Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Curcumin-Loaded Nanoparticle-Enriched Hydrogels
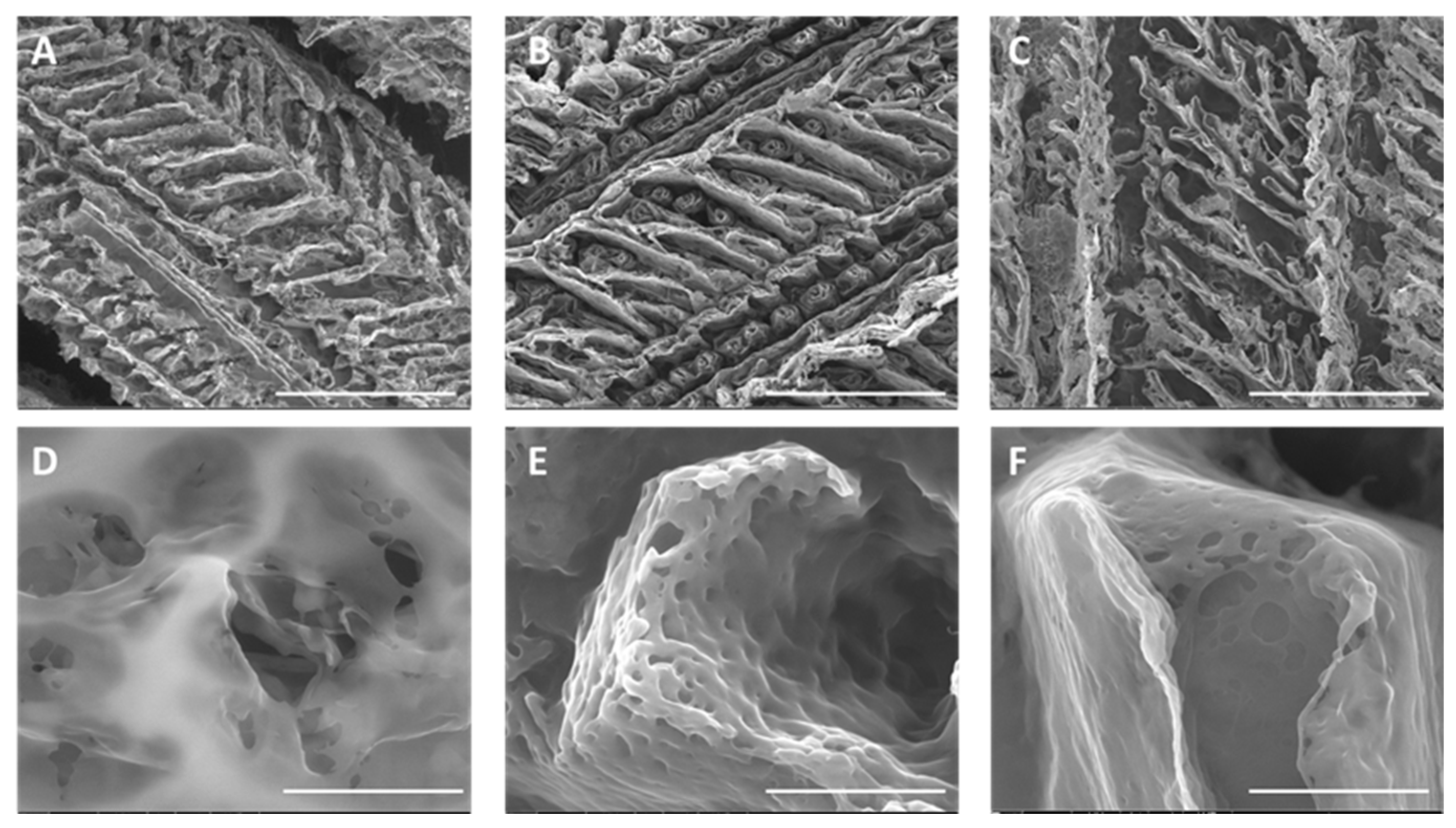
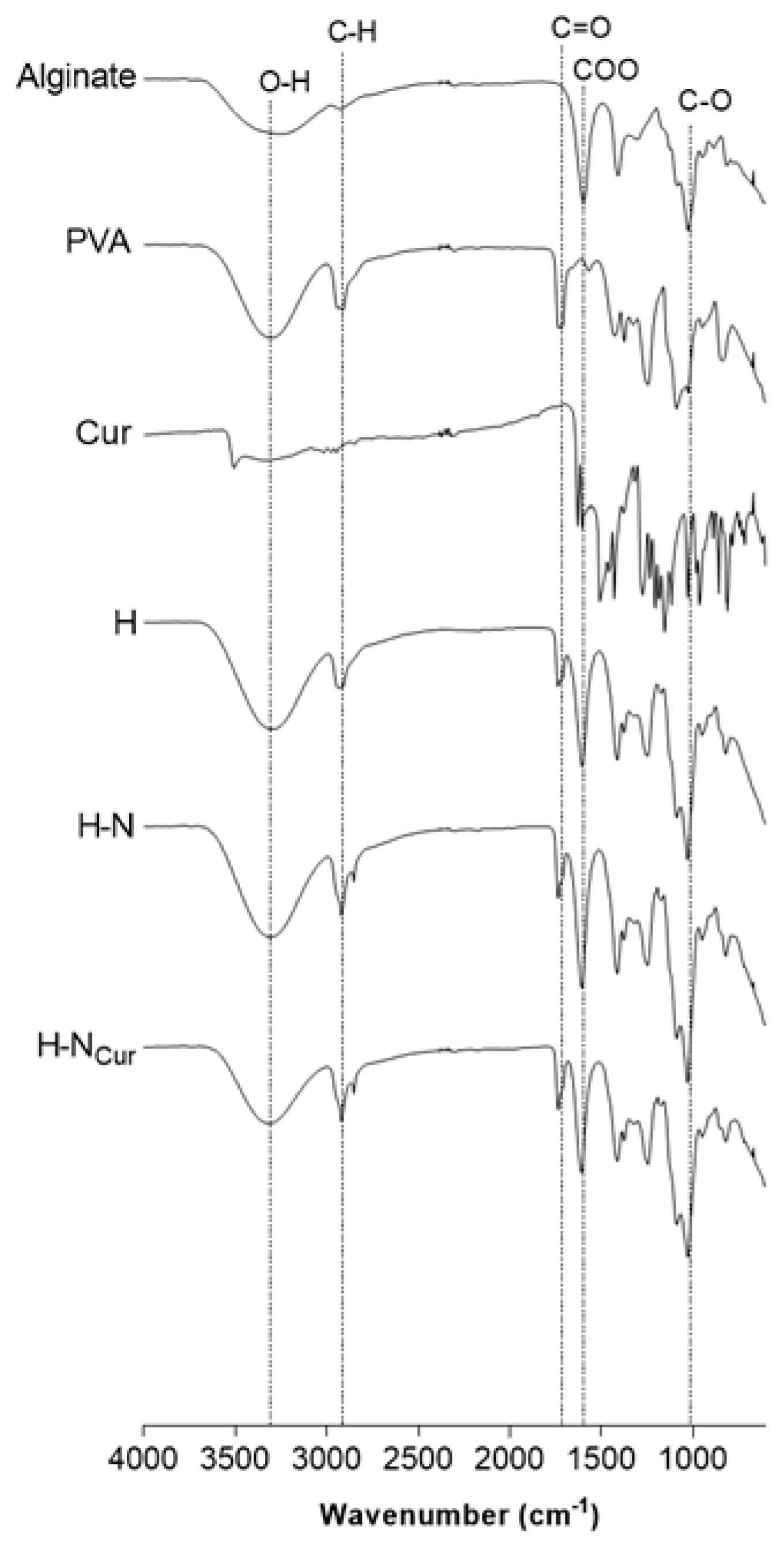
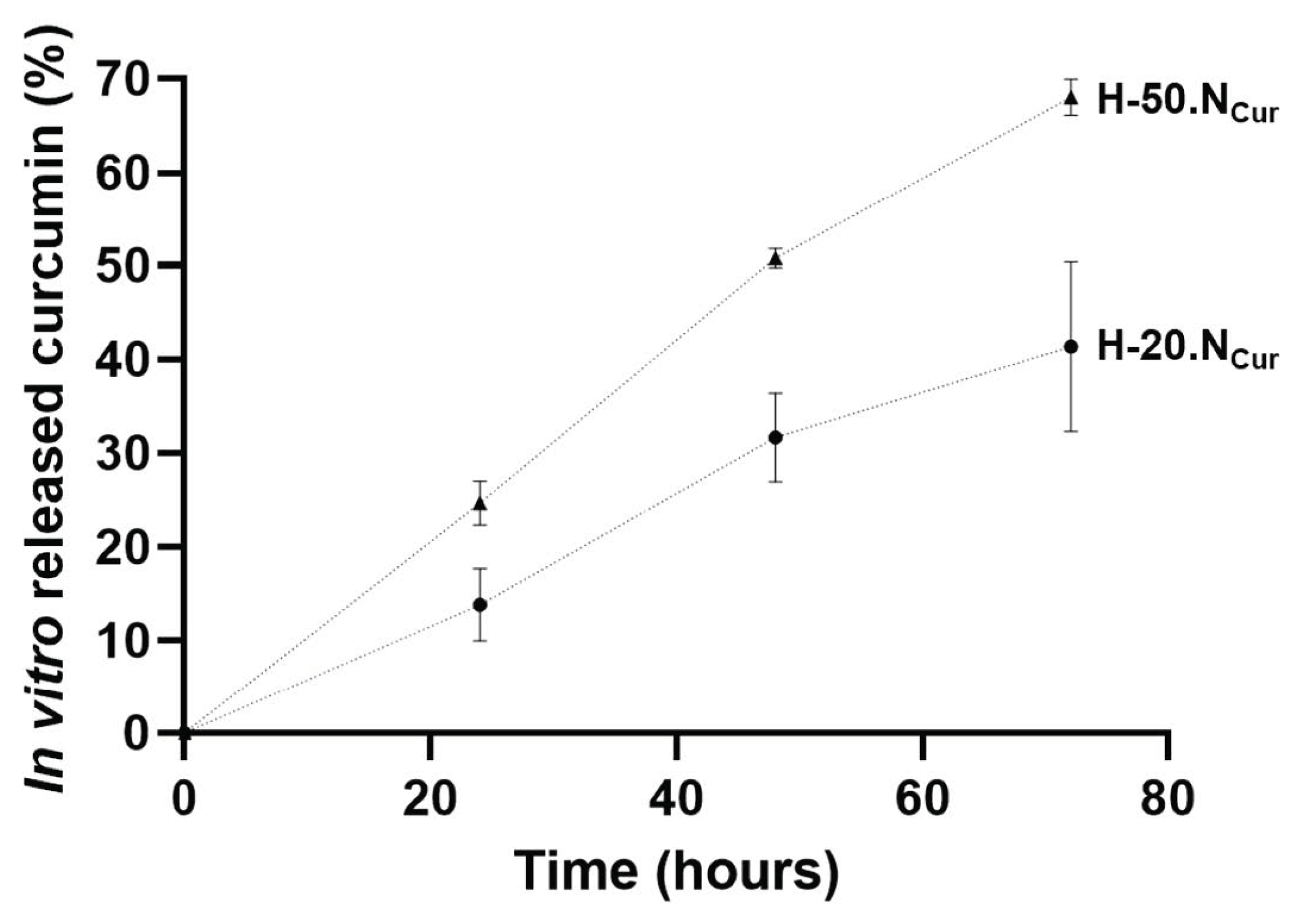
2.1.1. Hydrogel Production and Physicochemical Properties
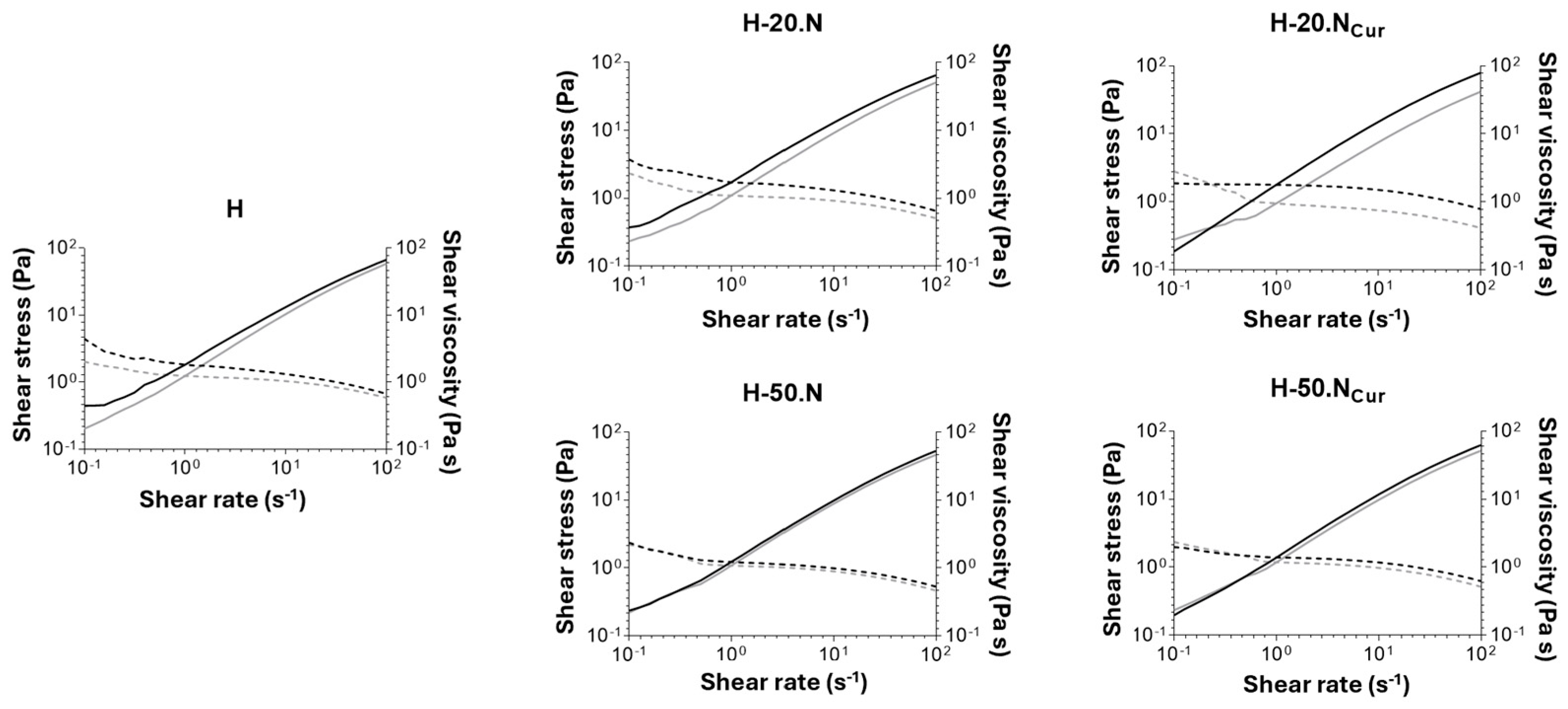
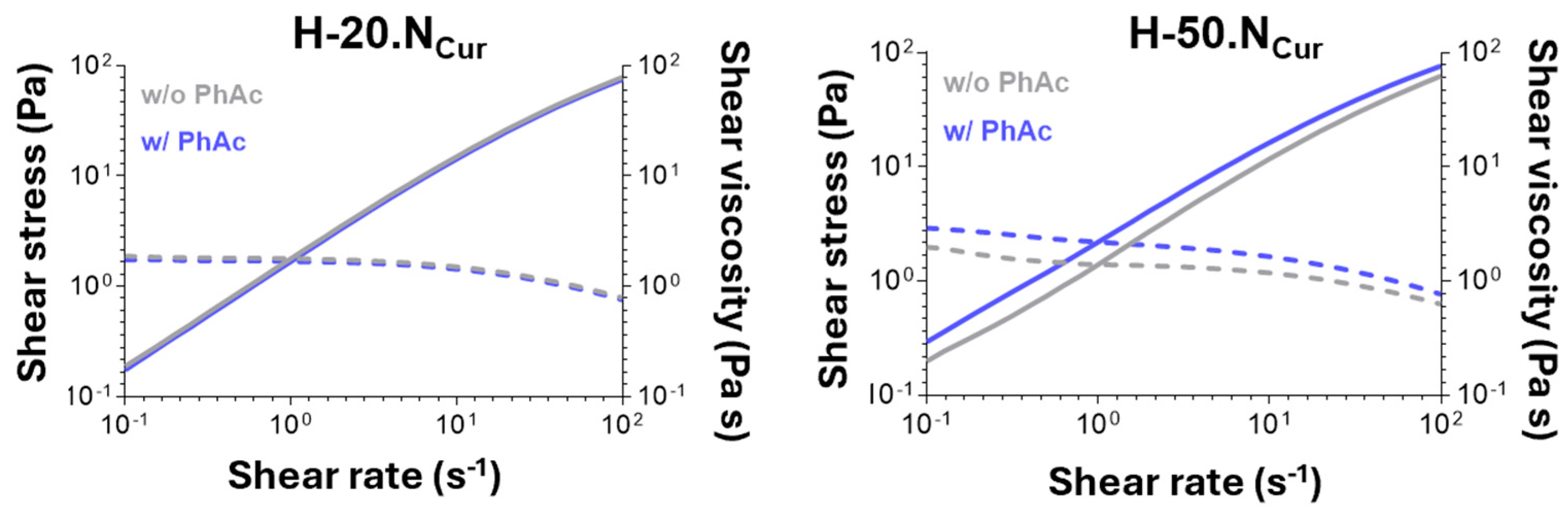
2.1.2. Hydrogels’ Mechanical Properties
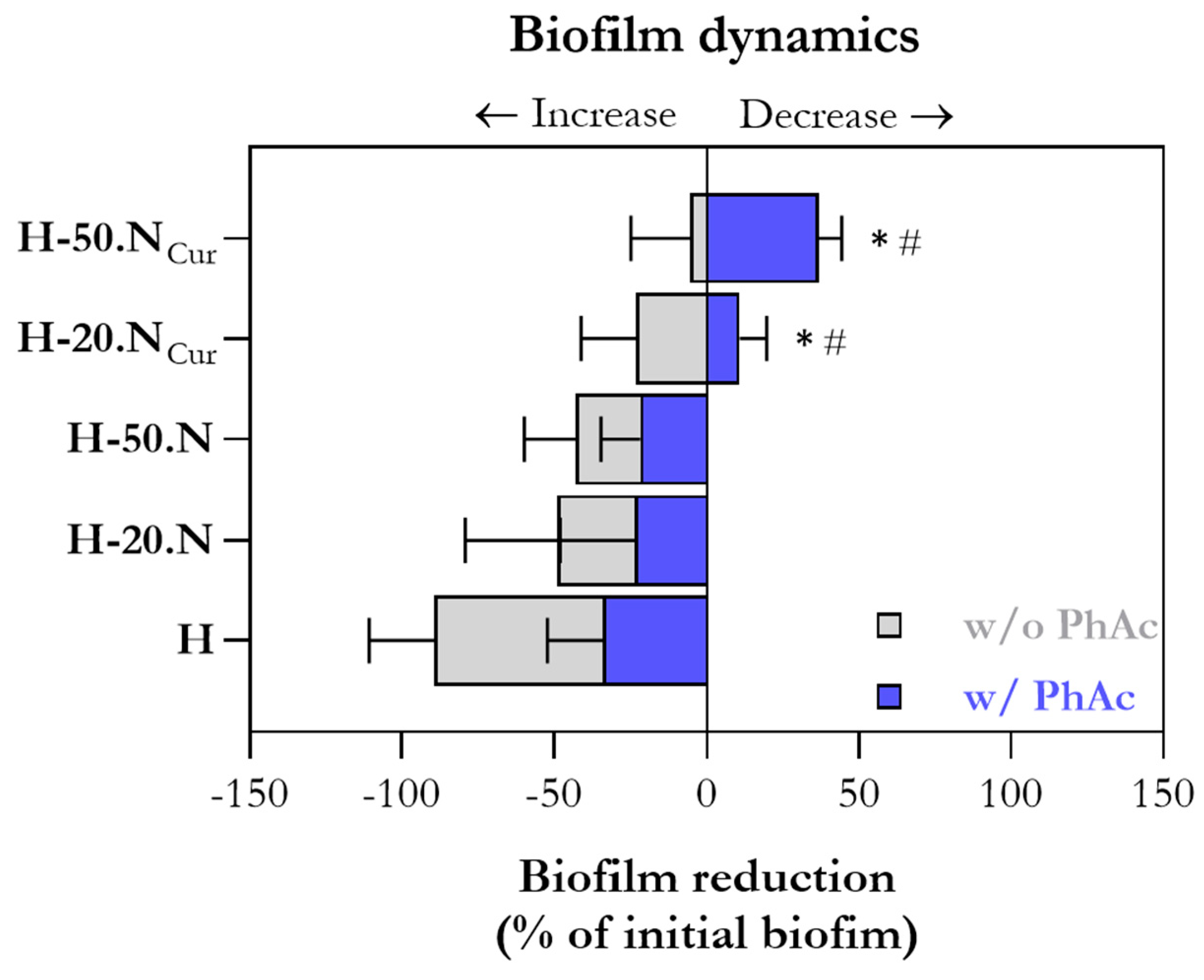
2.2. Hydrogel Antimicrobial Activity Mediated by Photoactivation
2.3. Hydrogel Cytocompatibility Mediated by Photoactivation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Solid Lipid Nanoparticles
4.2.2. Preparation of Hydrogels Enriched with Lipid Nanoparticles
4.2.3. Physicochemical Characterization of the Hydrogels
Morphological Analysis
Rheology Studies
Chemical Interactions Analysis by Fourier Transform Infrared Spectroscopy
4.2.4. In Vitro Curcumin Release from the Hydrogels
4.2.5. Antibiofilm Activity
4.2.6. In Vitro Cytocompatibility
Cell Culture Conditions
Metabolic Activity
Cell Morphology
4.2.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MEM | α-minimum essential medium |
| BSA | bovine serum albumin |
| CHX | chlorhexidine |
| CFU | colony-forming units |
| CUR | curcumin |
| E. faecalis | Enterococcus faecalis |
| FBS | fetal bovine serum |
| f-actin | filamentous actin |
