Development of Biomimetic Edible Scaffolds for Cultured Meat Based on the Traditional Freeze-Drying Method for Ito-Kanten (Japanese Freeze-Dried Agar)
Abstract
1. Introduction
2. Results
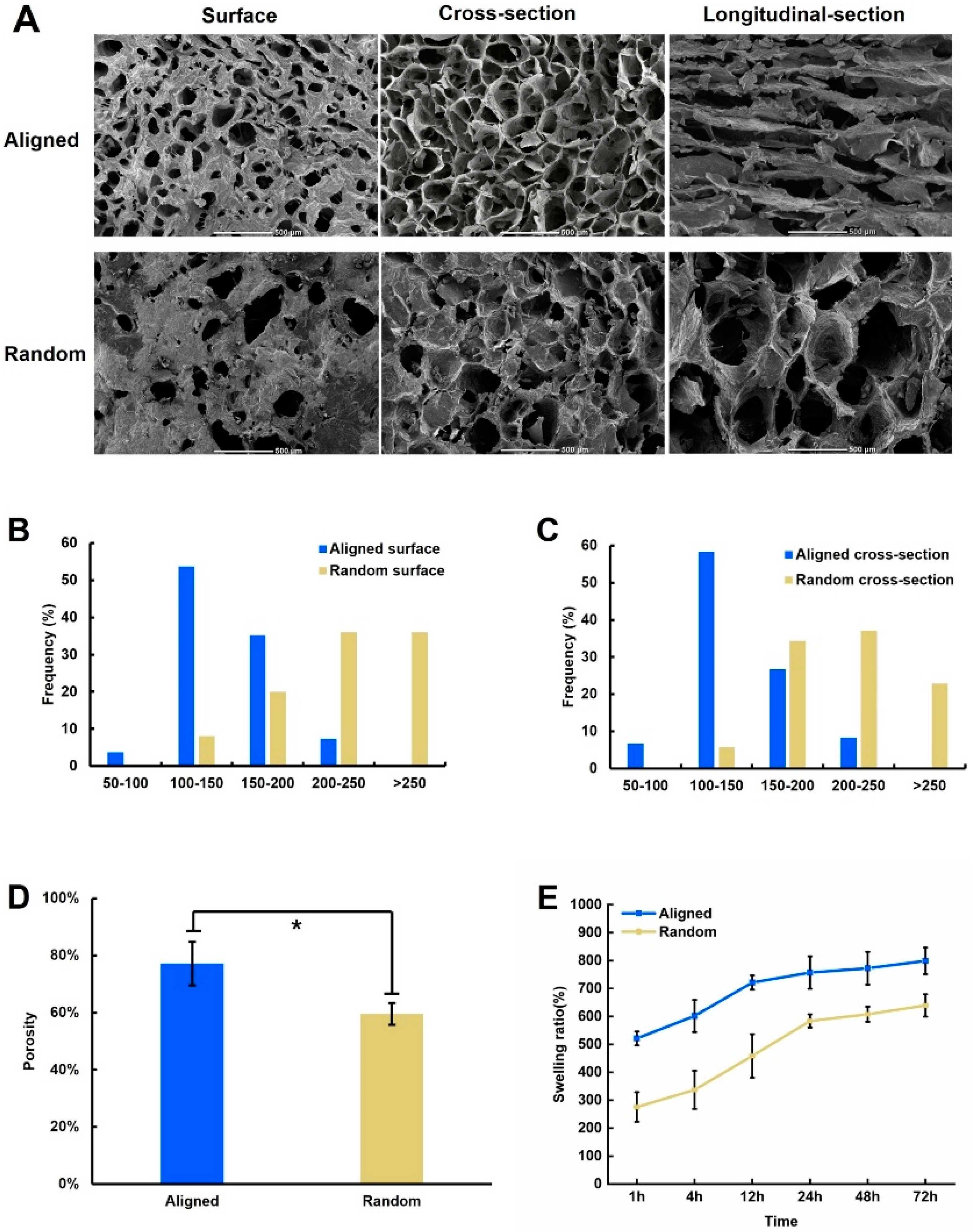
2.1. Morphology of the SPI/CA/SA Scaffold
2.2. Swelling Ratio
2.3. Anisotropy
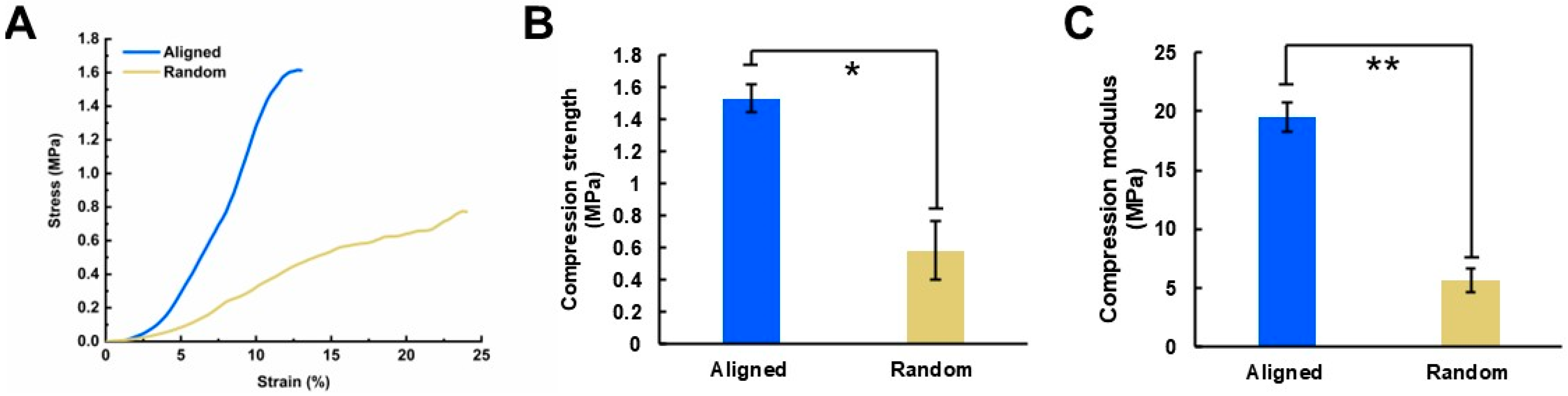
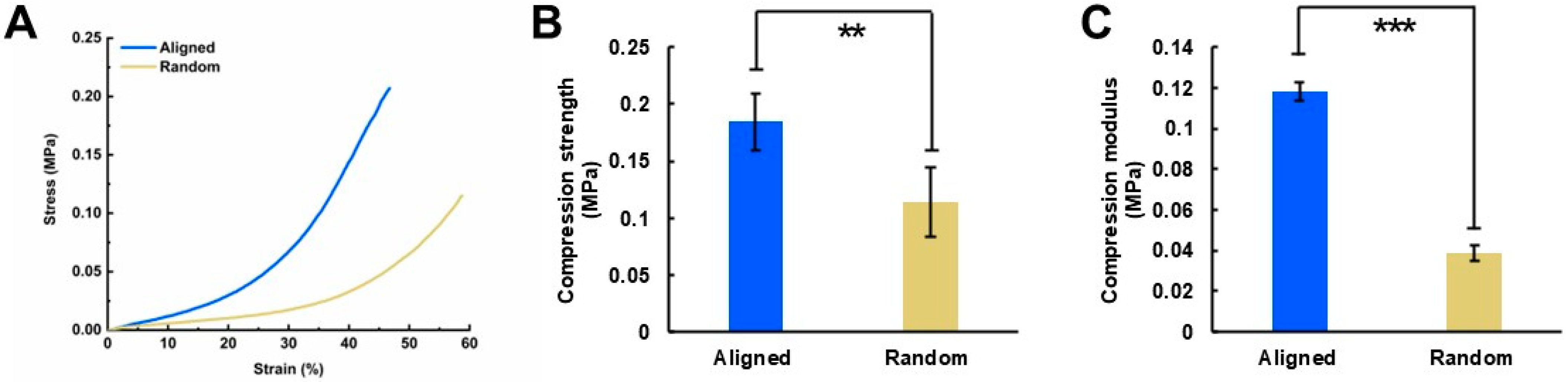
2.4. Mechanical Properties
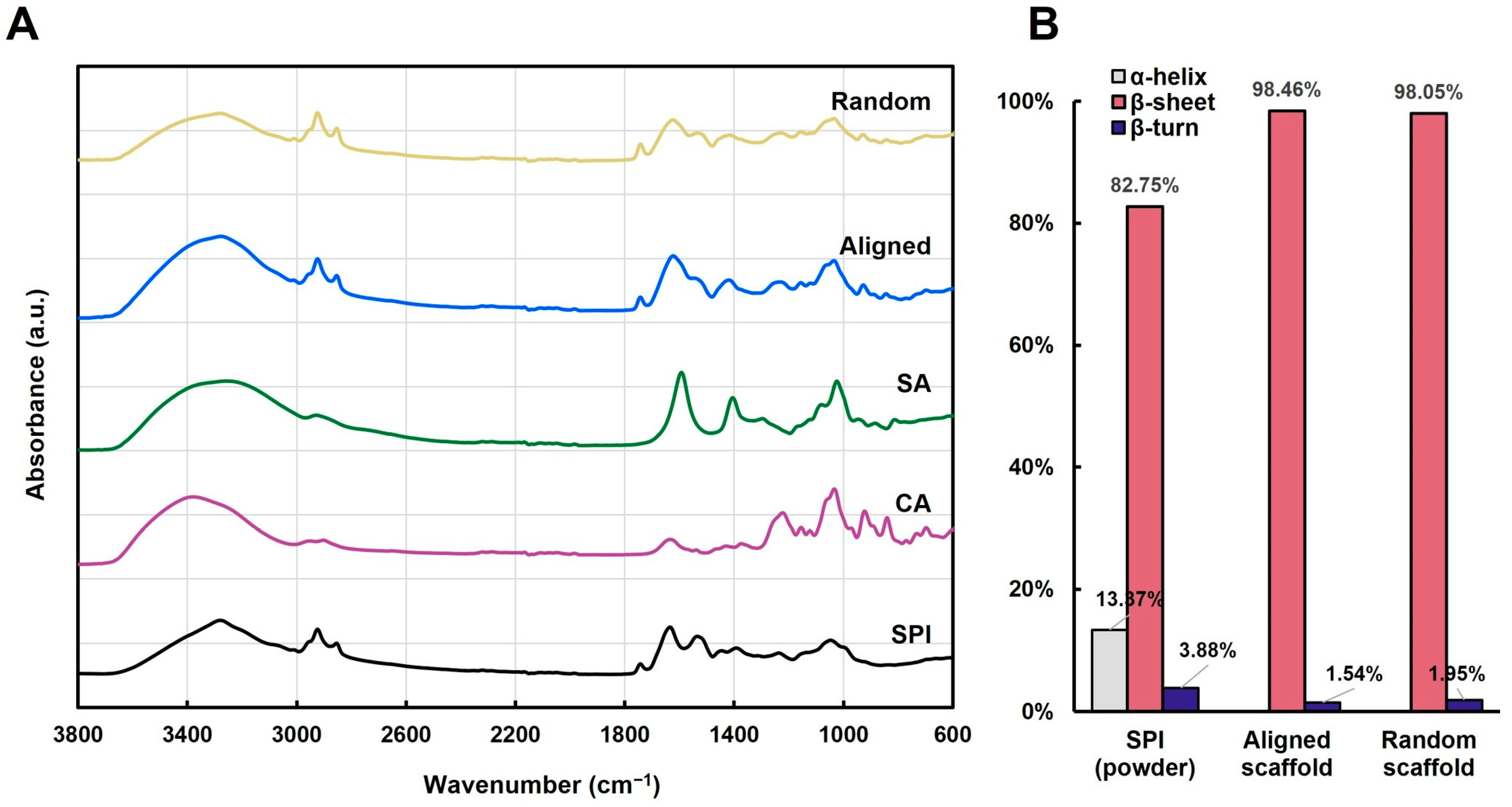
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. Secondary Structure Analysis
2.7. Cell Morphology
2.8. Cell Growth
2.9. Cell Differentiation
3. Discussion
3.1. Use of Scaffolds for Cultured Meat Production and Design Requirements
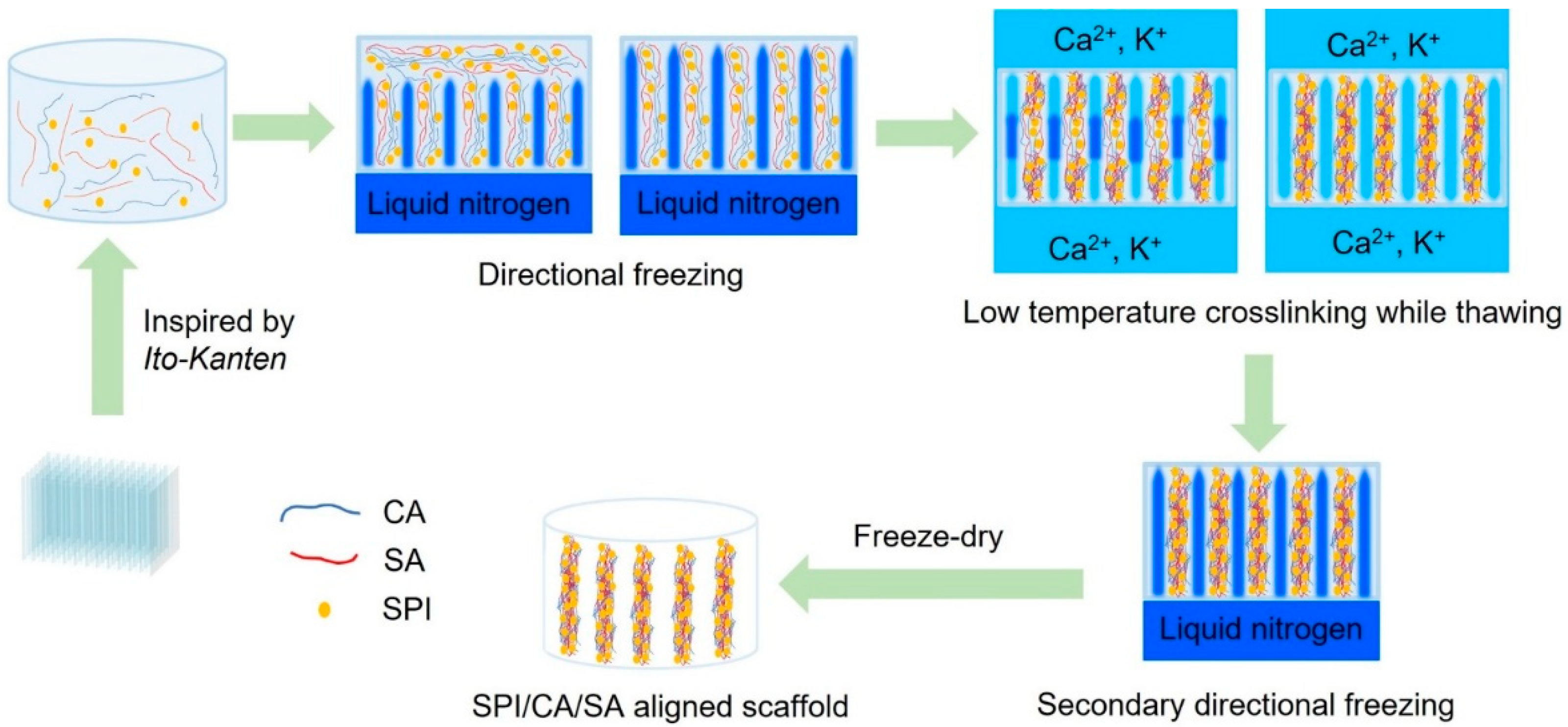
3.2. Materials and Fabrication Methods of Scaffolds
3.3. Structural Analysis and Mechanical Properties
3.4. Effects of Scaffold Porosity on Cell Proliferation
3.5. Comparison of Static and Dynamic Culture Conditions
3.6. Effect of Scaffold Structure on Cell Differentiation
3.7. Application Potential of Scaffolds
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Preparation of the SPI/CA/SA Cryogel Scaffolds
5.3. Characterization
5.3.1. Scanning Electron Microscopy (SEM)
5.3.2. Measurement of Anisotropy
5.3.3. Porosity and Swelling Ratio
5.3.4. Mechanical Characterization
5.4. Cell Culture
5.5. Immunofluorescent Staining
5.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPI | soy protein isolate |
| CA | carrageenan |
| SA | sodium alginate |
| SEM | scanning electron microscopy |
| PBS | Phosphate-buffered saline |
| FTIR | Fourier-transform infrared |
References
- Tuomisto, H.L.; de Mattos, M.J.T. Environmental impacts of cultured meat production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef]
