Unlocking the Rich Potential of a Soft Gel-Cream Enriched with Royal Jelly for Topical Use
Abstract
:1. Introduction
2. Results and Discussion
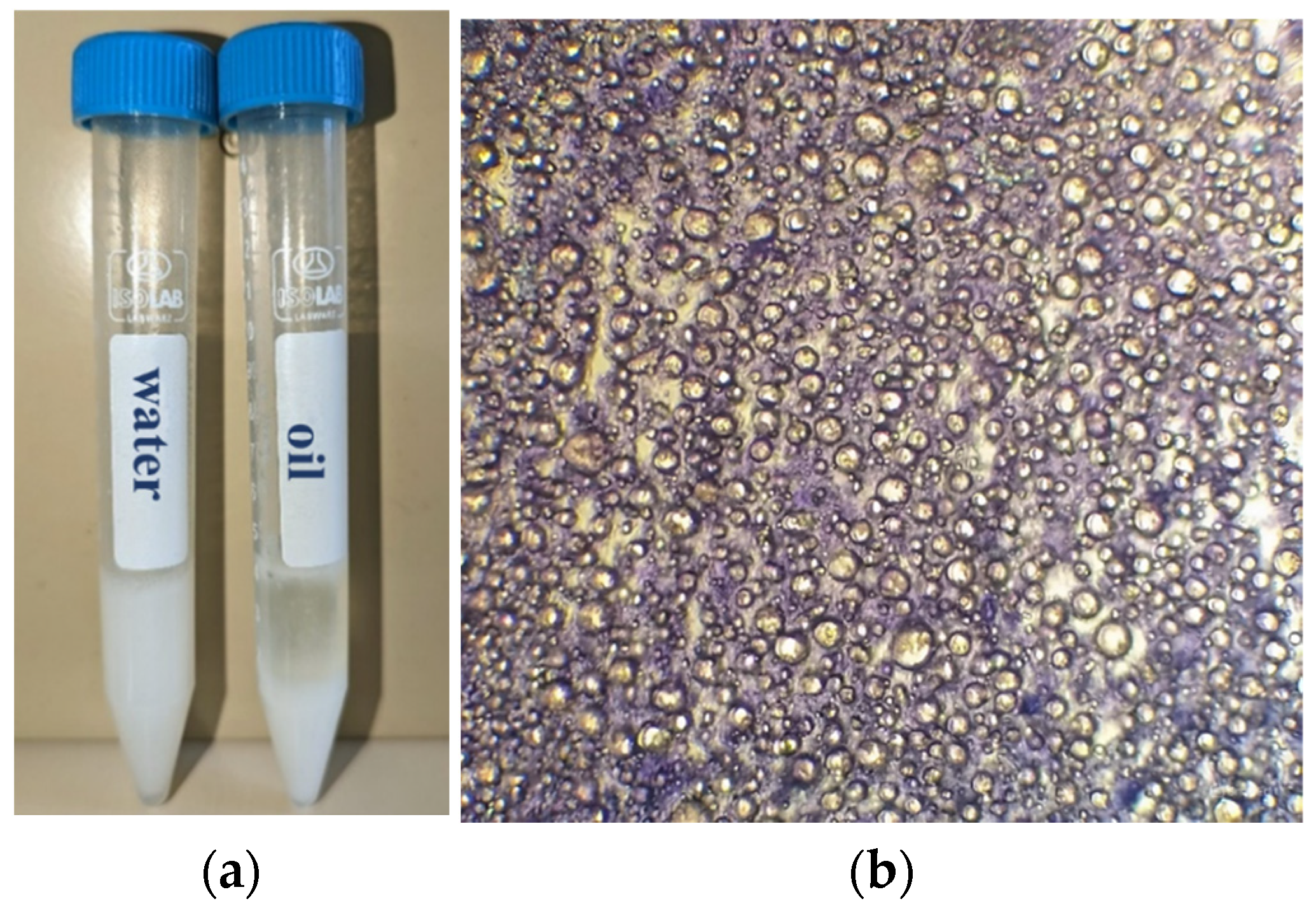
2.1. Synthesis and Evaluation of RJ-Based Formulation
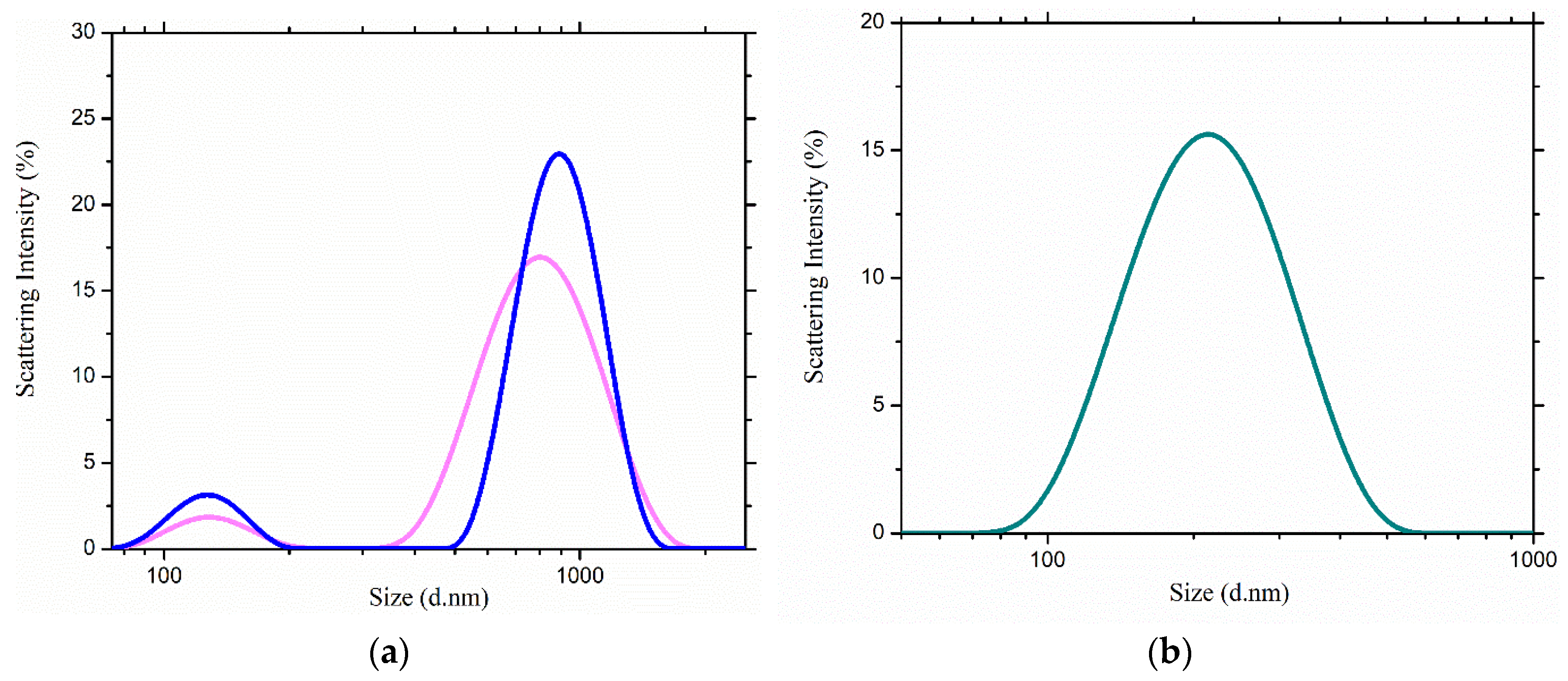
2.2. Scattering Measurements
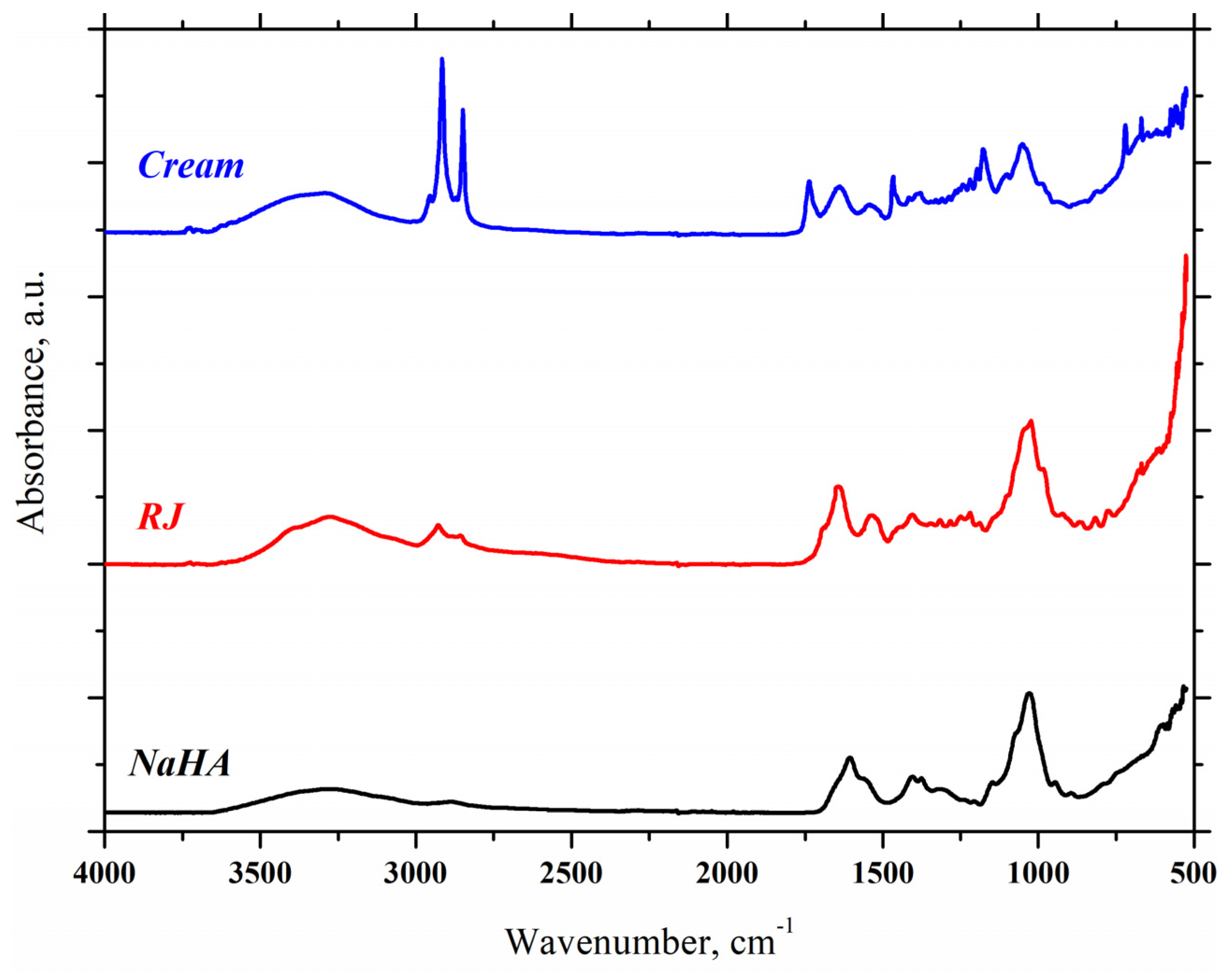
2.3. FTIR-ATR
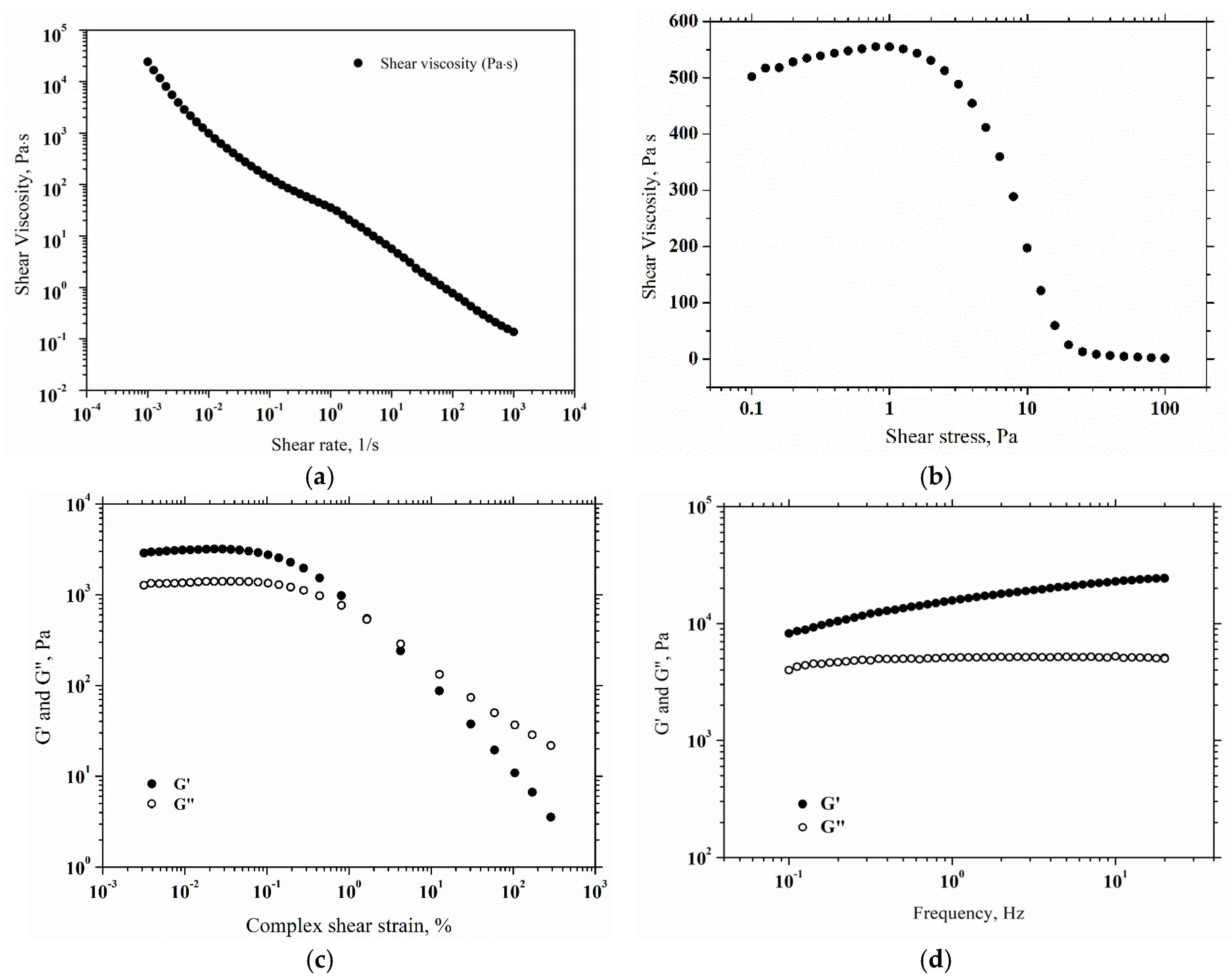
2.4. Rheological Properties
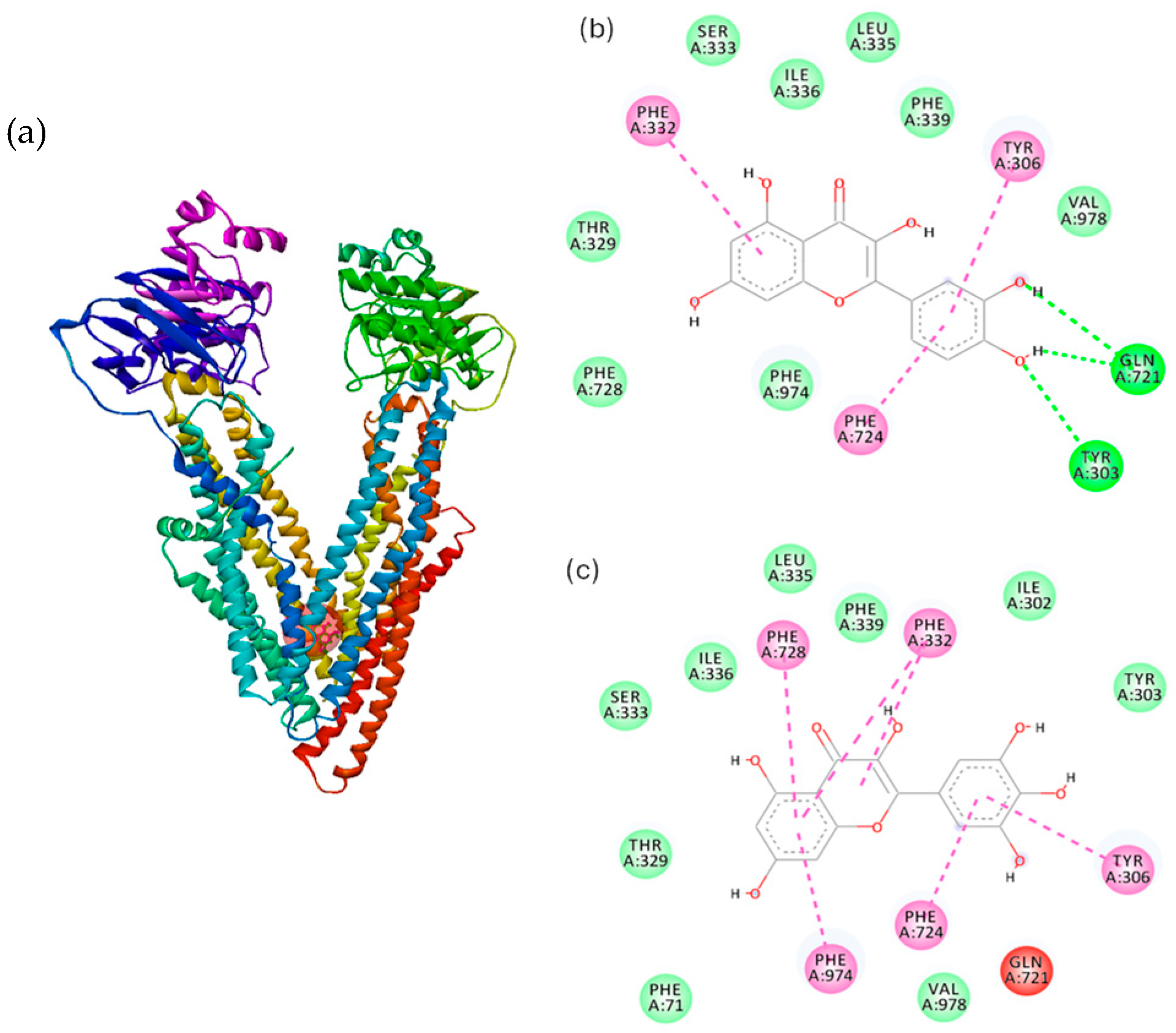
2.5. Molecular Docking Study
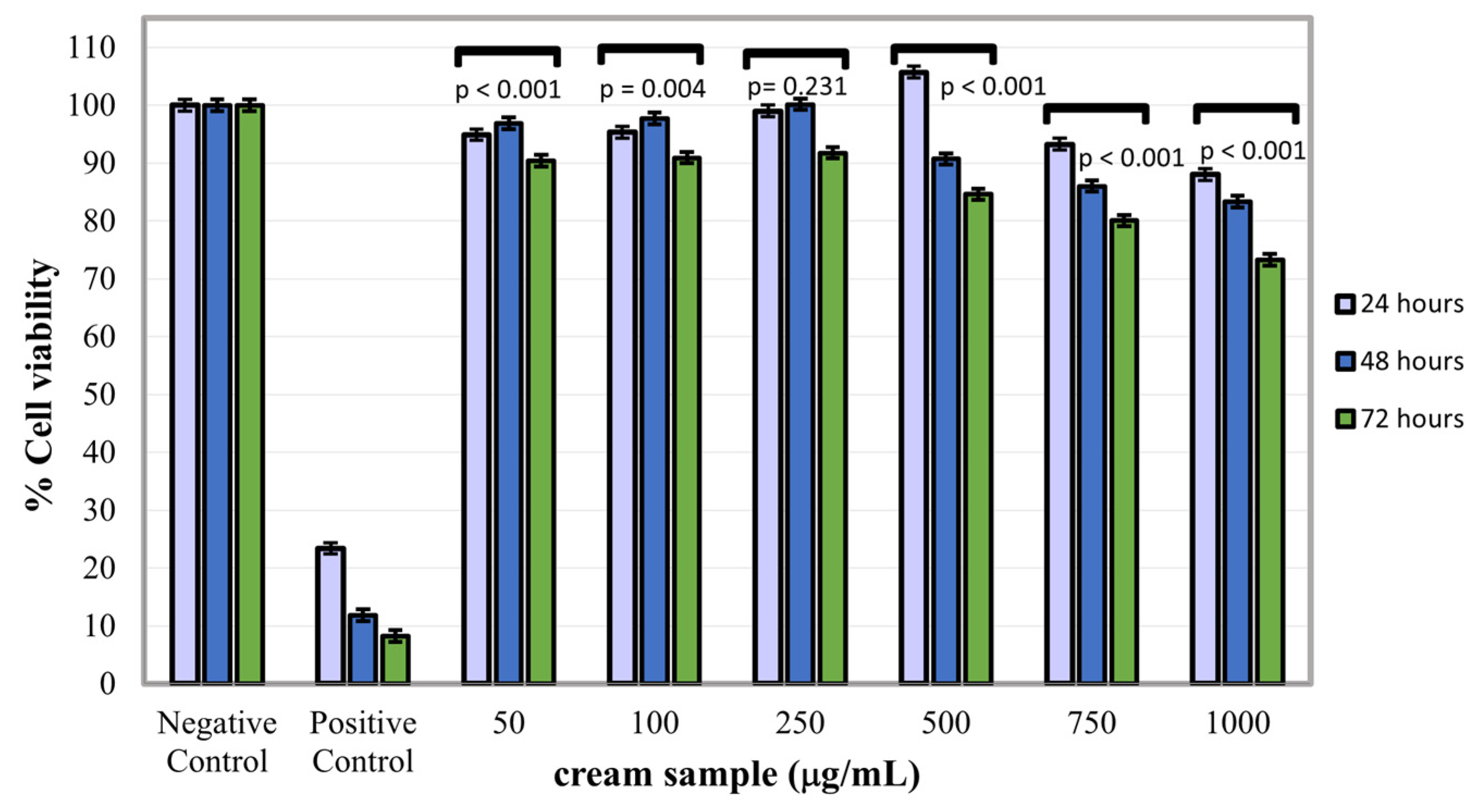
2.6. Cytotoxicity Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation and Evaluation of RJ-Soft Gel-Cream
4.3. Determination of Saponification and Acid Values
4.4. Identification Tests
4.5. Dynamic Light Scattering (DLS)
4.6. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
4.7. Rheology
4.8. Molecular Docking
4.9. Cytotoxicity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Royal Jelly: Biological Action and Health Benefits. Int. J. Mol. Sci. 2024, 25, 6023. [Google Scholar] [CrossRef]
- Park, H.M.; Cho, M.H.; Cho, Y.; Kim, S.Y. Royal Jelly Increases Collagen Production in Rat Skin after Ovariectomy. J. Med. Food 2012, 15, 568–575. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 19. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.D.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-Gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Amin, M.L. P-Glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef]
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen. Molecules 2021, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 13, 2012. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Diseases Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Yang, X.; Zhai, G. Current research on hyaluronic acid-drug bioconjugates. Eur. J. Med. Chem. 2014, 86, 310–317. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 458280. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size matters: Molecular weight specificity of hyaluronan effects in cell biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 518. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient Treatment of Atopic Dermatitis: Latest Evidence and Clinical Considerations. Drugs Context 2018, 7, 212530. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Arouri, A.; Lauritsen, K.E.; Nielsen, H.L.; Mouritsen, O.G. Effect of fatty acids on the permeability barrier of model and biological membranes. Chem. Phys. Lipids 2016, 200, 139–146. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Fatty acids in cell signaling: Historical perspective and future outlook. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 57–62. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. Chemik 2014, 68, 103–110. [Google Scholar]
- Lanigan, R.S.; Yamarik, T.A. Final Report on the Safety Assessment of Sorbitan Caprylate, Sorbitan Cocoate, Sorbitan Diisostearate, Sorbitan Dioleate, Sorbitan Distearate, Sorbitan Isostearate, Sorbitan Olivate, Sorbitan Sesquiisostearate, Sorbitan Sesquistearate, and Sorbitan Triis. Int. J. Toxicol. 2002, 21 (Suppl. S1), S93–S112. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Olczyk, P. Chapter 2—Bee Products and Skin Therapy. In Bee Products and Their Applications in the Food and Pharmaceutical Industries; Boyacioglu, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 25–62. ISBN 978-0-323-85400-9. [Google Scholar]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef]
- Low, L.K.; Ng, C.S. Analysis of Oils: Determination of Saponification Value; Marine Fisheries Research Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 1987. [Google Scholar]
- Low, L.K.; Ng, C.S. Analysis of Oils: Determination of Acid Value; Marine Fisheries Research Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 1987. [Google Scholar]
- Priyadarsini, S.; Kumar, P.R.; Margesan, M.T. Formulation and evaluation of a herbal antibacterial cream from ethyl acetate extract of leaves of Spinacia oleracea Linn. against Aeromonas skin and soft tissue infections. Int. J. Green Pharm. 2018, 12, S537–S542. [Google Scholar]
- Garti, H.; Agbemafle, R. Physicochemical properties and fatty acid composition of shea butter from Tamale, Northern Ghana. UDS Int. J. Dev. 2020, 3, 35–40. [Google Scholar]
- Evans, W.C.; Evans, D. Chapter 19—Hydrocarbons and Derivatives. In Trease and Evans’ Pharmacognosy (Sixteenth Edition); Evans, W.C., Ed.; Saunders: Philadelphia, PA, USA, 2009; pp. 173–193. ISBN 978-0-7020-2933-2. [Google Scholar]