| FTIR | Fourier-transform infrared spectroscopy |
| hGF | Human gingival fibroblasts |
| H | hydrogel |
| H-20.N | hydrogel containing 20% (v/v) empty SLN |
| H-50.N | hydrogel containing 50% (v/v) empty SLN |
| H-20.NCur | hydrogel containing 20% (v/v) CUR-loaded SLN |
| H-50.NCur | hydrogel containing 50% (v/v) CUR-loaded SLN |
| Non-PhAc | non-photoactivated |
| PBS | phosphate-buffered saline |
| PDT | photodynamic therapy |
| PhAc | photoactivated |
| PI | propidium iodide |
| PVA | poly(vinyl alcohol) |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
| SLNs | solid lipid nanoparticles |
| TSA | Tryptic Soy Agar |
| TSB | Tryptic Soy Broth |
References
- Minhaco, V.M.T.R.; Maquera Huacho, P.M.; Mancim Imbriani, M.J.; Tonon, C.C.; Chorilli, M.; Rastelli, A.N.D.S.; Spolidorio, D.M.P. Improving Antimicrobial Activity against Endodontic Biofilm after Exposure to Blue Light-Activated Novel Curcumin Nanoparticle. Photodiagnosis Photodyn. Ther. 2023, 42, 103322. [Google Scholar] [CrossRef] [PubMed]
- Ravazzi, T.P.Q.; De Jesus, I.M.; De Oliveira Santos, G.P.; Reis, T.A.; Rosa, L.P.; Rosa, F.C.S. The Effects of Antimicrobial Photodynamic Therapy (aPDT) with Nanotechnology-Applied Curcumin and 450nm Blue Led Irradiation on Multi-Species Biofilms in Root Canals. Lasers Med. Sci. 2023, 38, 254. