caffeine as an Active Molecule in Cosmetic Products for Hair Loss: Its Mechanisms of Action in the Context of Hair Physiology and Pathology. Molecules 2025, 30, 167. [Google Scholar] [CrossRef]
- Ko, J.; Kim, J.-Y.; Kim, J.; Yoon, J.S. Anti-Oxidative and Anti-Adipogenic Effects of Caffeine in an In Vitro Model of Graves’ Orbitopathy. Endocr. J. 2020, 67, 439–447. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Chu, C.; Couroucli, X.; Moorthy, B.; Lingappan, K. Differential Concentration-Specific Effects of Caffeine on Cell Viability, Oxidative Stress, and Cell Cycle in Pulmonary Oxygen Toxicity In Vitro. Biochem. Biophys. Res. Commun. 2014, 450, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M. 6—Functional Requirements of Wound Repair Biomaterials. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: London, UK, 2011; pp. 155–173. ISBN 978-1-84569-700-6. [Google Scholar]
- Zakzak, K.; Semenescu, A.-D.; Moacă, E.-A.; Predescu, I.; Drăghici, G.; Vlaia, L.; Vlaia, V.; Borcan, F.; Dehelean, C.-A. Comprehensive Biosafety Profile of Carbomer-Based Hydrogel Formulations Incorporating Phosphorus Derivatives. Gels 2024, 10, 477. [Google Scholar] [CrossRef]
- Coneac, G.; Vlaia, V.; Olariu, I.; Muţ, A.M.; Anghel, D.F.; Ilie, C.; Popoiu, C.; Lupuleasa, D.; Vlaia, L. Development and Evaluation of New Microemulsion-Based Hydrogel Formulations for Topical Delivery of Fluconazole. AAPS PharmSciTech 2015, 16, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Segger, D.; Aßmus, U.; Brock, M.; Erasmy, J.; Finkel, P.; Fitzner, A.; Heuss, H.; Kortemeier, U.; Munke, S.; Rheinländer, T.; et al. Multicenter Study on Measurement of the Natural pH of the Skin Surface. Int. J. Cosmet. Sci. 2008, 30, 75. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Abd El-Aziz, F.E.A.; Abouelmagd, S.A.; Abd El-Hamid, B.N.; Hetta, H.F. Spidroin in Carbopol-based Gel Promotes Wound Healing in Earthworm’s Skin Model. Drug Dev. Res. 2019, 80, 1051–1061. [Google Scholar] [CrossRef]
- Xu, H.; Wen, Y.; Chen, S.; Zhu, L.; Feng, R.; Song, Z. Paclitaxel Skin Delivery by Micelles-Embedded Carbopol 940 Hydrogel for Local Therapy of Melanoma. Int. J. Pharm. 2020, 587, 119626. [Google Scholar] [CrossRef]