- Ruoff, K.; Devant, J.M.; Hansman, G. Natural Extracts, Honey, and Propolis as Human Norovirus Inhibitors. Sci. Rep. 2022, 12, 8116. [Google Scholar] [CrossRef] [PubMed]
- Botezan, S.; Baci, G.-M.; Bagameri, L.; Pașca, C.; Dezmirean, D.S. Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects. Molecules 2023, 28, 1510. [Google Scholar] [CrossRef]
- Hashemirad, F.-S.; Behfar, M.; Kavoosi, G. Proximate composition, physico-chemical, techno-functional, amino acid profile, fatty acid profile, nutritional quality, antioxidant, anti-amylase and anti-lipase properties of bee bread, royal jelly, and bee propolis. LWT 2024, 200, 116190. [Google Scholar] [CrossRef]
- Kokalj Ladan, M.; Straus, J.; Tavčar Benković, E.; Kreft, S. FT-IR-based method for rutin, quercetin and quercitrin quantification in different buckwheat (Fagopyrum) species. Sci. Rep. 2017, 7, 7226. [Google Scholar] [CrossRef]
- Gilli, R.; Kacuráková, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.d.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus Zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.-K.; Kim, J.-H.; Kweon, D.-K.; Lee, J.-W. Degradation of hyaluronic acid powder by electron beam irradiation, gamma ray irradiation, microwave irradiation and thermal treatment: A comparative study. Carbohydr. Polym. 2010, 79, 1080–1085. [Google Scholar] [CrossRef]
- Cebi, N.; Bozkurt, F.; Yilmaz, M.; Sagdic, O. An evaluation of FTIR spectroscopy for prediction of royal jelly content in hive products. J. Apic. Res. 2020, 59, 1707009. [Google Scholar] [CrossRef]
- Lazarevska, S.; Makreski, P. Insights into the infrared and raman spectra of fresh and lyophilized Royal Jelly and protein degradation IR spectroscopy study during heating. Maced. J. Chem. Chem. Eng. 2015, 34, 87–93. [Google Scholar] [CrossRef]
- Tarantilis, P.; Pappas, C.; Alissandrakis, E.; Harizanis, P.; Polissiou, M. Monitoring of royal jelly protein degradation during storage using Fourier-transform infrared (FTIR) spectroscopy. J. Apic. Res. 2012, 51, 185–192. [Google Scholar] [CrossRef]
