Dichromated Gelatin in Optics
Abstract
1. Introduction
2. Physics and Chemistry of DCG
2.1. Colloids and Gelatin
2.2. Gelatin Sensitizers: Ammonium, Sodium, and Potassium Dichromates
2.3. Photochemical Processes Taking Place in the DCG Layer
2.4. Dark Reaction
2.5. DCG Films’ Spectral Sensitivity
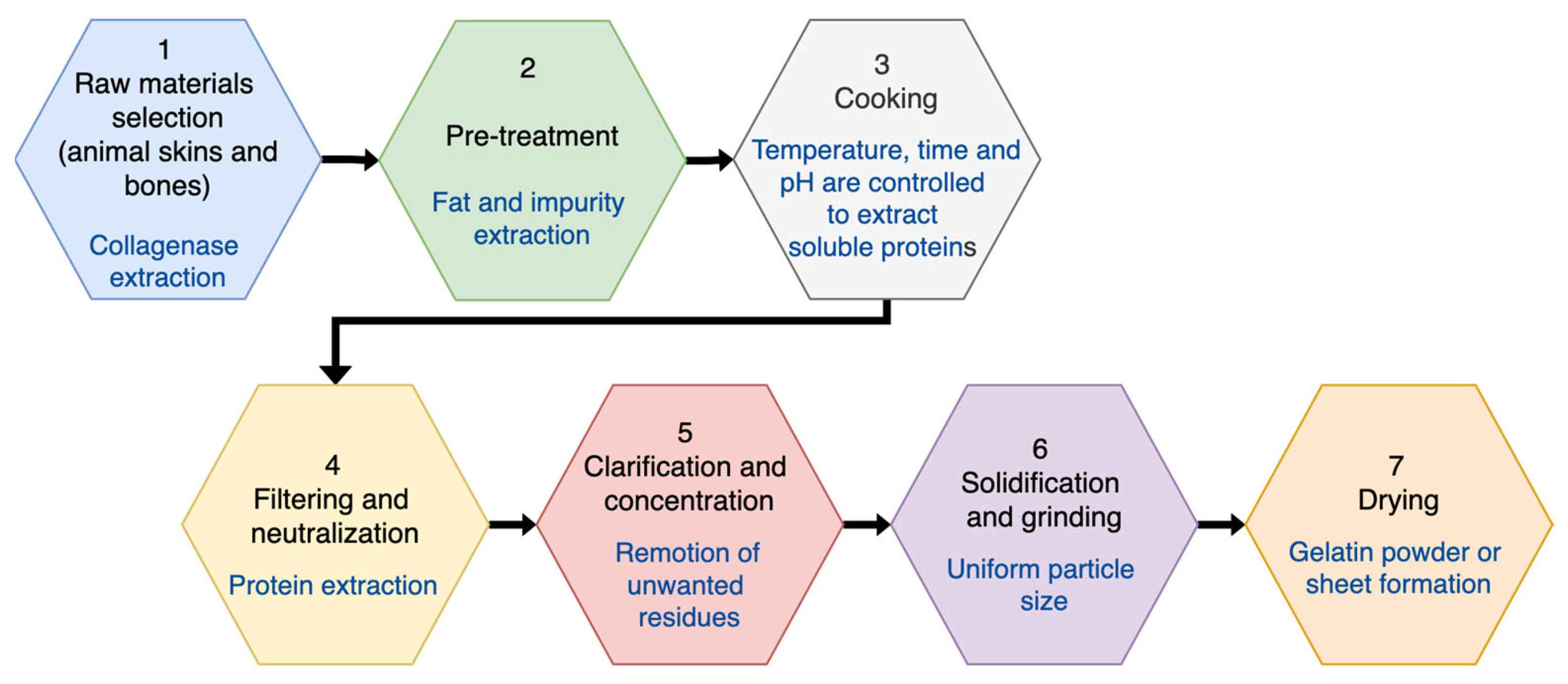
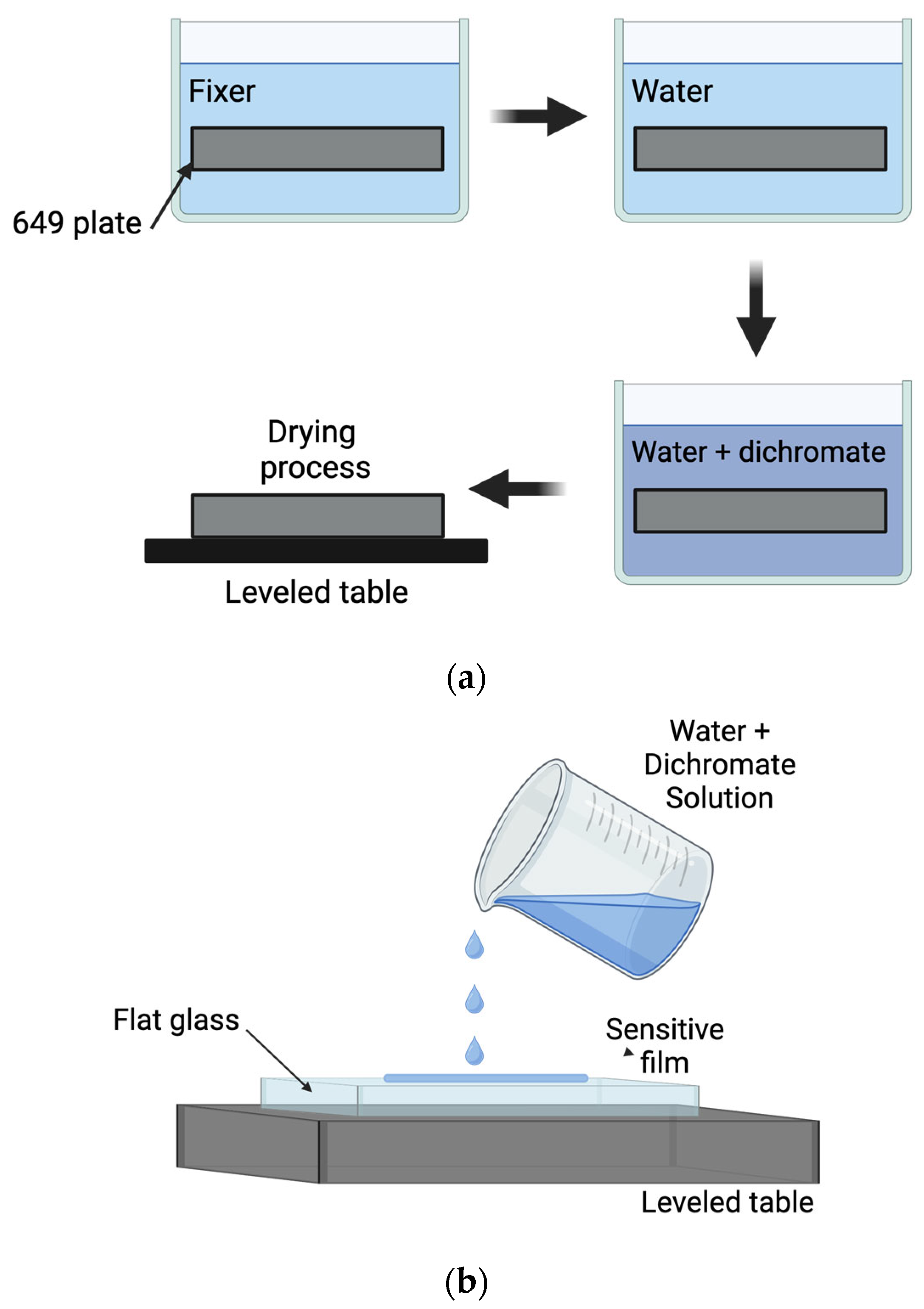
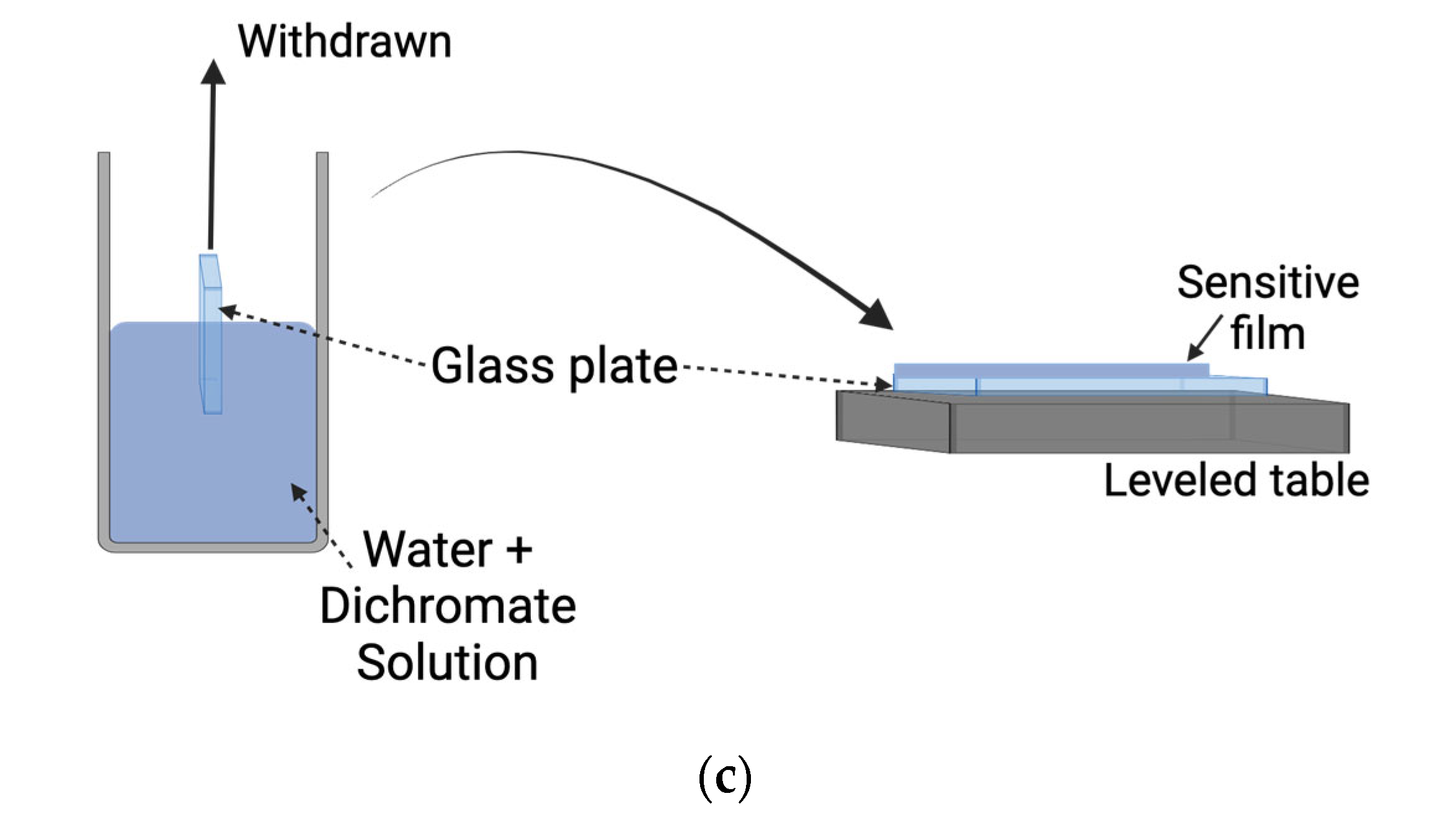
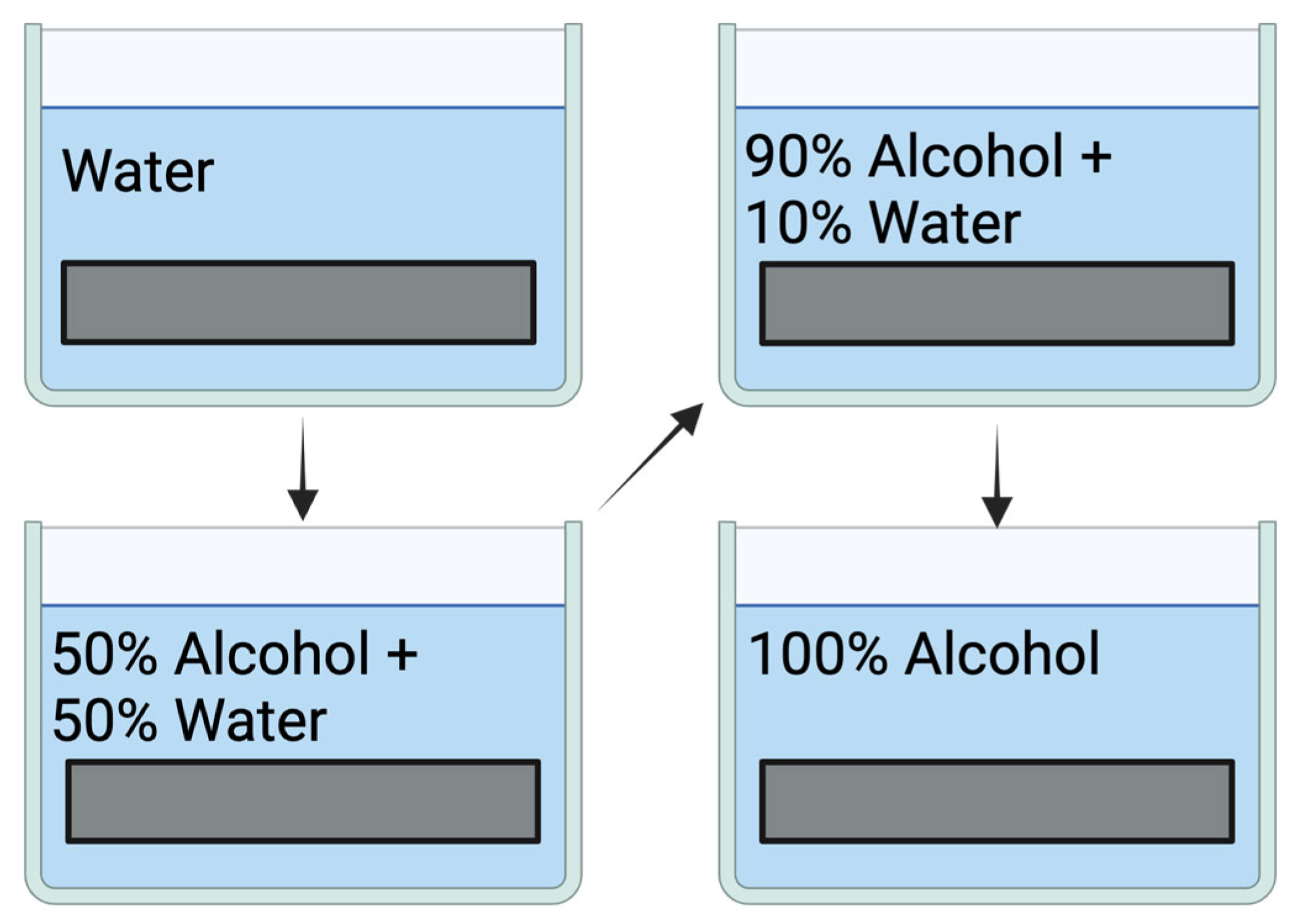
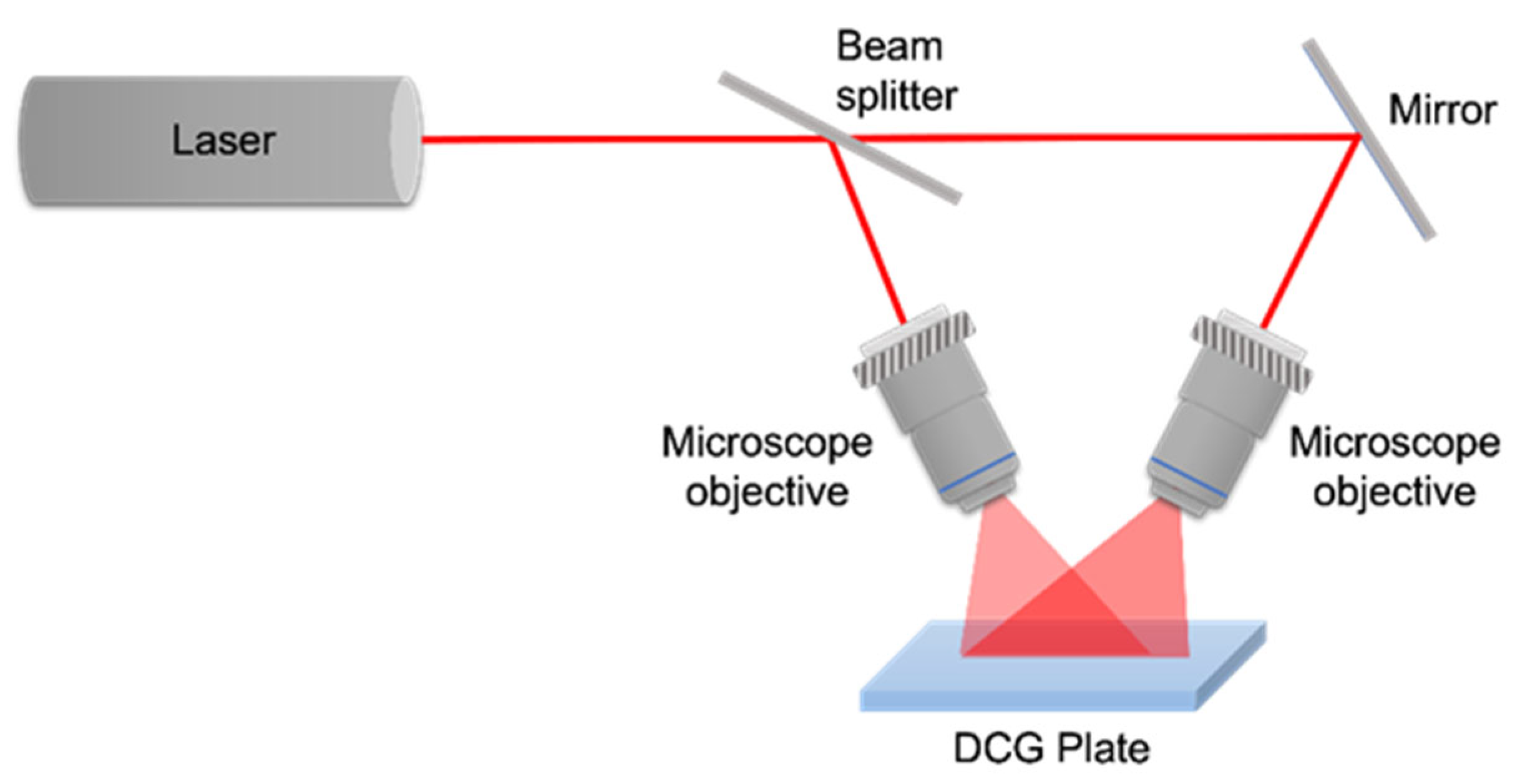
3. DCG Film Fabrication and Processing—Recording Gratings
4. Special Developments of DCG Films
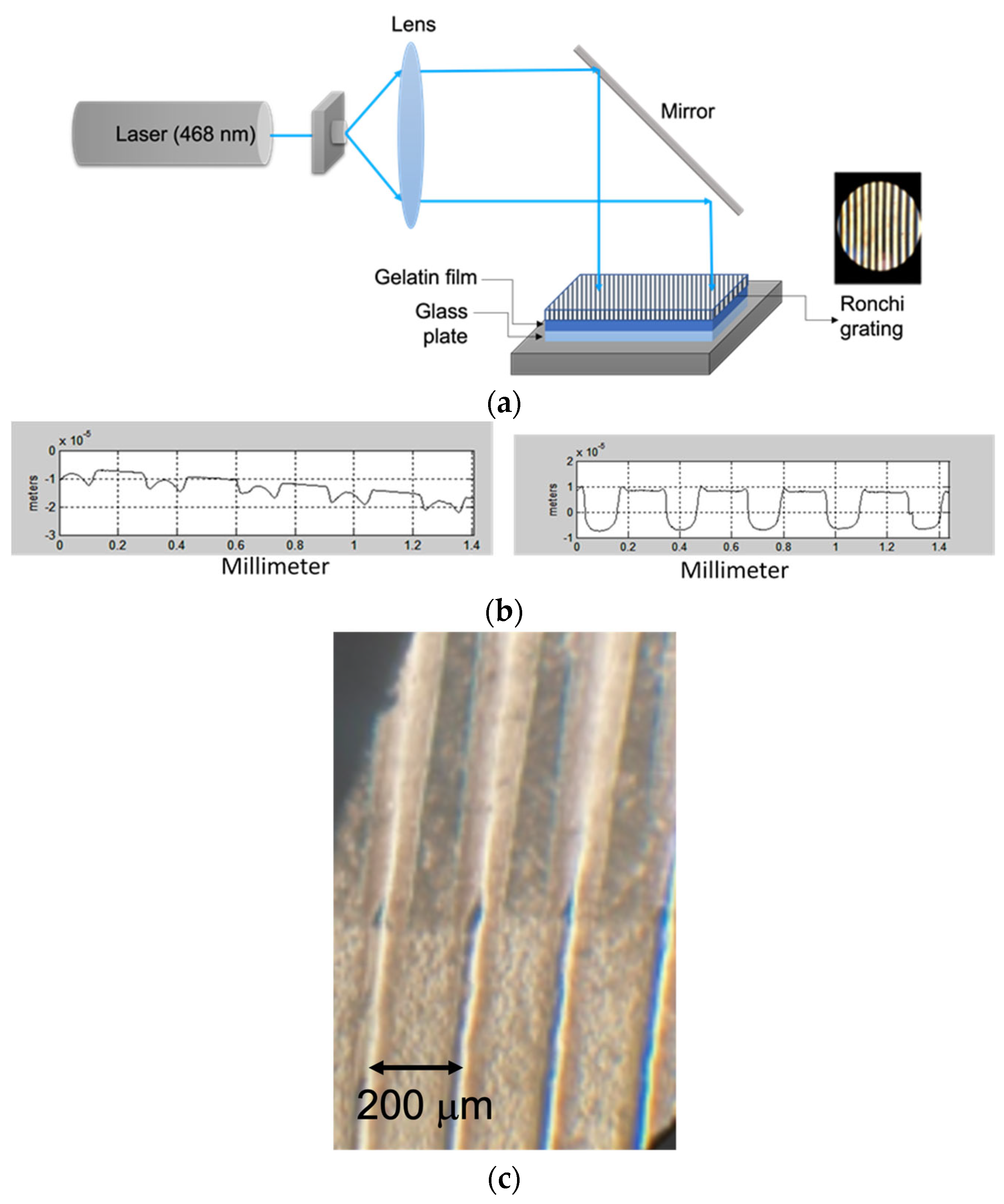
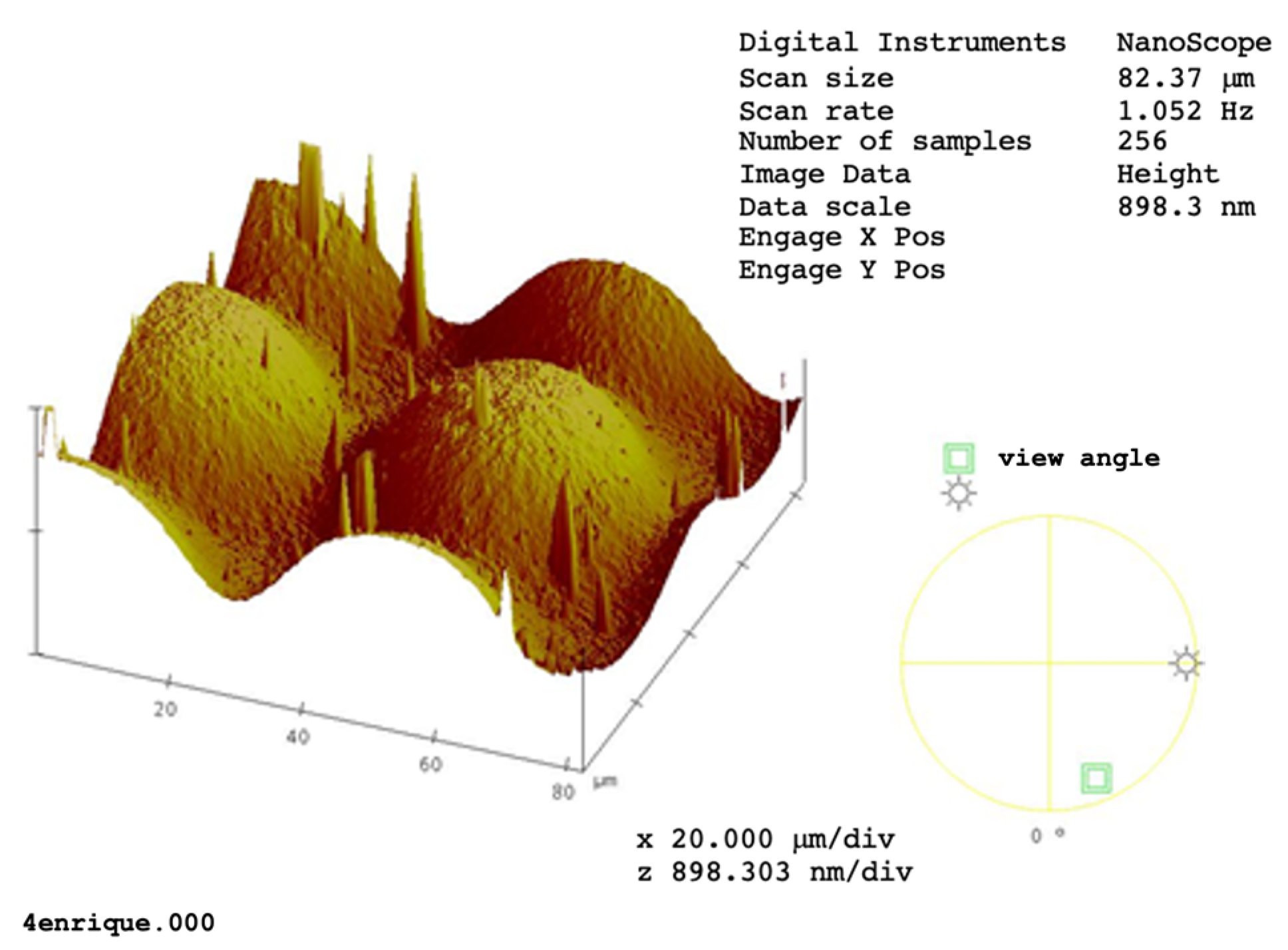
5. Characterization of DCG Films
6. Holography in Real Time with DCG Films
7. Solar Concentrators with DCG Films
8. Control of Spectral Position and Bandwidth
9. DCG in Light Sources
10. Optical Elements Made with DCG
11. DCG Gratings Used in Hygrometers
12. Photopolymers
13. Recent Applications of DCG Plates
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosar, J. Light-Sensitive Systems: Chemistry and Application of Nonsilver Halide Photographic Processes; Wiley: Hoboken, NJ, USA, 1965. [Google Scholar]
- Strutt, J.W.I. Preliminary note on the reproduction of diffraction-gratings by means of photography. Proc. R. Soc. Lond. 1872, 20, 414–417. [Google Scholar] [CrossRef]
- Shankoff, T.A. Phase Holograms in Dichromated Gelatin. Appl. Opt. 1978, 7, 2101–2106. [Google Scholar] [CrossRef]
- Pawluczyk, R.; Billard, T.C.; Quaglia, A.; Vienneau, T.; Hockley, B.S. Characterization Of DCG Holograms During The Production Process: Some Practical Aspects. In Proceedings of the SPIE International Symposium on Optical Engineering and Industrial Sensing for Advance Manufacturing Technologies, Dearborn, MI, USA, 28–29 June 1989. [Google Scholar] [CrossRef]
- Grzymala, R.; Keinonen, T. Dark self-enhancement in dichromated-gelatin gratings: A detailed study. Appl. Opt. 1999, 38, 7222–7227. [Google Scholar] [CrossRef] [PubMed]
- James, T.H. The Theory of the Photographic Process, 4th ed.; Macmillan: New York, NY, USA, 1977. [Google Scholar]
- Cómo se hace? Cómo Se Fabrica La Gelatina? [Proceso En Fábrica]; YouTube, [Publication Date 28 Feb 2023]. Available online: https://www.youtube.com/watch?v=rRyAPR0b37I (accessed on 19 March 2025).
- Smith, H.M. (Ed.) Holographic Recording Materials; Springer: New York, NY, USA, 1977; pp. 75–99. [Google Scholar]
- Page, B.J.; Loar, G.W. Chromium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2004; Volume 6, pp. 526–571. [Google Scholar] [CrossRef]