- Veryser, L.; Boonen, J.; Mehuys, E.; Roche, N.; Remon, J.-P.; Peremans, K.; Burvenich, C.; De Spiegeleer, B. Transdermal Evaluation of Caffeine in Different Formulations and Excipients. J. Caffeine Res. 2013, 3, 41–46. [Google Scholar] [CrossRef]
- Higuchi, T.K.; Connors, A. Phase Solubility Techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–212. [Google Scholar]
- Musa, K.H.; Abdullah, A.; Kuswandi, B.; Hidayat, M.A. A Novel High Throughput Method Based on the DPPH Dry Reagent Array for Determination of Antioxidant Activity. Food Chem. 2013, 141, 4102–4106. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Advances in the Analytical Methods for Determining the Antioxidant Properties of Honey: A Review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 36–42. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Knorst, M.T. Desenvolvimento e avaliação da estabilidade física de loções O/A contendo filtros solares. Rev. Bras. Cienc. Farm. 2006, 42, 531–537. [Google Scholar] [CrossRef]
- Mendes Aciole, I.H.; De Andrade Júnior, F.P.; Vilar Cordeiro, L.; Pereira de Souza, J.B. Aloe Gel: Manipulation and Characterization of Physical-Chemical Quality Adjustment. Rev. Colomb. Cienc. Quím. Farm. 2020, 49, 790–805. [Google Scholar] [CrossRef]

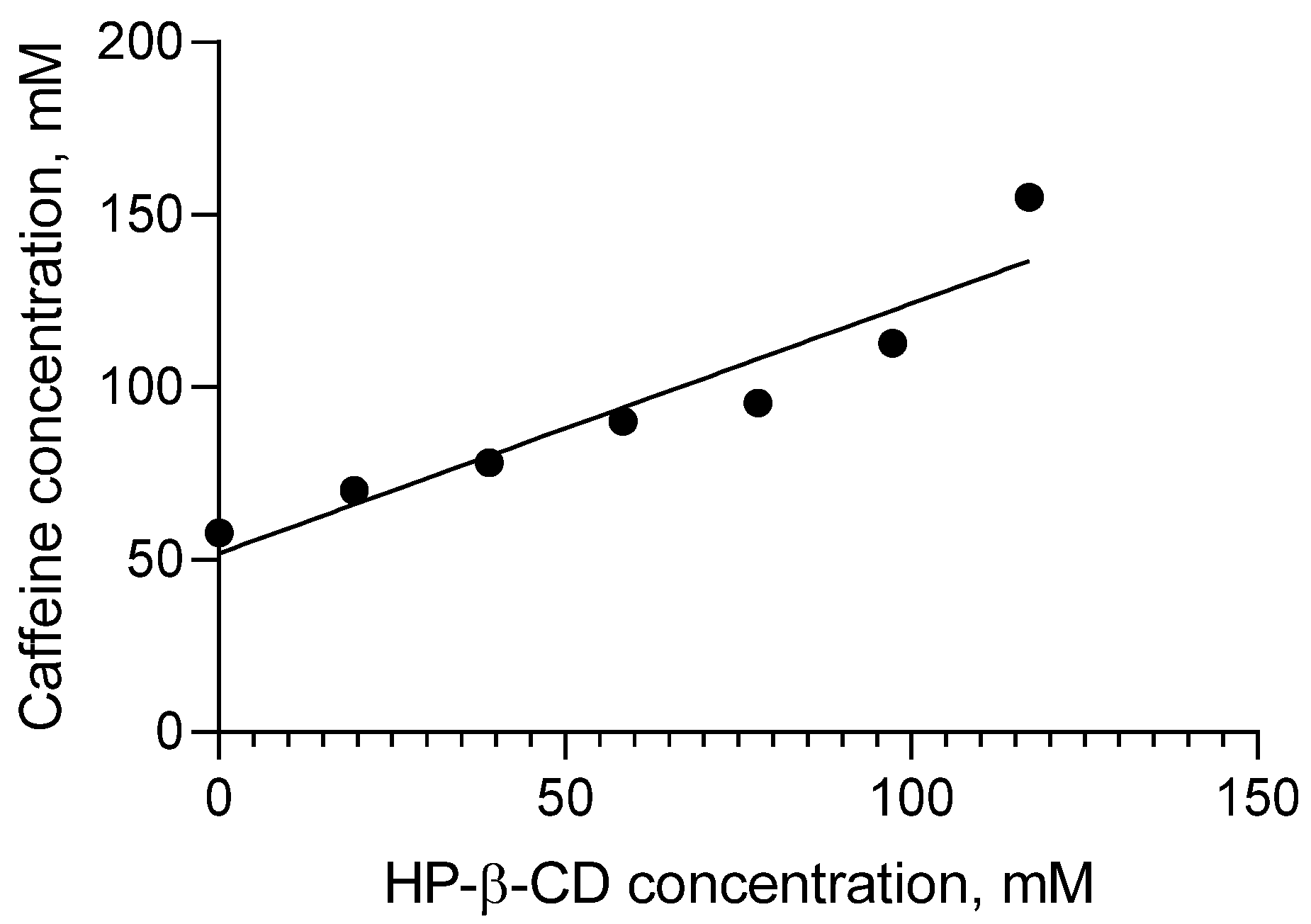


| S0, M | K1:1, M−1 | CE |
|---|---|---|
| 57.7 | 37.44 | 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, L.; Kalampalika, E.; Yordanov, Y.; Petrov, P.D.; Tzankova, V.; Yoncheva, K. Formulation of Caffeine–Hydroxypropyl-β-Cyclodextrin Complex in Hydrogel for Skin Treatment. Gels 2025, 11, 326. https://doi.org/10.3390/gels11050326
Radeva L, Kalampalika E, Yordanov Y, Petrov PD, Tzankova V, Yoncheva K. Formulation of Caffeine–Hydroxypropyl-β-Cyclodextrin Complex in Hydrogel for Skin Treatment. Gels. 2025; 11(5):326. https://doi.org/10.3390/gels11050326
Chicago/Turabian StyleRadeva, Lyubomira, Eleftheria Kalampalika, Yordan Yordanov, Petar D. Petrov, Virginia Tzankova, and Krassimira Yoncheva. 2025. "Formulation of Caffeine–Hydroxypropyl-β-Cyclodextrin Complex in Hydrogel for Skin Treatment" Gels 11, no. 5: 326. https://doi.org/10.3390/gels11050326
APA StyleRadeva, L., Kalampalika, E., Yordanov, Y., Petrov, P. D., Tzankova, V., & Yoncheva, K. (2025). Formulation of Caffeine–Hydroxypropyl-β-Cyclodextrin Complex in Hydrogel for Skin Treatment. Gels, 11(5), 326. https://doi.org/10.3390/gels11050326









