Regulation of the Properties of the Hierarchical Porous Structure of Alumophosphate Molecular Sieves AEL by Reaction Gels Prepared with Different Templates
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Preparation of Aluminophosphate Gels
4.2. Crystallization of AlPO4-11 Molecular Sieves
4.3. Material Analysis Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermeiren, W.; Gilson, J.-P. Impact of Zeolites on the Petroleum and Petrochemical Industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Potter, M.E. Down the Microporous Rabbit Hole of Silicoaluminophosphates: Recent Developments on Synthesis, Characterization, and Catalytic Applications. ACS Catal. 2020, 10, 9758–9789. [Google Scholar] [CrossRef]
- Hartmann, M.; Elangovan, S.P. Catalysis with Microporous Aluminophosphates and Silicoaluminophosphates Containing Transition Metals. In Advances in Nanoporous Materials; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, ISBN 978-0-444-53179-7. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-53064-6. [Google Scholar]
- Endregard, M.; Nicholson, D.G.; Stöcker, M.; Beagley, B. Cobalticenium Ions Adsorbed on Large-Pore Aluminophosphate VPI-5 Studied by X-Ray Absorption, 13C Solid-State NMR and FTIR Spectroscopy. J. Mater. Chem. 1995, 5, 485–491. [Google Scholar] [CrossRef]
- Ganschow, M.; Schulz-Ekloff, G.; Wark, M.; Wendschuh-Josties, M.; Wöhrle, D. Microwave-Assisted Preparation of Uniform Pure and Dye-Loaded AlPO4-5 Crystals with Different Morphologies for Use as Microlaser Systems. J. Mater. Chem. 2001, 11, 1823–1827. [Google Scholar] [CrossRef]
- García-Carmona, J.; Fanovich, M.A.; Llibre, J.; Rodríguez-Clemente, R.; Domingo, C. Processing of Microporous VPI-5 Molecular Sieve by Using Supercritical CO2: Stability and Adsorption Properties. Microporous Mesoporous Mater. 2002, 54, 127–137. [Google Scholar] [CrossRef]
- Van Heyden, H.; Mintova, S.; Bein, T. AlPO-18 Nanocrystals Synthesized under Microwave Irradiation. J. Mater. Chem. 2006, 16, 514–518. [Google Scholar] [CrossRef]