- Norseth, T. The carcinogenicity of chromium. Environ. Health Perspect. 1981, 40, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.A.; Salem, H. The toxicology of chromium with respect to its chemical speciation: A review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and Carcinogenicity of Chromium Compounds in Humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chro-mium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Kołaciński, Z.; Kołacinski, Z.; Kostrzewski, P.; Kruszewska, S.; Raźniewska, G.; Mielczarska, J. Acute Potassium Dichromate Poisoning: A Toxicokinetic Case Study. J. Toxicol. Clin. Toxicol. 1999, 37, 785–791. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Mfarrej, M.F.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of chromium in plants and its repercussion in animals and humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef]
- Xiong, L.-W.; Liu, S.; Peng, B.-X. Mechanism of Hologram Formation in Dichromated Gelatin with X-Ray Photoelectron Spectroscopy. Appl. Opt. 1998, 37, 3678–3684. [Google Scholar]
- Slangen, P.; Martinez, C.; Weber, G.; Lion, Y. Measurement of chromium content in dichromated gelatin by x-ray fluorescence. Appl. Opt. 1993, 32, 6132–6136. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Ose, T.; Sasaki, M.; Honda, K. Holgram formation with red light in methylene blue sensitized dichromated gelatin. Appl. Opt. 1976, 15, 556–558. [Google Scholar] [CrossRef]
- Pirodda, L.; Moriconi, M. An effective processing agent for dichromated gelatin. Opt. Commun. 1988, 65, 7–10. [Google Scholar] [CrossRef]
- Calixto, S.; Piazza, V.; Garnica, G. Surface Profile Studies of Photoinduced Gratings Made with DCG Films with Optional Papain Development. Gels 2022, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Billard, T.C.; Pawluczyk, R.; Hockley, B.S. The Sensitization Process of Dichromated Gelatin. In Proceedings of the SPIE 1051, Practical Holography III, OE/LASE’89, Los Angeles, CA, USA, 15–20 January 1989. [Google Scholar] [CrossRef]
- Pascual, I.; Belendez, A.; Fimia, A. Analysis of the holographic reciprocity law for di-chromated gelatin. Appl. Opt. 1992, 31, 3200–3201. [Google Scholar] [CrossRef]
- Calixto, S.; Lessard, R.A. Real-time holography with undeveloped dichromated gelatin films. Appl. Opt. 1984, 23, 1989–1994. [Google Scholar] [CrossRef]
- Newell, J.C.; Solymar, L.; Ward, A.A. Holograms in dichromated gelatin: Real-time effects. Appl. Opt. 1985, 24, 4460–4466. [Google Scholar] [CrossRef] [PubMed]
- Denisyuk, Y.N.; Ganzherli, N.M.; Maurer, I.A. Recording of deep three-dimensional holograms in gel-like layers of dichromated gelatin. In Proceedings of the SPIE 2688, Holographic Materials II, Photonics West’96, San Jose, CA, USA, 27 January–2 February 1996. [Google Scholar] [CrossRef]
- Denisyuk, Y.N.; Ganzherli, N.M.; Maurer, I.M.; Pisarevskaya, S.A. Thick-layer glycerin-containing bi-chromated gelatin for recording volume holograms. Tech. Phys. Lett. 1997, 23, 279–280. [Google Scholar] [CrossRef]