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Mousa, H.A.; Kataia, M.M.M.S.; Afifi, N.N. Functionalized Surface of PLGA Nanoparticles in Thermosensitive Gel to Enhance the Efficacy of Antibiotics against Antibiotic Resistant Infections in Endodontics: A Randomized Clinical Trial. Int. J. Pharm. X 2023, 6, 100219. [Google Scholar] [CrossRef]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus Faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-Activated Nanoparticles: An In Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Plotino, G.; Chiniforush, N.; Bahador, A. Dual Wavelength Irradiation Antimicrobial Photodynamic Therapy Using Indocyanine Green and Metformin Doped with Nano-Curcumin as an Efficient Adjunctive Endodontic Treatment Modality. Photodiagnosis Photodyn. Ther. 2020, 29, 101628. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Kazemian, H.; Chiniforush, N.; Hosseini, N.; Pourakbari, B.; Azizollahi, A.; Rezaei, F.; Bahador, A. Exploring Different Photosensitizers to Optimize Elimination of Planktonic and Biofilm Forms of Enterococcus Faecalis from Infected Root Canal during Antimicrobial Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2018, 24, 206–211. [Google Scholar] [CrossRef]
- Persadmehr, A.; Torneck, C.D.; Cvitkovitch, D.G.; Pinto, V.; Talior, I.; Kazembe, M.; Shrestha, S.; McCulloch, C.A.; Kishen, A. Bioactive Chitosan Nanoparticles and Photodynamic Therapy Inhibit Collagen Degradation In Vitro. J. Endod. 2014, 40, 703–709. [Google Scholar] [CrossRef]
- Kara, O.; Seseogullari Dirihan, R.; Sayin Ozel, G.; Tezvergil Mutluay, A.; Usumez, A. Inhibition of Cathepsin-K and Matrix Metalloproteinase by Photodynamic Therapy. Dent. Mater. 2021, 37, e485–e492. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Boreak, N.; Al Mahde, R.Z.; Otayn, W.A.; Alamer, A.Y.; Alrajhi, T.; Jafri, S.; Sharwani, A.; Swaidi, E.; Abozoah, S.; Mowkly, A.A.M. Exploring Plant-Based Compounds as Alternatives for Targeting Enterococcus Faecalis in Endodontic Therapy: A Molecular Docking Approach. Int. J. Mol. Sci. 2024, 25, 7727. [Google Scholar] [CrossRef]
- Devaraj, S.; Jagannathan, N.; Neelakantan, P. Antibiofilm Efficacy of Photoactivated Curcumin, Triple and Double Antibiotic Paste, 2% Chlorhexidine and Calcium Hydroxide against Enterococcus Fecalis in Vitro. Sci. Rep. 2016, 6, 24797. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic Therapy in Endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Grenho, L.; Fernandes, M.H.; Lima, S.A.C. Curcumin-Loaded Lipid Nanoparticles: A Promising Antimicrobial Strategy Against Enterococcus Faecalis in Endodontic Infections. Pharmaceutics 2025, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Amaral, M.H.; González-Mira, E.; Santos, D.; Ferreira, D. Solid