- Shutilov, R.A.; Grenev, I.V.; Kikhtyanin, O.V.; Gavrilov, V.Y. Adsorption of Molecular Hydrogen on Aluminophosphate Zeolites at 77 K. Kinet. Catal. 2012, 53, 137–144. [Google Scholar] [CrossRef]
- Weiß, Ö.; Loerke, J.; Wüstefeld, U.; Marlow, F.; Schüth, F. Host–Guest Interactions and Laser Activity in AlPO4-5/Laser Dye Composites. J. Solid. State Chem. 2002, 167, 302–309. [Google Scholar] [CrossRef]
- Yao, M.; Wang, T.; Yao, Z.; Duan, D.; Chen, S.; Liu, Z.; Liu, R.; Lu, S.; Yuan, Y.; Zou, B.; et al. Pressure-Driven Topological Transformations of Iodine Confined in One-Dimensional Channels. J. Phys. Chem. C 2013, 117, 25052–25058. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.; Xu, J.; Deng, F.; Yan, W.; Sharma, R.P.; Xu, R. Encapsulation of Bulky Solvent Molecules into the Channels of Aluminophosphate Molecular Sieve and Its Negative Influence on the Thermal Stability of Open-Framework. Inorg. Chem. Commun. 2018, 91, 67–71. [Google Scholar] [CrossRef]
- Carreon, M.L.; Li, S.; Carreon, M.A. AlPO-18 Membranes for CO2/CH4 Separation. Chem. Commun. 2012, 48, 2310. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lucero, J.; Zong, Z.; Elsaidi, S.K.; Thallapally, P.K.; Carreon, M.A. Microporous Crystalline Membranes for Kr/Xe Separation: Comparison Between AlPO-18, SAPO-34, and ZIF-8. ACS Appl. Nano Mater. 2018, 1, 463–470. [Google Scholar] [CrossRef]
- Wang, B.; Gao, F.; Zhang, F.; Xing, W.; Zhou, R. Highly Permeable and Oriented AlPO-18 Membranes Prepared Using Directly Synthesized Nanosheets for CO2 /CH4 Separation. J. Mater. Chem. A 2019, 7, 13164–13172. [Google Scholar] [CrossRef]
- Yang, W.; Sun, W.; Zhao, S.; Yin, X. Single-Walled Carbon Nanotubes Prepared in Small AlPO4-5 and CoAlPO-5 Molecular Sieves by Low-Temperature Hydrocracking. Microporous Mesoporous Mater. 2016, 219, 87–92. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, B.; Liu, X.; Li, J. Cyclohexane Oxidation over AFI Molecular Sieves: Effects of Cr, Co Incorporation and Crystal Size. Catal. Sci. Technol. 2015, 5, 3394–3402. [Google Scholar] [CrossRef]