- Rao, K.M.; Choi, S.M.; Han, S.S. A review on directional muscle cell growth in scaffolding biomaterials with aligned porous structures for cultivated meat production. Food Res. Int. 2023, 168, 112755. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-based meat analogues. In Sustainable Meat Production and Processing; Academic Press: Cambridge, MA, USA, 2019; pp. 103–126. [Google Scholar] [CrossRef]
- Chen, D.; Jones, O.G.; Campanella, O.H. Plant protein-based fibers: Fabrication, characterization, and potential food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 4554–4578. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Furuhashi, M.; Morimoto, Y.; Shima, A.; Nakamura, F.; Ishikawa, H.; Takeuchi, S. Formation of contractile 3D bovine muscle tissue for construction of millimetre-thick cultured steak. NPJ Sci. Food 2021, 5, 6. [Google Scholar] [CrossRef]
- Wei, Z.; Dai, S.; Huang, J.; Hu, X.; Ge, C.; Zhang, X.; Yang, K.; Shao, P.; Sun, P.; Xiang, N. Soy protein amyloid fibril scaffold for cultivated meat application. ACS Appl. Mater. Interfaces 2023, 15, 15108–15119. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Levenberg, S. Tissue engineering for clean meat production. Front. Sustain. Food Syst. 2019, 3, 46. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Ding, X.; Ding, S.; Tang, C.; Zeng, X.; Wang, J.; Zhou, G. Programmable scaffolds with aligned porous structures for cell cultured meat. Food Chem. 2024, 430, 137098. [Google Scholar] [CrossRef]
- Jahangirian, H.; Azizi, S.; Rafiee-Moghaddam, R.; Baratvand, B.; Webster, T.J. Status of plant protein-based green scaffolds for regenerative medicine applications. Biomolecules 2019, 9, 619. [Google Scholar] [CrossRef]
- Alesaeidi, S.; Kahrizi, M.S.; Ghorbani Tajani, A.; Hajipour, H.; Ghorbani, M. Soy protein isolate/sodium alginate hybrid hydrogel embedded with hydroxyapatite for tissue engineering. J. Polym. Environ. 2023, 31, 396–405. [Google Scholar] [CrossRef]