- Ghadimi-Garjan, R.; Javadi, A.; Jafarizadeh-Malmiri, H.; Anarjan, N.; Mirzaei, H. Lyophilized royal jelly preparation in nanoscale and evaluation of its physicochemical properties and bactericidal activity. Food Sci. Nutr. 2023, 11, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chophi, R.; Kaur, H.; Singh, R. Differentiation of Cosmetic Foundation Creams Using Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy: A Rapid and Non-Destructive Approach in Trace Evidence Analysis. J. Forensic Sci. 2020, 65, 751–761. [Google Scholar] [CrossRef]
- Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Novel phase change materials for thermal energy storage: Evaluation of tropical tree fruit oils. Biotechnol. Rep. 2019, 24, e00359. [Google Scholar] [CrossRef]
- McKenna, B.M.; Lyng, J.G. 6—Introduction to Food Rheology and Its Measurement. In Woodhead Publishing Series in Food Science, Technology and Nutrition; McKenna, B.M.B.T.-T., Ed.; Woodhead Publishing: Cambridge, UK, 2003; pp. 130–160. ISBN 978-1-85573-673-3. [Google Scholar]
- Simões, A.; Miranda, M.; Cardoso, C.; Vitorino, F.V.; Vitorino, C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Adeyeye, M.C.; Jain, A.C.; Ghorab, M.K.M.; Reilly, W.J. Viscoelastic evaluation of topical creams containing microcrystalline cellulose/sodium carboxymethyl cellulose as stabilizer. AAPS PharmSciTech 2002, 3, 8. [Google Scholar] [CrossRef]
- Chiarentin, L.; Cardoso, C.; Miranda, M.; Vitorino, C. Rheology of Complex Topical Formulations: An Analytical Quality by Design Approach to Method Optimization and Validation. Pharmaceutics 2023, 15, 1810. [Google Scholar] [CrossRef]
- Hashimoto, N.; Nakamichi, N.; Yamazaki, E.; Oikawa, M.; Masuo, Y.; Schinkel, A.H.; Kato, Y. P-Glycoprotein in skin contributes to transdermal absorption of topical corticosteroids. Int. J. Pharm. 2017, 521, 365–373. [Google Scholar] [CrossRef]
- Ueda, T.; Waverczak, W.; Ward, K.; Sher, N.; Ketudat, M.; Schmidt, R.J.; Messing, J. Mutations of the 22- and 27-KD Zein Promoters Affect Transactivation by the Opaque-2 Protein. Plant Cell 1992, 4, 701–709. [Google Scholar] [CrossRef]
- Saeki, T.; Ueda, K.; Tanigawara, Y.; Hori, R.; Komano, T. Human P-glycoprotein transports cyclosporin A and FK506. J. Biol. Chem. 1993, 268, 6077–6080. [Google Scholar] [CrossRef] [PubMed]