Lipid Nanoparticles (SLN)—Based Hydrogels as Potential Carriers for Oral Transmucosal Delivery of Risperidone: Preparation and Characterization Studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.L.P. Evaluation of Photodynamic Therapy on Nanoparticles and Films Loaded-Nanoparticles Based on Chitosan/Alginate for Curcumin Delivery in Oral Biofilms. Int. J. Biol. Macromol. 2023, 240, 124489. [Google Scholar] [CrossRef]
- De Araújo, L.P.; Immich, F.; Da Rosa, W.L.D.O.; Da Silva, A.F.; Lund, R.G.; Piva, E. Current Perspectives on Calcium Silicate-Based Intracanal Medicaments: A Scoping Review of Clinical and Laboratory Evidence. J. Dent. 2024, 149, 105311. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a Polyvinyl Alcohol/Sodium Alginate Hydrogel-Based Scaffold Incorporating bFGF-Encapsulated Microspheres for Accelerated Wound Healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Candry, P.; Godfrey, B.J.; Wang, Z.; Sabba, F.; Dieppa, E.; Fudge, J.; Balogun, O.; Wells, G.; Winkler, M.-K.H. Tailoring Polyvinyl Alcohol-Sodium Alginate (PVA-SA) Hydrogel Beads by Controlling Crosslinking pH and Time. Sci. Rep. 2022, 12, 20822. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of Green Tea Polyphenol Nanospheres in PVA/Alginate Hydrogel for Promoting Wound Healing of Diabetic Rats by Regulating PI3K/AKT Pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ran, F.; Li, C.; Hao, Z.; He, H.; Dai, L.; Wang, J.; Yang, W. A Lignin Silver Nanoparticles/Polyvinyl Alcohol/Sodium Alginate Hybrid Hydrogel with Potent Mechanical Properties and Antibacterial Activity. Gels 2024, 10, 240. [Google Scholar] [CrossRef]
- Farazin, A.; Mohammadimehr, M.; Ghasemi, A.H.; Naeimi, H. Design, Preparation, and Characterization of CS/PVA/SA Hydrogels Modified with Mesoporous Ag2O/SiO2 and Curcumin Nanoparticles for Green, Biocompatible, and Antibacterial Biopolymer Film. RSC Adv. 2021, 11, 32775–32791. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Y.; Pan, Y.; Yu, M.; Lin, X.; Mondal, A.K. Biocompatible, Injectable, and Self-Healing Poly(N-vinylpyrrolidone)/Carboxymethyl Cellulose Hydrogel for Drug Release. ACS Omega 2024, 9, 5854–5861. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G.; et al. Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing. Antioxidants 2021, 10, 725. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic Property in Pharmaceutical Formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Cai, J.; Zhong, H.; Tang, W.; Wen, F.; Lv, Y.; Huang, X.; Luo, J.; Li, P. Multiple Response Behaviors of Curcumin-Loaded Ammonium Alginate/Polyvinyl Alcohol Hydrogel and Its Application. Biomass Conv. Bioref. 