- Park, S.K.; Rhee, C.O.; Bae, D.H.; Hettiarachchy, N.S. Mechanical properties and water-vapor permeability of soy-protein films affected by calcium salts and glucono-delta-lactone. J. Agric. Food Chem. 2001, 49, 2308–2312. [Google Scholar] [CrossRef]
- Varghese, J.S.; Chellappa, N.; Fathima, N.N. Gelatin-carrageenan hydrogels: Role of pore size distribution on drug delivery process. Colloids Surf. B Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.T.N.T.; Nguyen, B.T.; Renou, F.; Nicolai, T. Structure and rheological properties of carrageenans extracted from different red algae species cultivated in Cam Ranh Bay, Vietnam. J. Appl. Phycol. 2019, 31, 1947–1953. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Geng, C.; Xue, Z.; Xia, Y.; Qin, Y.; Zhang, G. Study on the preparation and flame retardant properties of an eco-friendly potassium-calcium carrageenan fiber. Carbohydr. Polym. 2019, 206, 420–427. [Google Scholar] [CrossRef]
- Fujita, S.; Wakuda, Y.; Matsumura, M.; Suye, S.-I. Geometrically customizable alginate hydrogel nanofibers for cell culture platforms. J. Mater. Chem. B 2019, 7, 6556–6563. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Chen, X.; Chen, Y.; Ding, S.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; et al. Chitosan-sodium alginate-collagen/gelatin three-dimensional edible scaffolds for building a structured model for cell cultured meat. Int. J. Biol. Macromol. 2022, 209, 668–679. [Google Scholar] [CrossRef]
- Arora, A.; Kothari, A.; Katti, D.S. Pore orientation mediated control of mechanical behavior of scaffolds and its application in cartilage-mimetic scaffold design. J. Mech. Behav. Biomed. Mater. 2015, 51, 169–183. [Google Scholar] [CrossRef]