- Esther Leena Preethi, M.; Umasankari, A.; H.Rekha, C.; Palanichamy, M.; Sivakumar, T.; Pandurangan, A. Selective Oxidation of Cyclohexane to KA Oil Over Ce-Alpo-18 Molecular Sieves. Int. J. Eng. Technol. 2018, 7, 352. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Zhang, Y.; Li, D.; Gao, F.; Feng, C.; Wen, S.; Ruan, S. Humidity Sensor Based on AlPO4-5 Zeolite with High Responsivity and Its Sensing Mechanism. Sens. Actuators B Chem. 2015, 212, 242–247. [Google Scholar] [CrossRef]
- Ristić, A.; Logar, N.Z.; Henninger, S.K.; Kaučič, V. The Performance of Small-Pore Microporous Aluminophosphates in Low-Temperature Solar Energy Storage: The Structure–Property Relationship. Adv. Funct. Mater. 2012, 22, 1952–1957. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schossig, P.; Henning, H.-M. Novel Sorption Materials for Solar Heating and Cooling. Energy Procedia 2012, 30, 279–288. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Z.; Zhu, Z.; Li, A.; Li, G.; Wang, X. Evaluation of Iron-Containing Aluminophosphate Molecular Sieve Catalysts Prepared by Different Methods for Phenol Hydroxylation. Catal. Lett. 2013, 143, 657–665. [Google Scholar] [CrossRef]
- Chen, J.; Thomas, J.M. MAPO-18 (M [Triple Bond, Length Half m-Dash] Mg, Zn, Co): A New Family of Catalysts for the Conversion of Methanol to Light Olefins. J. Chem. Soc. Chem. Commun. 1994, 603–604. [Google Scholar] [CrossRef]
- Mishra, T.; Parida, K.M.; Rao, S.B. Transition Metal Promoted AlPO4 Catalyst 2. The Catalytic Activity of M0.05Al0.95PO4 for Alcohol Conversion and Cumene Cracking/Dehydrogenation Reactions. Appl. Catal. A Gen. 1998, 166, 115–122. [Google Scholar] [CrossRef]