2024, 14, 16121–16139. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Saouaf, O.A.; Lopez Hernandez, H.; Appel, E.A. Gelation and Yielding Behavior of POLYMER–NANOPARTICLE Hydrogels. J. Polym. Sci. 2021, 59, 2854–2866. [Google Scholar] [CrossRef]
- Willis, J.A.; Trevino, A.; Nguyen, C.; Benjamin, C.C.; Yakovlev, V.V. Photodynamic Therapy Minimally Affects HEMA-DMAEMA Hydrogel Viscoelasticity. Macromol. Biosci. 2023, 23, 2300124. [Google Scholar] [CrossRef]
- Pereira, J.A.; Polaquini, C.R.; Dos Santos, V.; Caiaffa, K.S.; Rabelo, R.L.; Theodoro, R.D.S.; Theodoro, L.H.; Regasini, L.O.; Duque, C. Antibiofilm and Cytotoxic Effect of 3,3′-Dihydroxycurcumin (DHC) as Photosensitizer Agent in Antimicrobial Photodynamic Therapy for Endodontic Purposes. Photodiagnosis Photodyn. Ther. 2021, 36, 102534. [Google Scholar] [CrossRef] [PubMed]
- Strazzi-Sahyon, H.B.; Cintra, L.T.A.; Nakao, J.M.; Takamiya, A.S.; Queiroz, I.O.A.; Dos Santos, P.H.; Oliveira, S.H.P.; Sivieri-Araujo, G. Cytotoxicity of Root Canal Irrigating Solutions and Photodynamic Therapy Using Curcumin Photosensitizer. Photodiagnosis Photodyn. Ther. 2022, 38, 102795. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Bedia, S.V.; Bedia, A.S.; Yang, H.J.; Dharmadhikari, S.; Abdulla, A.M.; Chaubal, T.V.; Bapat, P.R.; Abullais, S.S.; Wahab, S.; et al. Current Appraises of Therapeutic Applications of Nanocurcumin: A Novel Drug Delivery Approach for Biomaterials in Dentistry. Environ. Res. 2023, 238, 116971. [Google Scholar] [CrossRef] [PubMed]