- Lynch, H.A.; Johannessen, W.; Wu, J.P.; Jawa, A.; Elliott, D.M. Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon. J. Biomech. Eng. 2003, 125, 726–731. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Wardale, R.J.; Best, S.M.; Cameron, R.E. The effects of scaffold architecture and fibrin gel addition on tendon cell phenotype. J. Mater. Sci. Mater. Med. 2015, 26, 5349. [Google Scholar] [CrossRef][Green Version]
- Fuse, T.; Suzuki, T. Preparation and properties of agar sulfates. Agric. Biol. Chem. 1975, 39, 119–126. [Google Scholar] [CrossRef]
- Selby, H.H.; Whistler, R.L. Agar. In Industrial Gums, 3rd ed.; Whistler, R.L., Bemiller, J.N., Eds.; Academic Press: Cambridge, MA, USA, 1993; pp. 87–103. [Google Scholar] [CrossRef]
- Armisen, R.; Galatas, F. Production, properties and uses of agar. In Production and Utilization of Products from Commercial Seaweeds; FAO Fisheries Technical Paper No. 288, FAO: Rome, Italy, 1987. [Google Scholar]
- Araki, C. Chemical studies of agar-agar. I. Nippon Kagaku Kaishi 1937, 58, 1085–1088. (In Japanese) [Google Scholar] [CrossRef]
- Hayashi, K.; Hiramitsu, T. Separation of agarose and agaropectin from agar. Nippon Shokuhin Kogyo Gakkaishi 1970, 17, 575–580. (In Japanese) [Google Scholar] [CrossRef]
- Fuse, T.; Yoshii, H. Effect of the chemical composition on gelation in agar. Nippon Nōgeikagaku Kaishi 1974, 48, 451–457. (In Japanese) [Google Scholar] [CrossRef]
- Hu, T.; Shi, M.; Zhao, X.; Liang, Y.; Bi, L.; Zhang, Z.; Liu, S.; Chen, B.; Duan, X.; Guo, B. Biomimetic 3D Aligned Conductive Tubular Cryogel Scaffolds with Mechanical Anisotropy for 3D Cell Alignment, Differentiation and In Vivo Skeletal Muscle Regeneration. Chem. Eng. J. 2022, 428, 131017. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Q.; Dong, W.; Liu, S.; Zhang, H.; Gu, Y. Alginate/gelatin-based hydrogel with soy protein/peptide powder for 3D printing tissue-engineering scaffolds to promote angiogenesis. Macromol. Biosci. 2022, 22, e2100413. [Google Scholar] [CrossRef]