- Yadav, R.; Sakthivel, A. Silicoaluminophosphate Molecular Sieves as Potential Catalysts for Hydroisomerization of Alkanes and Alkenes. Appl. Catal. A Gen. 2014, 481, 143–160. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Wang, C.; Lv, G.; Wang, D.; Ma, H.; Tian, Z. Direct Synthesis of Shaped MgAPO-11 Molecular Sieves and the Catalytic Performance in n-Dodecane Hydroisomerization. RSC Adv. 2021, 11, 25364–25374. [Google Scholar] [CrossRef]
- Höchtl, M.; Jentys, A.; Vinek, H. Alkane Conversion over Pd/SAPO Molecular Sieves: Influence of Acidity, Metal Concentration and Structure. Catal. Today 2001, 65, 171–177. [Google Scholar] [CrossRef]
- Deldari, H. Suitable Catalysts for Hydroisomerization of Long-Chain Normal Paraffins. Appl. Catal. A Gen. 2005, 293, 1–10. [Google Scholar] [CrossRef]
- Akhmedov, V.M.; Al-Khowaiter, S.H. Recent Advances and Future Aspects in the Selective Isomerization of High n-Alkanes. Catal. Rev. 2007, 49, 33–139. [Google Scholar] [CrossRef]
- Chen, Z.; Song, W.; Zhu, S.; Lai, W.; Yi, X.; Fang, W. Synthesis of a Multi-Branched Dandelion-like SAPO-11 by an In Situ Inoculating Seed-Induced-Steam-Assisted Conversion Method (SISAC) as a Highly Effective Hydroisomerization Support. RSC Adv. 2017, 7, 4656–4666. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Xu, Y.; Dong, Y.; Lai, W.; Fang, W.; Yi, X. Fabrication of Nano-Sized SAPO-11 Crystals with Enhanced Dehydration of Methanol to Dimethyl Ether. Catal. Commun. 2018, 103, 1–4. [Google Scholar] [CrossRef]
- Jin, D.; Ye, G.; Zheng, J.; Yang, W.; Zhu, K.; Coppens, M.-O.; Zhou, X. Hierarchical Silicoaluminophosphate Catalysts with Enhanced Hydroisomerization Selectivity by Directing the Orientated Assembly of Premanufactured Building Blocks. ACS Catal. 2017, 7, 5887–5902. [Google Scholar] [CrossRef]
- Jin, D.; Li, L.; Ye, G.; Ding, H.; Zhao, X.; Zhu, K.; Coppens, M.-O.; Zhou, X. Manipulating the Mesostructure of Silicoaluminophosphate SAPO-11 Tumbling-Assisted, Oriented Assembly Crystallization: A Pathway to Enhance Selectivity in Hydroisomerization. Catal. Sci. Technol. 2018, 8, 5044–5061. [Google Scholar] [CrossRef]