- Vigovky, Y.N.; Malov, A.N.; Malov, S.N.; Fetschenko, S.; Konop, S. New dichromated gelatin technologies for diffraction optical element fabrication. In Proceedings of the SPIE 3347, Optical Information Science and Technology (OIST97): Optical Recording Mechanisms and Media, Optical Information Science and Technology, Moscow, Russia, 27–30 August 1997. [Google Scholar] [CrossRef]
- Maka, A.O.M.; Alabid, J.M. Solar energy technology and its roles in sustainable development. Clean Energy 2022, 6, 476–483. [Google Scholar] [CrossRef]
- Shanks, K.; Senthilarasu, S.; Mallick, T.K. Optics for concentrating photovoltaics: Trends, limits and opportunities for materials and design. Renew. Sustain. Energy Rev. 2016, 60, 394–407. [Google Scholar] [CrossRef]
- Hull, J.; Lauer, J.; Broadbent, D. Holographic solar concentrators. Energy 1987, 12, 209–215. [Google Scholar] [CrossRef]
- Ferrara, M.A.; Striano, V.; Coppola, G. Volume Holographic Optical Elements as Solar Concentrators: An Overview. Appl. Sci. 2019, 9, 193. [Google Scholar] [CrossRef]
- Collados, M.V.; Chemisana, D.; Atencia, J. Holographic solar energy systems: The role of optical elements. Renew. Sustain. Energy Rev. 2016, 59, 130–140. [Google Scholar] [CrossRef]
- Pratheep, H.R.; Balamurugan, A. A review of holographic optical elements in solar concentrator applications. Int. J. Adv. Res. Ideas Innov. Technol. 2018, 4, 214–222. [Google Scholar]
- Vorndran, S.D.; Chrysler, B.; Wheelwright, B.; Angel, R.; Holman, Z.; Kostuk, R. Off-axis holographic lens spectrum-splitting photovoltaic system for direct and diffuse solar energy conversion. Appl. Opt. 2016, 55, 7522–7529. [Google Scholar] [CrossRef]
- Zhang, D.; Vorndran, S.; Russo, J.M.; Gordon, M.; Kostuk, R.K. Ultra light-trapping filters with broadband reflection holograms. Opt. Express 2012, 20, 14260–14271. [Google Scholar] [CrossRef]
- Ranjan, R.; Khan, A.; Chakraborty, N.R.; Yadav, H.L. Use of holographic lenses recorded in dichromated gelatin film for PV concentrator applications to minimize solar tracking in Energy Problems and Environmental Engineering. In Proceedings of the 3rd WSEAS International Conference on Energy Planning, Energy Saving, Environmental Education, Canary Islands, Spain, 1–3 July 2009; Perlovsky, L., Dionysiou, D.D., Zadeh, L.A., Kostic, M.M., Gonzalez-Concepcion, C., Jaberg, H., Sopian, K., Eds.; WSEAS Press: Athens, Greece, 2009; pp. 49–52, ISBN 978-960-474-093-2. [Google Scholar]
- Imenes, A.G.; Mills, D.R. Spectral beam splitting technology for increased conversion efficiency in solar concentrating systems: A review. Sol. Energy Mater. Sol. Cells 2004, 84, 19–69. [Google Scholar] [CrossRef]