- Caillard, R.; Remondetto, G.E.; Subirade, M. Physicochemical properties and microstructure of soy protein hydrogels co-induced by Maillard type cross-linking and salts. Food Res. Int. 2009, 42, 98–106. [Google Scholar] [CrossRef]
- Zou, P.-R.; Hu, F.; Ni, Z.-J.; Zhang, F.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Effects of phosphorylation pretreatment and subsequent transglutaminase cross-linking on physicochemical, structural, and gel properties of wheat gluten. Food Chem. 2022, 392, 133296. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Xue, W.; Lee, L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008, 22, 568–575. [Google Scholar] [CrossRef]
- Chen, X.; Ru, Y.; Chen, F.; Wang, X.; Zhao, X.; Ao, Q. FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll. 2013, 31, 435–437. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Miyajima, H.; Fujita, S. Porous edible cryogel inspired by the production of Shimitofu (freeze-dried tofu) for high-density cell culture in cultured meat. Food Hydrocoll. 2025, 163, 111155. [Google Scholar] [CrossRef]
- Mandal, B.B.; Gil, E.S.; Panilaitis, B.; Kaplan, D.L. Laminar silk scaffolds for aligned tissue fabrication. Macromol. Biosci. 2013, 13, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.-S.; Park, S.-H.; Cebe, P.; Kaplan, D.L. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef]
- Xu, H.; Cai, S.; Sellers, A.; Yang, Y. Electrospun ultrafine fibrous wheat glutenin scaffolds with three-dimensionally random organization and water stability for soft tissue engineering. J. Biotechnol. 2014, 184, 179–186. [Google Scholar] [CrossRef]
- Chen, E.J.; Novakofski, J.; Jenkins, W.K.; O’Brien, W.D. Young’s modulus measurements of soft tissues with application to elasticity imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1996, 43, 191–194. [Google Scholar] [CrossRef]