- Guo, L.; Bao, X.; Fan, Y.; Shi, G.; Liu, H.; Bai, D. Impact of Cationic Surfactant Chain Length during SAPO-11 Molecular Sieve Synthesis on Structure, Acidity, and n-Octane Isomerization to Di-Methyl Hexanes. J. Catal. 2012, 294, 161–170. [Google Scholar] [CrossRef]
- Fernandes, A.; Ribeiro, F.; Lourenço, J.; Gabelica, Z. An Elegant Way to Increase Acidity in SAPOs: Use of Methylamine as Co-Template during Synthesis. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, pp. 281–284. ISBN 978-0-444-53297-8. [Google Scholar]
- Liu, P.; Ren, J.; Sun, Y. Effect of Template Content on the Physicochemical Characterization and Catalytic Performance of SAPO-11 for the Hydroisomerization of n-Tetradecane. J. Fuel Chem. Technol. 2008, 36, 610–615. [Google Scholar] [CrossRef]
- Luan, H.; Wu, Q.; Wu, J.; Meng, X.; Xiao, F.-S. Templates for the Synthesis of Zeolites. Chin. J. Struct. Chem. 2024, 43, 100252. [Google Scholar] [CrossRef]
- Tapp, N.J.; Milestone, N.B.; Bibby, D.M. Synthesis of AIPO4-11. Zeolites 1988, 8, 183–188. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Guo, S.; Zhao, L.; Xiang, H. Optimization of the Synthesis of SAPO-11 for the Methylation of Naphthalene with Methanol by Varying Templates and Template Content. J. Braz. Chem. Soc. 2013, 24, 1180–1187. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.-L.; Dong, P.; Yuan, G.; Xu, K. Characterization and Hydroisomerization Performance of SAPO-11 Molecular Sieves Synthesized in Different Media. Appl. Catal. A Gen. 2007, 332, 46–55. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Cherepanova, S.V.; Fayzullina, Z.R.; Serebrennikov, D.V.; Khalilov, L.M.; Prosochkina, T.R.; Kutepov, B.I. Crystallization of SAPO-11 Molecular Sieves Prepared from Silicoaluminophosphate Gels Using Boehmites with Different Properties. Gels 2023, 9, 123. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Yakovenko, R.E.; Kolyagin, Y.G.; Serebrennikov, D.V.; Vildanov, F.S.; Prosochkina, T.R.; Kutepov, B.I. Relation between Morphology and Porous Structure of SAPO-11 Molecular Sieves and Chemical and Phase Composition of Silicoaluminophosphate Gels. Gels 2022, 8, 142. [Google Scholar] [CrossRef]