- Ludman, J.; Riccobono, J.; Reinhand, N.; Semenova, I.; Martin, J.; Tai, W.; Li, X.-L.; Syphers, G. Holographic solar concentrator for terrestrial photovoltaics. In Proceedings of the 1994 IEEE 1st World Conference on Photovoltaic Energy Conversion–WCPEC (A Joint Conference of PVSC, PVSEC and PSEC), Waikoloa, HI, USA, 5–9 December 1994; Volume 1, pp. 1208–1215. [Google Scholar] [CrossRef]
- Alfaro, E.; Vilardy, J.M.; Bastidas, M.; Lloret, T.; Morales-Vidal, M.; Pascual, I.; Jimenez Ruiz, C. Review of recording materials in holographic lenses for solar energy applications. In Proceedings of the SPIE 13015, Photosensitive Materials and their Applications III, 130151A, SPIE Photonics Europe, Strasbourg, France, 7–11 April 2024. [Google Scholar] [CrossRef]
- Kostuk, R.K.; Vorndran, S.D.; Zhang, D.; Russo, J.M.; Gordon, M. Holographic Diffraction-Through-Aperture Spectrum Splitting System and Method. U.S. Patent No. 10,514,485, 2019. Available online: https://patents.google.com/patent/US10514485B2 (accessed on 15 March 2025).
- Bloss, W.H.; Griesinger, M.; Reinhardt, E.R. Dispersive concentrating systems based on transmission phase holograms for solar applications. Appl. Opt. 1982, 21, 3739–3742. [Google Scholar] [CrossRef]
- Ludman, J.E.; Riccobono, J.; Semenova, I.V.; Reinhand, N.O.; Tai, W.; Li, X.; Syphers, G.; Rallis, E.; Sliker, G.; Martín, J. The optimization of a holographic system for solar power generation. Sol. Energy 1997, 60, 1–9. [Google Scholar] [CrossRef]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a Photosensitive Material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef]
- Schuette, H.; Dederichs, V.; Stojanoff, C.G. Methods for influencing the optical properties of dichromated gelatin films. In Proceedings of the SPIE 2262, Optical Thin Films IV: New Developments, SPIE’s 1994 International Symposium on Optics, Imaging, and Instrumentation, San Diego, CA, USA, 24–29 July 1994. [Google Scholar] [CrossRef]
- Markova, B.; Nazarova, D.; Sharlandjiev, P. Control of the spectral position of dichromated gelatin reflection holograms. Appl. Opt. 2011, 50, 5534–5537. [Google Scholar] [CrossRef] [PubMed]
- Stojanoff, C.G.; Froening, P.; Schulat, J. Use of filler material in DCG films for predictable shift of the spectral characteristics of holograms. In Proceedings of the SPIE 3638, Holographic Materials V, Electronic Imaging’99, San Jose, CA, USA, 23–29 January 1999. [Google Scholar] [CrossRef]
- Stojanoff, C.G. Effects of the film manufacturing procedure and development process on the holographic properties of HOE in DCG. In Proceedings of the SPIE 5290, Practical Holography XVIII: Materials and Applications, Electronic Imaging 2004, San Jose, CA, USA, 19–21 January 2004. [Google Scholar] [CrossRef]
- Coleman, D.J.; Magariños, J. Controlled shifting of the spectral response of reflection holograms. Appl. Opt. 1981, 20, 2600–2601. [Google Scholar] [CrossRef] [PubMed]