- Palmberger, T.F.; Hombach, J.; Bernkop-Schnürch, A. Thiolated chitosan: Development and in vitro evaluation of an oral delivery system for acyclovir. Int. J. Pharm. 2008, 348, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.R.; Chang, C.; Kearbey, J.D.; Yasuda, K.; Schuetz, E.G.; Miller, D.D.; Dalton, J.T.; Swaan, P.W. Structural Determinants of P-Glycoprotein-Mediated Transport of Glucocorticoids. Pharm. Res. 2003, 20, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, C.; Graff, C.L.; Pollack, G.M. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem. Pharmacol. 2004, 67, 269–276. [Google Scholar] [CrossRef]
- Suzuki, T.; Zaima, C.; Moriki, Y.; Fukami, T.; Tomono, K. P-glycoprotein mediates brain-to-blood efflux transport of buprenorphine across the blood–brain barrier. J. Drug Target. 2007, 15, 67–74. [Google Scholar] [CrossRef]
- Kim, W.Y.; Benet, L.Z. P-glycoprotein (P-gp/MDR1)-Mediated Efflux of Sex-Steroid Hormones and Modulation of P-gp Expression In Vitro. Pharm. Res. 2004, 21, 1284–1293. [Google Scholar] [CrossRef]
- Tahara, K.; Kagawa, Y.; Takaai, M.; Taguchi, M.; Hashimoto, Y. Directional Transcellular Transport of Bisoprolol in P-glycoprotein-expressed LLC-GA5-COL150 Cells but not in Renal Epithelial LLC-PK1 Cells. Drug Metab. Pharmacokinet. 2008, 23, 340–346. [Google Scholar] [CrossRef]
- Koziolek, M.J.; Riess, R.; Geiger, H.; Thévenod, F.; Hauser, I.A. Expression of multidrug resistance P-glycoprotein in kidney allografts from cyclosporine A-treated patients. Kidney Int. 2001, 60, 156–166. [Google Scholar] [CrossRef]
- Hoffmaster, K.A.; Turncliff, R.Z.; LeCluyse, E.L.; Kim, R.B.; Meier, P.J.; Brouwer, K.L.R. P-glycoprotein Expression, Localization, and Function in Sandwich-Cultured Primary Rat and Human Hepatocytes: Relevance to the Hepatobiliary Disposition of a Model Opioid Peptide. Pharm. Res. 2004, 21, 1294–1302. [Google Scholar] [CrossRef]
- Greiner, B.; Eichelbaum, M.; Fritz, P.; Kreichgauer, H.P.; von Richter, O.; Zundler, J.; Kroemer, H.K. The Role of Intestinal P-Glycoprotein in the Interaction of Digoxin and Rifampin. J. Clin. Investig. 1999, 104, 147–153. [Google Scholar] [CrossRef]