- Ratheesh, G.; Shi, M.; Lau, P.; Xiao, Y.; Vaquette, C. Effect of dual pore size architecture on in vitro osteogenic differentiation in additively manufactured hierarchical scaffolds. ACS Biomater. Sci. Eng. 2021, 7, 2615–2626. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Wang, Z.; Gao, H.; Chen, S.; Cong, Y.; Yang, L.; Wen, S.; Cheng, D.; He, J.; et al. Radially porous nanocomposite scaffolds with enhanced capability for guiding bone regeneration in vivo. Adv. Funct. Mater. 2022, 32, 180931. [Google Scholar] [CrossRef]
- Bai, H.; Wang, D.; Delattre, B.; Gao, W.; De Coninck, J.; Li, S.; Tomsia, A.P. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect. Acta Biomater. 2015, 20, 113–119. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Jaipaew, J.; Wangkulangkul, P.; Meesane, J.; Raungrut, P.; Puttawibul, P. Mimicked cartilage scaffolds of silk fibroin/hyaluronic acid with stem cells for osteoarthritis surgery: Morphological, mechanical, and physical clues. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Montaser, A.S.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-C.; Glusac, J.; Levi, S.; Zernov, A.; Baruch, L.; Davidovich-Pinhas, M.; Fishman, A.; Machluf, M. Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleogel-based fat substitute. Nat. Commun. 2023, 14, 2942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, H.; Wu, Y.; Yin, H.; Mao, X.; Li, N.; Guo, H.; Chang, Y.; Jiang, X.; Ai, Q.; et al. Scalable production of muscle and adipose cell-laden microtissues using edible macroporous microcarriers for 3D printing of cultured fish fillets. Nat. Commun. 2025, 16, 1740. [Google Scholar] [CrossRef]