- McGrew, S.P. Color Control In Dichromated Gelatin Reflection Holograms. In Proceedings of the SPIE 0215, Recent Advances in Holography, 1980 Los Angeles Technical Symposium, Los Angeles, CA, USA, 4–7 February 1980. [Google Scholar] [CrossRef]
- McCartney, D.J.; Payne, D.B.; Duncan, S.S. Position-tunable holographic filters in dichromated gelatin for use in single-mode-fiber demultiplexers. Opt. Lett. 1985, 10, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.-H.; Lu, W.; Tam, W.-Y.; Wong, K.L.G. Lasing from dye-doped icosahedral quasicrystals in dichromate gelatin emulsions. Opt. Exp. 2009, 17, 7275–7284. [Google Scholar] [CrossRef]
- Navarrete-García, E.; Calixto, S. Continuous surface relief micro-optical elements fabricated on photographic emulsions by use of binary and halftone masks. Opt. Mater. 2003, 23, 501–512. [Google Scholar] [CrossRef]
- Calixto, S.; Andres, M.V. Water Vapor Sensors Based on the Swelling of Relief Gelatin Gratings. Adv. Mater. Sci. Eng. 2015, 2015, 584324. [Google Scholar] [CrossRef]
- Calixto, S.; Lougnot, D.; Naydenova, I. Light sensitive materials: Silver Halide Emulsions, Photoresist and Photopolymers. In Handbook of Optical Engineering, 2nd ed.; Malacara, D., Thompson, B.J., Eds.; Marcel Dekker: New York, NY, USA, 2001; Chapter 25. [Google Scholar]
- Murray, M.; Naydenova, I.; Martin, S. Review of recent advances in photosensitive polymer materials and requirements for transmission diffractive optical elements for LED light sources. Opt. Mater. Express 2023, 13, 3481–3501. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Liu, A.; Weng, Y.; Li, X. Volume holographic waveguide display with large field of view using a Au-NPs dispersed acrylate-based photopolymer. Opt. Mater. Express 2020, 10, 312. [Google Scholar] [CrossRef]
- Shen, Z.; Weng, Y.; Zhang, Y.; Wang, C.; Liu, A.; Li, X. Holographic recording performance of acrylate-based photopolymer under different preparation conditions for waveguide display. Polymers 2021, 13, 936. [Google Scholar] [CrossRef]
- Guo, B.; Wang, M.; Zhang, D.; Sun, M.; Bi, Y.; Zhao, Y. High refractive index monomers for improving the holographic recording performance of two-stage photopolymers. ACS Appl. Mater. Interfaces 2023, 15, 24827–24835. [Google Scholar] [CrossRef]
- Rogers, B.; Martin, S.; Naydenova, I. Study of the effect of methyldiethanolamine initiator on the recording properties of acrylamide based photopolymer. Polymers 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Li, W.; Shi, Z.; Chen, H.; Jiang, X. Effect of monomers on the holographic properties of poly(vinylalcohol)-based photopolymers. ACS Appl. Polym. Mater. 2020, 2, 5208–5218. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, W.; Zhou, X.; Wu, W.; Liu, Q.; Peng, H.; Zhu, J.; Smalyukh, I.I.; Xie, X. Holographic polymer nanocomposites with simultaneously boosted diffraction efficiency and upconversion photoluminescence. Compos. Sci. Technol. 2019, 181, 107705. [Google Scholar] [CrossRef]