- Sasongko, L.; Link, J.M.; Muzi, M.; Mankoff, D.A.; Yang, X.; Collier, A.C.; Shoner, S.C.; Unadkat, J.D. Imaging P-glycoprotein Transport Activity at the Human Blood-brain Barrier with Positron Emission Tomography. Clin. Pharmacol. Ther. 2005, 77, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, M.A.; Watson, J.D.; Murison, J.G. Neonatal Murine Epidermal Cells Express a Functional Multidrug-Resistant Pump. J. Investig. Dermatol. 2000, 115, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Höller, D.; Schiffer, R.; Frankenberg, S.; Neis, M.; Merk, H.F.; Jugert, F.K. Expression of Multiple Cytochrome P450 Enzymes and Multidrug Resistance-Associated Transport Proteins in Human Skin Keratinocytes. J. Investig. Dermatol. 2001, 116, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Triel, C.; Vestergaard, M.E.; Bolund, L.; Jensen, T.G.; Jensen, U.B. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp. Cell Res. 2004, 295, 79–90. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Ling, V. Positively Cooperative Sites for Drug Transport by P-Glycoprotein with Distinct Drug Specificities. Eur. J. Biochem. 1997, 250, 130–137. [Google Scholar] [CrossRef]
- Martin, C.; Berridge, G.; Higgins, C.F.; Mistry, P.; Charlton, P.; Callaghan, R. Communication between Multiple Drug Binding Sites on P-Glycoprotein. Mol. Pharmacol. 2000, 58, 624–632. [Google Scholar] [CrossRef]
- Singh, A.; Patel, S.K.; Kumar, P.; Das, K.C.; Verma, D.; Sharma, R.; Tripathi, T.; Giri, R.; Martins, N.; Garg, N. Quercetin Acts as a P-Gp Modulator via Impeding Signal Transduction from Nucleotide-Binding Domain to Transmembrane Domain. J. Biomol. Struct. Dyn. 2022, 40, 4507–4515. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, S.; Fan, X.; Chen, Y.; Zou, X.; Hu, M.; Wang, B.; Jin, J.; Wang, X.; Hu, J.; et al. Inhibitory Effects of Flavonoids on P-Glycoprotein in Vitro and in Vivo: Food/Herb-Drug Interactions and Structure-Activity Relationships. Toxicol. Appl. Pharmacol. 2019, 369, 49–59. [Google Scholar] [CrossRef]
- Veiga-Matos, J.; Morales, A.I.; Prieto, M.; Remião, F.; Silva, R. Study Models of Drug–Drug Interactions Involving P-Glycoprotein: The Potential Benefit of P-Glycoprotein Modulation at the Kidney and Intestinal Levels. Molecules 2023, 28, 7532. [Google Scholar] [CrossRef]
- Mohana, S.; Ganesan, M.; Agilan, B.; Karthikeyan, R.; Srithar, G.; Beaulah Mary, R.; Ananthakrishnan, D.; Velmurugan, D.; Rajendra Prasad, N.; Ambudkar, S.V. Screening dietary flavonoids for the reversal of P-glycoprotein-mediated multidrug resistance in cancer. Mol. BioSyst. 2016, 12, 2458–2470. [Google Scholar] [CrossRef]