- Juhas, M.; Bursac, N. Roles of adherent myogenic cells and dynamic culture in engineered muscle function and maintenance of satellite cells. Biomaterials 2014, 35, 9438–9446. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Rimington, R.P.; Capel, A.J.; Chaplin, K.F.; Fleming, J.W.; Bandulasena, H.C.H.; Bibb, R.J.; Christie, S.D.R.; Lewis, M.P. Differentiation of bioengineered skeletal muscle within a 3D printed perfusion bioreactor reduces atrophic and inflammatory gene expression. ACS Biomater. Sci. Eng. 2019, 5, 5525–5538. [Google Scholar] [CrossRef]
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.W.; Sinskey, A.J.; Springs, S.L. Large-scale cultured meat production: Trends, challenges and promising biomanufacturing technologies. Biomaterials 2022, 280, 121274. [Google Scholar] [CrossRef]



| Sample | SPI | CA | SA | Salt Solution |
|---|---|---|---|---|
| Aligned scaffold | 63% | 20% | 17% | CaCl2/KCl 0.5 M each |
| Random scaffold | 63% | 20% | 17% | CaCl2/KCl 0.5 M each |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, P.; Miyajima, H.; Fujita, S. Development of Biomimetic Edible Scaffolds for Cultured Meat Based on the Traditional Freeze-Drying Method for Ito-Kanten (Japanese Freeze-Dried Agar). Gels 2025, 11, 299. https://doi.org/10.3390/gels11040299
Xia P, Miyajima H, Fujita S. Development of Biomimetic Edible Scaffolds for Cultured Meat Based on the Traditional Freeze-Drying Method for Ito-Kanten (Japanese Freeze-Dried Agar). Gels. 2025; 11(4):299. https://doi.org/10.3390/gels11040299
Chicago/Turabian StyleXia, Ping, Hiroki Miyajima, and Satoshi Fujita. 2025. "Development of Biomimetic Edible Scaffolds for Cultured Meat Based on the Traditional Freeze-Drying Method for Ito-Kanten (Japanese Freeze-Dried Agar)" Gels 11, no. 4: 299. https://doi.org/10.3390/gels11040299
APA StyleXia, P., Miyajima, H., & Fujita, S. (2025). Development of Biomimetic Edible Scaffolds for Cultured Meat Based on the Traditional Freeze-Drying Method for Ito-Kanten (Japanese Freeze-Dried Agar). Gels, 11(4), 299. https://doi.org/10.3390/gels11040299