- Serebrennikov, D.V.; Zabirov, A.R.; Saliev, A.N.; Yakovenko, R.E.; Prosochkina, T.R.; Fayzullina, Z.R.; Guskov, V.Y.; Kutepov, B.I.; Agliullin, M.R. Synthesis and Application of SAPO-11 Molecular Sieves Prepared from Reaction Gels with Various Templates in the Hydroisomerization of Hexadecane. Gels 2024, 10, 792. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Fayzullin, A.V.; Fayzullina, Z.R.; Kutepov, B.I. The Role of Intermediate Phases in the Crystallization of Aluminophosphate Sieves on Examples of AlPO-11 and AlPO-41. Crystals 2023, 13, 227. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Khairullina, Z.R.; Faizullin, A.V.; Kutepov, B.I. Crystallization of AlPO4-11 Aluminophosphate from Various Aluminum Sources. Pet. Chem. 2019, 59, 349–353. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Lazarev, V.V.; Kutepov, B.I. Influence of the Formation Conditions of Aluminophosphate Gels on the Morphology and Pore Structure of Molecular Sieve AlPO4-11. Russ. Chem. Bull. 2021, 70, 47–55. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Shamanaeva, I.A.; Zabirov, A.R.; Lazarev, V.V.; Maistrenko, V.N.; Kutepov, B.I. Influence of the Nature of the Al Source on the Properties of the Initial Reaction Gels for Crystallization of Molecular Sieve AlPO4-11. Pet. Chem. 2022, 62, 291–300. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Y. Examining the Self-Assembly of Microporous Material AlPO4-11 by Dry-Gel Conversion. J. Phys. Chem. C 2007, 111, 15236–15243. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Arzumanov, S.S.; Gerasimov, E.Y.; Grigorieva, N.G.; Bikbaeva, V.R.; Serebrennikov, D.V.; Khalilov, L.M.; Kutepov, B.I. Crystal Engineering of SAPO-11 Sieves by Forming Intermediate Phases. CrystEngComm 2023, 25, 3096–3107. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Z.; Sun, K.; Guo, M.; Guo, Q.; Song, Y.; Li, W.; Li, C. In Situ UV Raman Spectroscopic Study on the Synthesis Mechanism of AlPO-5. Angew. Chem. 2009, 121, 8899–8903. [Google Scholar] [CrossRef]