- Hu, Y.; Kowalski, B.A.; Mavila, S.; Podgórski, M.; Sinha, J.; Sullivan, A.C.; McLeod, R.R.; Bowman, C.N. Holographic photopolymer material with high dynamic range (δn) via thiol–ene click chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44103–44109. [Google Scholar] [CrossRef]
- Galli, P.; Evans, R.A.; Bertarelli, C.; Bianco, A. Cyclic allylic sulfide based photopolymer for holographic recording showing high refractive index modulation. J. Polym. Sci. 2021, 59, 1399–1413. [Google Scholar] [CrossRef]
- Galli, P.; Evans, R.A.; Bertarelli, C.; Bianco, A. Holographic photopolymer with high sulfur content for high refractive index modulation. In Proceedings of the SPIE 11774, Holography: Advances and Modern Trends VII, SPIE Optics + Optoelectronics, 2021, Online, 19–29 April 2021; p. 1177404. [Google Scholar]
- Mavila, S.; Sinha, J.; Hu, Y.; Podgórski, M.; Shah, P.K.; Bowman, C.N. High refractive index photopolymers by thiol–yne “click” polymerization. ACS Appl. Mater. Interfaces 2021, 13, 15647–15658. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Yadav, H.L. Dichromated gelatin, an efficient material for the fabrication of wavelength selective holographic solar concentrators for high-efficiency operation. Mater. Today Proc. 2022, 56, 94–99. [Google Scholar] [CrossRef]
- Jeanneau, A.; Bianco, A.; Clawson, A.; Frangiamore, M.; Pearson, E.; Pinard, L.; Schmoll, J.; Richard, J.; Giroud, R.; Laurent, F.; et al. Characterization of two ultraviolet–blue volume-phase holographic gratings based on dichromated gelatin and photopolymer recording materials. J. Astron.Telesc.Instrum. Syst. 2024, 10, 040501. [Google Scholar] [CrossRef]
- Arkhipov, A.V.; Ganzherli, N.M.; Gulyaev, S.N.; Maurer, I.A. High-frequency relief-phase holographic gratings on gelatin-containing photosensitive media. J. Opt. Technol. 2023, 90, 125–130. [Google Scholar] [CrossRef]
- Allegro, I.; Bonal, V.; Mamleyev, E.R.; Villalvilla, J.M.; Quintana, J.A.; Jin, Q.; Díaz-García, M.A.; Lemmer, U. Distributed Feedback Lasers by Thermal Nanoimprint of Perovskites Using Gelatin Gratings. ACS Appl. Mater. Interfaces 2023, 15, 8436–8445. [Google Scholar] [CrossRef]
- Murić, B.D.; Pantelić, D.V.; Radmilović, M.D.; Savić-Šević, S.N.; Vasović, V.O. Characterization and Optimization of Real-Time Photoresponsive Gelatin for Direct Laser Writing. Polymers 2022, 14, 2350. [Google Scholar] [CrossRef] [PubMed]