- Itoh, T.; Degawa, T.; Ito, Y.; Akao, Y.; Okumura, N. Role of Royal Jelly Treated Adipose-Derived Stem-Extracellular Vesicles on Fibroblast Proliferation, Migration, and Collagen Production. Dermatol. Ther. 2023, 2023, 7950026. [Google Scholar] [CrossRef]
- Park, H.M.; Hwang, E.; Lee, K.G.; Han, S.-M.; Cho, Y.; Kim, S.Y. Royal Jelly Protects against Ultraviolet B-Induced Photoaging in Human Skin Fibroblasts via Enhancing Collagen Production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Al-U’datt, D.G.F.; Alu’datt, M.H.; Tranchant, C.C.; Al-Dwairi, A.; Al-Shboul, O.; Almajwal, A.; Elsalem, L.; Jaradat, S.; Alzoubi, K.H.; Faleh, B.G.; et al. Royal Jelly Mediates Fibrotic Signaling, Collagen Cross-Linking and Cell Proliferation in Cardiac Fibroblasts. Biomed. Pharmacother. 2023, 164, 114922. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Varlan, A.; Hillebrand, M. Exploring the capabilities of TDDFT calculations to explain the induced chirality upon a binding process: A simple case, 3-carboxycoumarin. J. Mol. Struct. 2013, 1036, 341–349. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_C01 2016, Gaussian 16, Revision C.01; Gaussian, Inc.: Wallin, UK, 2016. [Google Scholar]
- Marques, S.M.; Šupolíková, L.; Molčanová, L.; Šmejkal, K.; Bednar, D.; Slaninová, I. Screening of Natural Compounds as P-Glycoprotein Inhibitors against Multidrug Resistance. Biomedicines 2021, 9, 357. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Dassault Systemes BIOVIA Discovery Studio Modeling Environment. Discov. Stud. Model. Environ. San Diego. 2019. Available online: https://www.3ds.com/products/biovia/discovery-studio (accessed on 13 April 2025).

| Phase A (Oil Phase > 70 °C) | Phase B (Water Phase > 70 °C) | Phase C (Humectants < 40 °C) |
|---|---|---|
| Shea butter | Ultrapure water | Royal jelly |
| Almond oil | Rose hydrosol | Sodium hyaluronate |
| Olliva emulsifier | Tocopherol | |
| Viola Odorata fragrance | ||
| Preservative |
| Components (INCI) | Country and Origin | Dosage (%) |
|---|---|---|
| Butyrospermum Parkii Butter | Burkina Faso, organic purified butter | 8 |