- Holmes, A.J.; Kirkby, S.J.; Ozin, G.A.; Young, D. Raman Spectra of the Unidimensional Aluminophosphate Molecular Sieves AlPO4-11, AlPO4-5, AlPO4-8, and VPI-5. J. Phys. Chem. 1994, 98, 4677–4682. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F.-S. Characterization of Aluminosilicate Zeolites by UV Raman Spectroscopy. Microporous Mesoporous Mater. 2001, 46, 23–34. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Kutepov, B.I. Selective Crystallization of AlPO4-41 Molecular Sieve in the Presence of Diethylamine. Pet. Chem. 2020, 60, 890–894. [Google Scholar] [CrossRef]
- Aguado, J.; Escola, J.M.; Castro, M.C. Influence of the thermal treatment upon the textural properties of sol–gel mesoporous γ-alumina synthesized with cationic surfactants. Microporous Mesoporous Mater. 2010, 128, 48–55. [Google Scholar] [CrossRef]
- Araujo, A.; Fernandes, V.; Silva, A.; Diniz, J. Evaluation of the ALPO-11 Crystallinity by Thermogravimetry. J. Therm. Anal. Calorim. 1999, 56, 151–157. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.-J.; Wu, W. Bifunctional Catalysts for the Hydroisomerization of N-Alkanes: The Effects of Metal–Acid Balance and Textural Structure. Catal. Sci. Technol. 2019, 9, 4162–4187. [Google Scholar] [CrossRef]
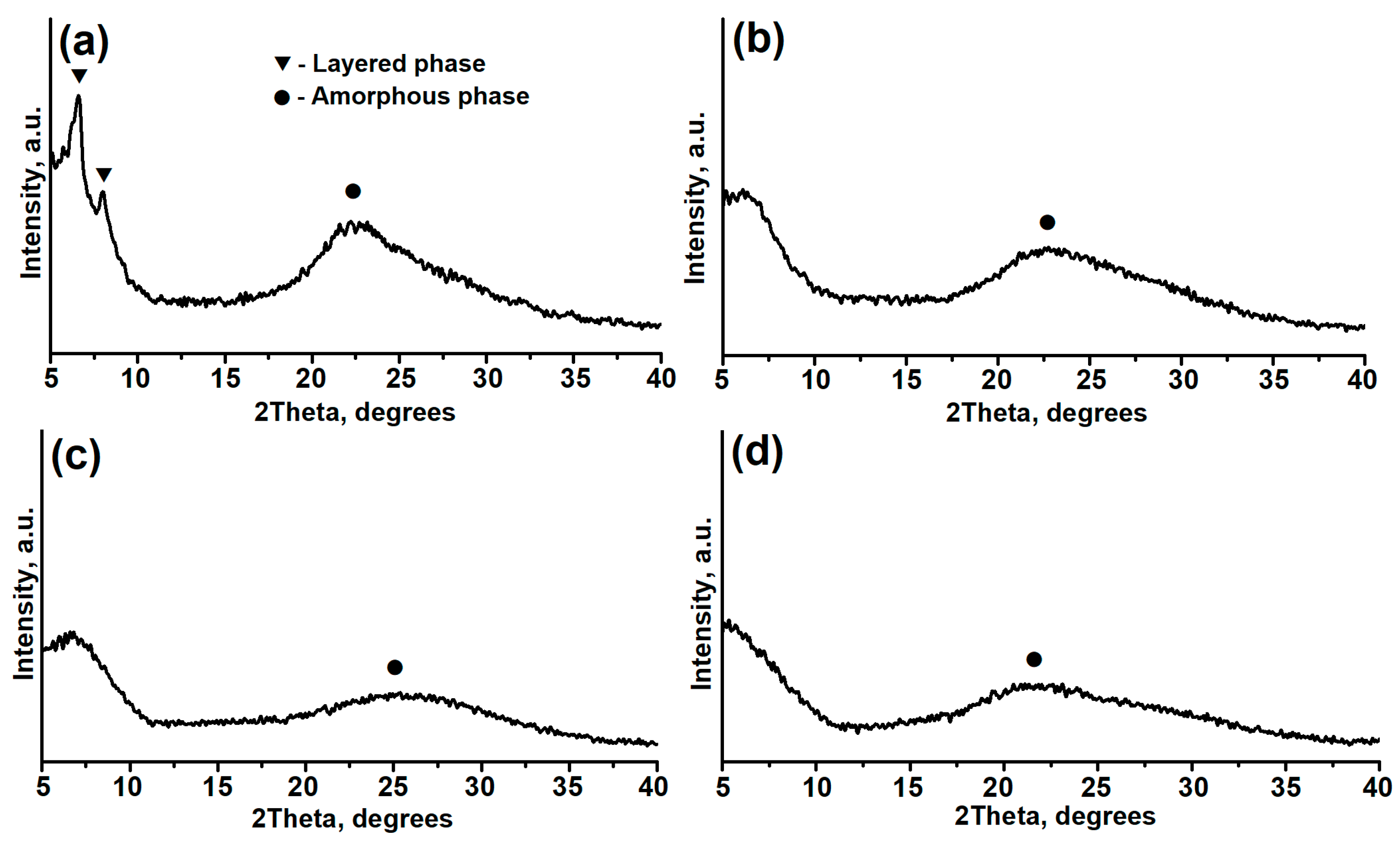
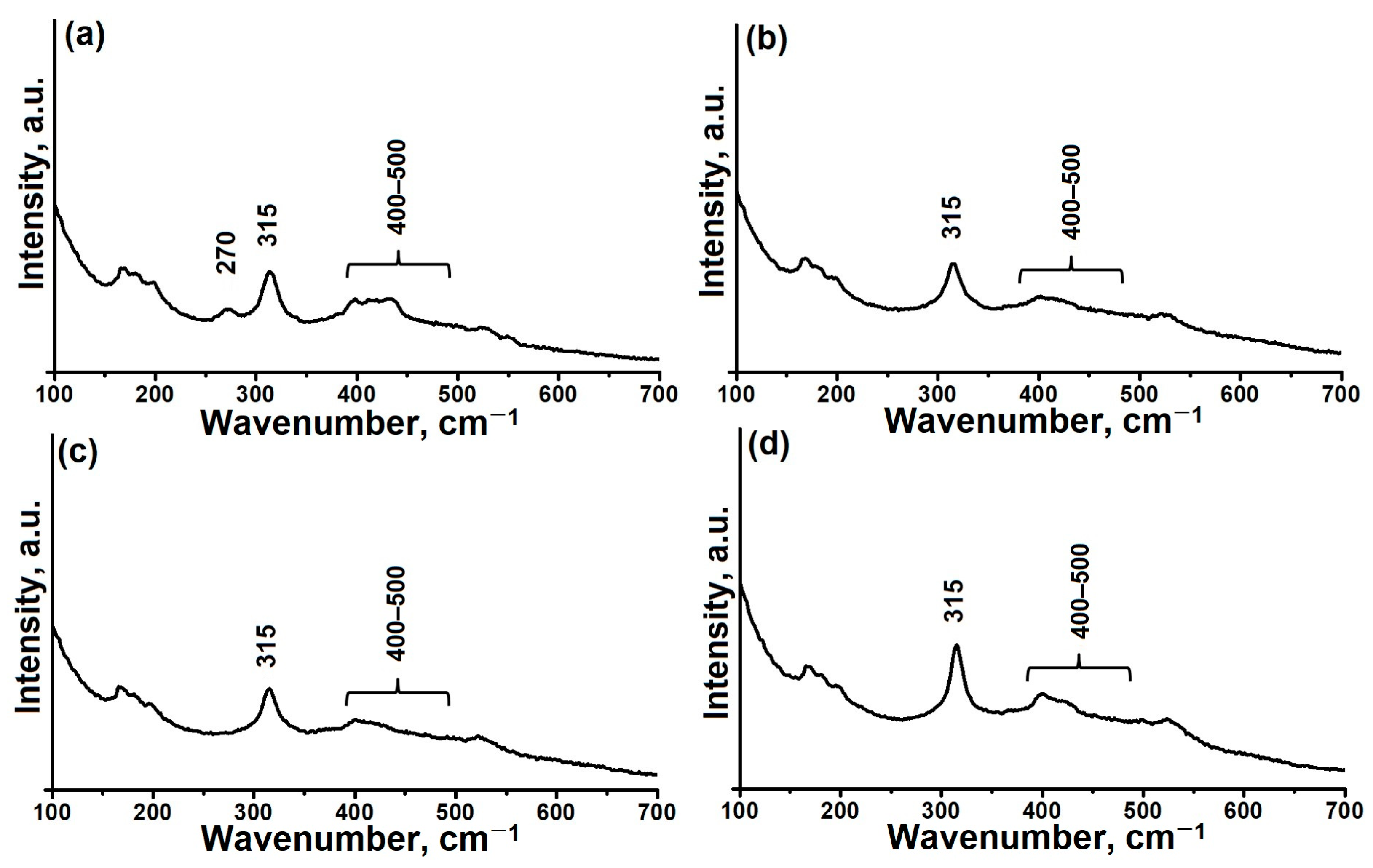


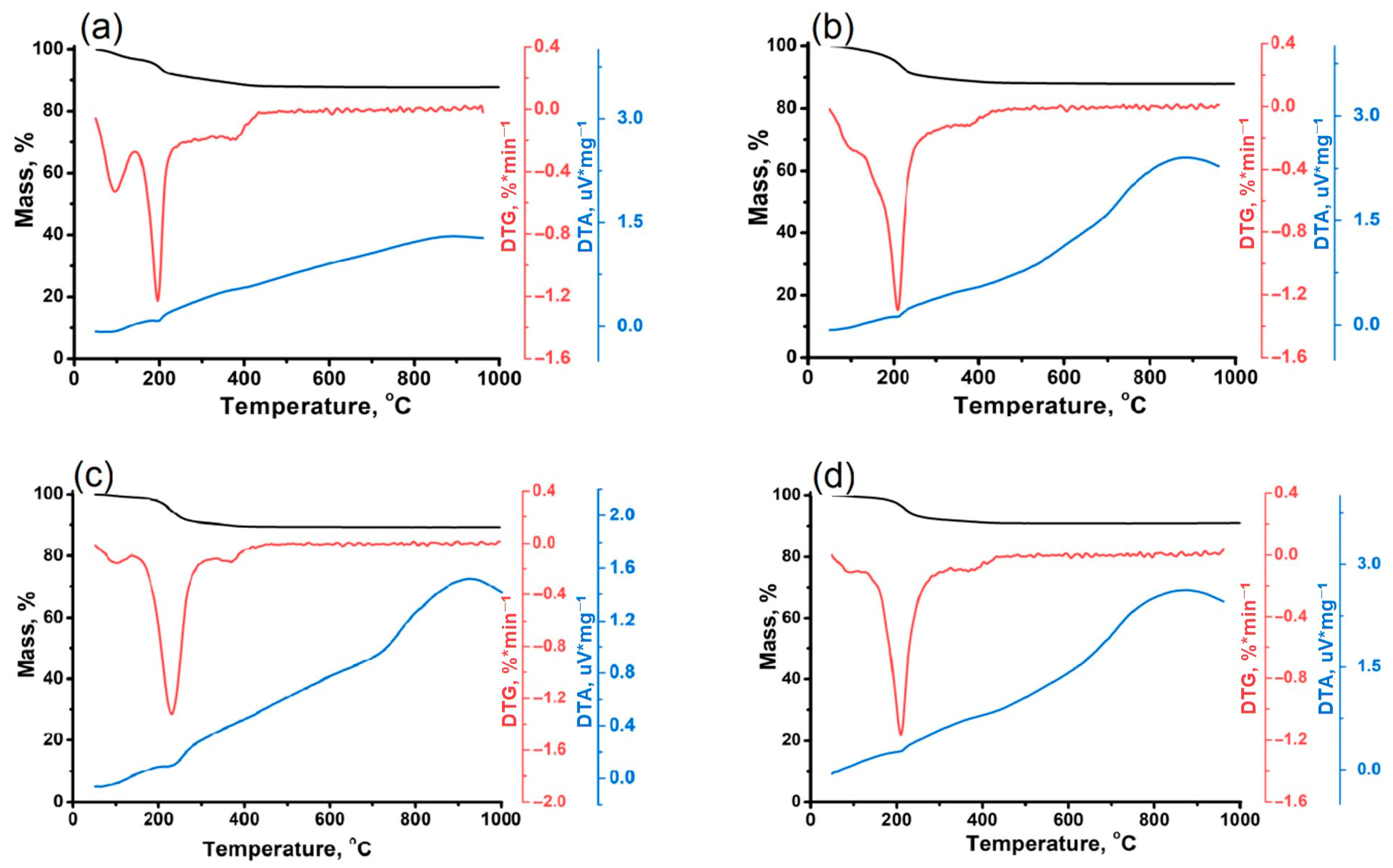

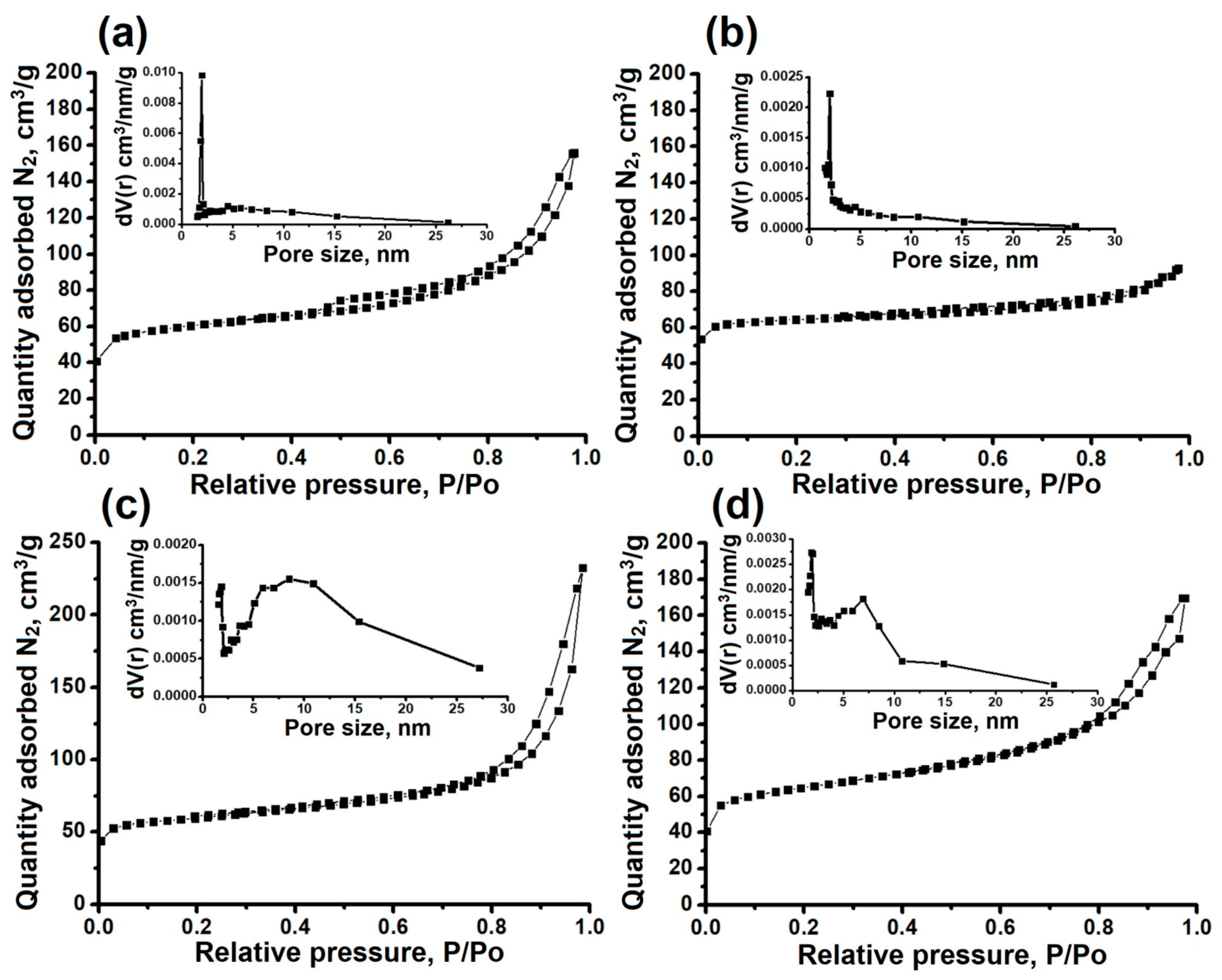

| Sample | Phase Composition | DC, % | Crystallization Yield, % |
|---|---|---|---|
| AlPO-11-DEA | AEL (90%) + AFO (10%) | - | 90 |
| AlPO-11-DPA | AEL | 92 | 89 |
| AlPO-11-DIPA | AEL | 91 | 85 |
| AlPO-11-DBA | AEL | 92 | 86 |
| Sample | Gel | AlPO4-11 |
|---|---|---|
| AlPO-11-DEA | Al1.00P0.99 | Al1.00P0.98 |
| AlPO-11-DPA | Al1.00P0.97 | Al1.00P0.99 |
| AlPO-11-DIPA | Al1.00P0.99 | Al1.00P0.99 |