- Ganzherli, N.M.; Gulyaev, S.N.; Maurer, I.A. Improvement of the technology for manufacturing relief holographic gratings on dichromated gelatin irradiated with short-wave UV radiation. Opt. Spectrosc. 2022, 130, 2011–2013. [Google Scholar] [CrossRef]
- Prokopova, D.V.; Eremchev, I.Y.; Losevsky, N.N.; Belousov, D.A.; Golubtsov, S.K.; Kotova, S.P.; Naumov, A.V. Diffractive Optical Elements for Three-Dimensional Nanoscopy Using Rotating Light Fields. Bull. Russ. Acad. Sci. Phys. 2024, 88, 1875–1880. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, A.; Khan, A.A.; Roy, S.; Yadav, H.L. Design and analysis of holographic optical elements for their use as couplers with appreciable efficiency at different optical transmission windows. Optik 2022, 261, 169184. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dhara, B.; Mallik, S.; Pathak, K.; Goswami, D.K.; Debnath, K.; Bhaktha, S.B. Temperature Tunable Optical Tamm State in Holographic Photonic Crystal. In Proceedings of the Frontiers in Optics + Laser Science 2023 (FiO, LS), Tacoma, WA, USA, 9–12 October 2023; Optica Publishing Group: Washington, DC, USA, 2023; p. JM4A–48. [Google Scholar]
- Farrando-Pérez, Á.; Villalvilla, J.M.; Quintana, J.A.; Boj, P.G.; Díaz-García, M.A. Top-Layer Resonator Organic Distributed Feedback Laser for Label-Free Refractive Index Sensing. Adv. Opt. Mater. 2024, 12, 2401284. [Google Scholar] [CrossRef]
- de León, Y.P.; Flores, B.M.; Ortiz-Gutiérrez, M.; Torres, J.C.I.; Cortes, M.P. Real-time characterization of gelatin doped with potassium dichromate as a photosensitive material. Optik 2001, 242, 167310. [Google Scholar] [CrossRef]
- Zhao, J.; Chrysler, B.D.; Kostuk, R.K. Design of a waveguide eye-tracking system operating in near-infrared with holographic optical elements. Opt. Eng. 2021, 60, 085101. [Google Scholar] [CrossRef]
- Li, y.; Yang, Q.; Xiong, J.; Yin, K.; Wu, D. 3D displays in augmented and virtual realities with holographic optical elements [Invited]. Opt. Express 2021, 29, 42696–42712. [Google Scholar] [CrossRef]
- Kress, B.; Shin, M. Diffractive and holographic optics as optical combiners in head mounted displays. In Proceedings of the 2013 ACM Conference on Pervasive and Ubiquitous Computing Adjunct Publication, Zurich, Switzerland, 8–12 September 2013; ACM: New York, NY, USA, 2013; pp. 1479–1482. [Google Scholar] [CrossRef]
- Calixto, S.; Scholl, M.S. Relief optical microelements fabricated with dichromated gelatin. Appl. Opt. 1997, 36, 2101–2106. [Google Scholar] [CrossRef]



| Concentration [%] | Thickness (μm) | Diffraction Efficiency [%] | Bandwidth [nm] |
|---|---|---|---|
| 2 | 12.5 | 90 | 16 |
| 2 | 15.2 | 95 | 16 |
| 5 | 12.8 | 97 | 17 |
| 5 | 14.9 | 99.3 | 20 |
| 10 | 12.3 | 99.5 | 25 |
| 10 | 16.1 | 99.4 | 22 |
| Wavelength and Conditions | Thickness [μm] | Grating Period [μm] | Sensitivity [mJ/cm²] |
|---|---|---|---|