| Prunus Amygdalus Dulcis Oil | Germany, organic unrefined oil | 12 |
| Olliva Emulsifier (cetearyl olivate and sorbitan olivate) | Italy, natural origin | 6 |
| Water | Germany, Mili-Q water | 50.5 |
| Hydrolyzed Rose Damascena Flower extract | Bulgaria, organic farming | 20 |
| Royal jelly powder | France, organic farming | 2 |
| High molecular weight sodium hyaluronate | China, plant sources | 0.4 |
| Tocopherol, Helianthus Annuus seed oil | Belgium, natural origin, | 0.5 |
| Viola Odorata flower extract | France, natural fragrance oil | 0.4 |
| Cosgard (benzyl alcohol, salicylic acid, glycerin, sorbic acid, water) | USA, cosmetic preservative | 0.6 |
| Polyphenol | Amino Acid Residue | Distance, Å | Type of Interaction | Binding Affinity, kcal/mol |
|---|---|---|---|---|
| quercetin | TYR303 | 2.83 | hydrogen bond | −7.6 |
| GLN721 | 3.09 | hydrogen bond | ||
| PHE724 | 3.88 | hydrophobic | ||
| TYR306 | 5.40 | hydrophobic | ||
| PHE332 | 5.49 | hydrophobic | ||
| myricetin | PHE724 | 4.03 | hydrophobic | −7.5 |
| PHE974 | 5.09 | hydrophobic | ||
| TYR306 | 5.38 | hydrophobic | ||
| PHE332 | 5.58 | hydrophobic | ||
| PHE728 | 5.93 | hydrophobic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxim, M.-E.; Toma, R.-M.; Aricov, L.; Leonties, A.-R.; Precupas, A.; Tatia, R.; Oprita, E.I. Unlocking the Rich Potential of a Soft Gel-Cream Enriched with Royal Jelly for Topical Use. Gels 2025, 11, 294. https://doi.org/10.3390/gels11040294
Maxim M-E, Toma R-M, Aricov L, Leonties A-R, Precupas A, Tatia R, Oprita EI. Unlocking the Rich Potential of a Soft Gel-Cream Enriched with Royal Jelly for Topical Use. Gels. 2025; 11(4):294. https://doi.org/10.3390/gels11040294
Chicago/Turabian StyleMaxim, Monica-Elisabeta, Raluca-Marieta Toma, Ludmila Aricov, Anca-Ruxandra Leonties, Aurica Precupas, Rodica Tatia, and Elena Iulia Oprita. 2025. "Unlocking the Rich Potential of a Soft Gel-Cream Enriched with Royal Jelly for Topical Use" Gels 11, no. 4: 294. https://doi.org/10.3390/gels11040294
APA StyleMaxim, M.-E., Toma, R.-M., Aricov, L., Leonties, A.-R., Precupas, A., Tatia, R., & Oprita, E. I. (2025). Unlocking the Rich Potential of a Soft Gel-Cream Enriched with Royal Jelly for Topical Use. Gels, 11(4), 294. https://doi.org/10.3390/gels11040294