| AlPO-11-DBA | Al1.00P0.97 | Al1.00P0.98 |
| Sample | M, % | Q, µV/mg | SDA/Unit Cell |
|---|---|---|---|
| AlPO-11-DEA | 7.50 | 0.087 | 4.1 |
| AlPO-11-DPA | 9.22 | 0.124 | 3.2 |
| AlPO-11-DIPA | 8.44 | 0.122 | 3.0 |
| AlPO-11-DBA | 7.18 | 0.275 | 2.1 |
| Sample | SBET, m2/g | SEX, m2/g | Vmicro, cm3/g | Vmeso, cm3/g |
|---|---|---|---|---|
| AlPO-11-DEA | 196 | 113 | 0.05 | 0.17 |
| AlPO-11-DIPA | 202 | 58 | 0.07 | 0.06 |
| AlPO-11-DPA | 225 | 96 | 0.07 | 0.25 |
| AlPO-11-DBA | 213 | 138 | 0.05 | 0.20 |
| AlPO-11-DIPA-micro | 195 | - | 0.07 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabirov, A.R.; Serebrennikov, D.V.; Kuvatova, R.Z.; Filippova, N.A.; Zilberg, R.A.; Travkina, O.S.; Agliullin, M.R. Regulation of the Properties of the Hierarchical Porous Structure of Alumophosphate Molecular Sieves AEL by Reaction Gels Prepared with Different Templates. Gels 2025, 11, 297. https://doi.org/10.3390/gels11040297
Zabirov AR, Serebrennikov DV, Kuvatova RZ, Filippova NA, Zilberg RA, Travkina OS, Agliullin MR. Regulation of the Properties of the Hierarchical Porous Structure of Alumophosphate Molecular Sieves AEL by Reaction Gels Prepared with Different Templates. Gels. 2025; 11(4):297. https://doi.org/10.3390/gels11040297
Chicago/Turabian StyleZabirov, Arthur R., Dmitry V. Serebrennikov, Rezeda Z. Kuvatova, Nadezhda A. Filippova, Rufina A. Zilberg, Olga S. Travkina, and Marat R. Agliullin. 2025. "Regulation of the Properties of the Hierarchical Porous Structure of Alumophosphate Molecular Sieves AEL by Reaction Gels Prepared with Different Templates" Gels 11, no. 4: 297. https://doi.org/10.3390/gels11040297
APA StyleZabirov, A. R., Serebrennikov, D. V., Kuvatova, R. Z., Filippova, N. A., Zilberg, R. A., Travkina, O. S., & Agliullin, M. R. (2025). Regulation of the Properties of the Hierarchical Porous Structure of Alumophosphate Molecular Sieves AEL by Reaction Gels Prepared with Different Templates. Gels, 11(4), 297. https://doi.org/10.3390/gels11040297