| 448 nm | 1 | 0.5 | 2.3 |
| 3 | 0.5 | 1.7 | |
| 7 | 0.5 | 2.3 | |
| 10 | 0.5 | 1.5 | |
| 15 | 0.5 | 1.9 | |
| 448 nm, 649F plates | 12 | 1.4 | 6.6 |
| 12 | 0.28 | 1.2 | |
| 12 | 0.16 | 1.6 | |
| 448 nm, 649F plates | 14 | 0.4–10 | 2.5 |
| 441 nm, 649F plates | 13 | ~0.5 | 5 |
| Reference | Presentation | Film Thickness [μm] | Exposure Wavelength [nm] | Sensitivity [mJ/cm²] | Resolution [lines/mm] | Diffraction Efficiency [%] |
|---|---|---|---|---|---|---|
| PMMA– titanocene | PMMA block | 500–3000 | 514 | 4000 | — | ~100 |
| Reference | Presentation | Film Thickness [μm] | Exposure Wavelength [nm] | Sensitivity [mJ/cm²] | Resolution [lines/mm] | Diffraction Efficiency [%] |
|---|---|---|---|---|---|---|
| Diluent + oligomers (FPK-488) | Liquid between glass plates | 20 | 300–500 | 20 | 1500–6000 | 80 |
| Diluent + oligomers (FPK-488) | Liquid between glass plates | 20 | 633 | 50 | — | 60 |
| Pre-polymerized multicomponents (PHG###) | Liquid between glass plates | 20–100 | 450–800 | 100–500 | >3000 | 80 |
| Reference | Presentation | Film Thickness [μm] | Exposure Wavelength [nm] | Sensitivity [mJ/cm²] | Resolution [lines/mm] | Diffraction Efficiency [%] |
|---|---|---|---|---|---|---|
| p-Vinylcarbazole | Dry film on glass | 2.5–7 | 488 | 50–500 | 800–2500 | 80 |
| PMMA | Dry film on glass | 100–200 | 488 | 7000 | 2000 | ~100 |
| DCPVA | Dry film on glass | 30–60 | 488 | 500 | 3000 | ~70 |
| DCPAA | Dry film on glass | 60 | 488 | 200 | 3000 | ~65 |
| FePVA | Dry film on glass | 60 | 488 | >15,000 | 3000 | 80 |
| Photopolymer | Reference | ∆nmax | Thickness [μm] | Wavelength [nm] | Spatial Frequency [lines/mm] |
|---|---|---|---|---|---|
| Acrylate | Shen et al. [56] | 0.08 | 15 | 532 | 4949 |
| Acrylate | Shen et al. [57] | 0.065 | 12 | 532 | N/A |
| Acrylate | Guo et al. [58] | 0.046 | 5 | 633 | 3250 |
| Acrylamide | Rogers et al. [59] | 0.005 | 36 | 633 | 800 |
| Acrylamide | Pi et al. [60] | N/A | 140 | 532 | N/A |
| Acrylamide | Zhang et al. [61] | 0.034 | 10 | 633 | 1333 |
| Thiol-‘X’ | Hu et al. [62] | 0.04 | 5–10 | 633 | 2000 |
| Thiol-‘X’ | Galli et al. [63,64] | 0.0346 | 16.2 | 633 | 1200 |
| Thiol-‘X’ | Mavila et al. [65] | 0.018 | 11 | 633 | 2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calixto, S.; Alfaro-Gomez, M. Dichromated Gelatin in Optics. Gels 2025, 11, 298. https://doi.org/10.3390/gels11040298
Calixto S, Alfaro-Gomez M. Dichromated Gelatin in Optics. Gels. 2025; 11(4):298. https://doi.org/10.3390/gels11040298
Chicago/Turabian StyleCalixto, Sergio, and Mariana Alfaro-Gomez. 2025. "Dichromated Gelatin in Optics" Gels 11, no. 4: 298. https://doi.org/10.3390/gels11040298
APA StyleCalixto, S., & Alfaro-Gomez, M. (2025). Dichromated Gelatin in Optics. Gels, 11(4), 298. https://doi.org/10.3390/gels11040298