| Hydrogel Code/Ingredient | Sodium Alginate | PVA 10% (w/v) | Water (mL) | Empty SLN (mL) | CUR-Loaded SLN (mL) |
|---|---|---|---|---|---|
| H | 0.25 g | 5 mL | 10 mL | – | – |
| H-20.N | 0.25 g | 5 mL | 8 mL | 2 mL | – |
| H-50.N | 0.25 g | 5 mL | 5 mL | 5 mL | – |
| H-20.NCur | 0.25 g | 5 mL | 8 mL | – | 2 mL |
| H-50.NCur | 0.25 g | 5 mL | 5 mL | – | 5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, S.; Grenho, L.; Fernandes, M.H.; Lima, S.A.C. Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy. Gels 2025, 11, 308. https://doi.org/10.3390/gels11050308
Ferreira S, Grenho L, Fernandes MH, Lima SAC. Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy. Gels. 2025; 11(5):308. https://doi.org/10.3390/gels11050308
Chicago/Turabian StyleFerreira, Sónia, Liliana Grenho, Maria H. Fernandes, and Sofia A. Costa Lima. 2025. "Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy" Gels 11, no. 5: 308. https://doi.org/10.3390/gels11050308
APA StyleFerreira, S., Grenho, L., Fernandes, M. H., & Lima, S. A. C. (2025). Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy. Gels, 11(5), 308. https://doi.org/10.3390/gels11050308









