From Basic to Breakthroughs: The Journey of Microfluidic Devices in Hydrogel Droplet Generation
Abstract
:1. Introduction
2. Biomedical Applications of Polymeric Nanoparticles
3. Hydrogel Particles Morphology
4. Microfluidics and Its Biomedical Applications
5. Geometric Configurations of Microfluidic Devices
6. Materials and Methods Used for Device Fabrication
7. Droplet Formation Through Microfluidics
8. Nanomedicines Based on Hydrogels Formed Through Microfluidics
9. Conclusions and Future Directions on the Application of Microfluidics in the Production of Nanomedicines
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Alam, M.K. Nanocarrier-Based Drug Delivery Systems using Microfluidic-Assisted Techniques. Adv. NanoBiomed Res. 2023, 3, 2300041. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; Derosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef]
- Kong, L.; Chen, R.; Wang, X.; Zhao, C.X.; Chen, Q.; Hai, M.; Chen, D.; Yang, Z.; Weitz, D.A. Controlled co-precipitation of biocompatible colorant-loaded nanoparticles by microfluidics for natural color drinks. Lab Chip 2019, 19, 2089–2095. [Google Scholar] [CrossRef]
- Min, Z.; Gao, J.; Yu, Y. The Roles of Mitochondrial SIRT4 in Cellular Metabolism. Front. Endocrinol. 2019, 9, 783. [Google Scholar] [CrossRef]
- Zhu, Z.; Geng, Y.; Yuan, Z.; Ren, S.; Liu, M.; Meng, Z.; Pan, D. A bubble-free microfluidic device for easy-to-operate immobilization, culturing and monitoring of zebrafish embryos. Micromachines 2019, 10, 168. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoscale nutrient delivery systems for food applications: Improving bioactive dispersibility, stability, and bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; McClements, D.J. Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability and protein release. Food Hydrocoll. 2016, 58, 308–315. [Google Scholar] [CrossRef]
- Assifaoui, A.; Loupiac, C.; Chambin, O.; Cayot, P. Structure of calcium and zinc pectinate films investigated by FTIR spectroscopy. Carbohydr. Res. 2013, 92, 1394–1402. [Google Scholar] [CrossRef]
- Uysal, B.; Madduma-Bandarage, U.S.K.; Jayasinghe, H.G.; Madihally, S. 3D-Printed Hydrogels from Natural Polymers for Biomedical Applications: Conventional Fabrication Methods, Current Developments, Advantages, and Challenges. Gels 2025, 11, 192. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Polymeric hydrogels as technology platform for drug delivery applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef]
- Nicol, E. Photopolymerized Porous Hydrogels. Biomacromolecules 2021, 22, 1325–1345. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan-based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G.; Dinescu, S.; Albu Kaya, M.; Chitoiu, L.; Ignat, S.; Kaya, D.A.; Costache, M. Collagen-Based Hydrogels and Their Applications for Tissue Engineering and Regenerative Medicine. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Déat-Lainé, E.; Hoffart, V.; Garrait, G.; Jarrige, J.F.; Cardot, J.M.; Subirade, M.; Beyssac, E. Efficacy of mucoadhesive hydrogel microparticles of whey protein and alginate for oral insulin delivery. Pharm. Res. 2013, 30, 721–734. [Google Scholar] [CrossRef]
- Elkhoury, K.; Koçak, P.; Kang, A.; Arab-Tehrany, E.; Ward, J.E.; Shin, S.R. Engineering smart targeting nanovesicles and their combination with hydrogels for controlled drug delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef]
- O’Donnell, K.; Boyd, A.; Meenan, B.J. Controlling fluid diffusion and release through mixed-molecular-weight poly(ethylene) glycol diacrylate (PEGDA) hydrogels. Materials 2019, 12, 3381. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Guo, C.; Shi, Y.; Li, L.C. Recent advances in polymeric microspheres for parenteral drug deliverypart 2. Expert. Opin. Drug Deliv. 2012, 9, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Env. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Carvalho, A.; Fernandes, A.R.; Baptista, P.V. Nanoparticles as Delivery Systems in Cancer Therapy: Focus on Gold Nanoparticles and Drugs. In Applications of Targeted Nano Drugs and Delivery Systems, 1st ed.; Shyam, S.M., Shivendu, R., Nandita, D., Raghvendra, K.M., Sabu, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 257–295. [Google Scholar] [CrossRef]
- Hamaloglu, K.Ö.; Çelebi, B.; Sag, E.; Tuncel, A. A new method for the synthesis of monodisperse-porous titania microbeads by using polymethacrylate microbeads as template. Micropor. Mesopor. Mat. 2015, 207, 17–26. [Google Scholar] [CrossRef]
- Hegab, R.A.; Pardue, S.; Shen, X.; Kevil, C.; Peppas, N.A.; Caldorera-Moore, M.E. Effect of network mesh size and swelling to the drug delivery from pH responsive hydrogels. J. Appl. Polym. Sci. 2020, 137, 48767. [Google Scholar] [CrossRef]
- Koetting, M.C.; Guido, J.F.; Gupta, M.; Zhang, A.; Peppas, N.A. PH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: Effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. J. Control. Release 2016, 221, 18–25. [Google Scholar] [CrossRef]
- Venkatesh, D.N.; Kumar, S.S.; Rajeshkumar, R. Sustained release microbeads of ritonavir: In vitro and in vivo evaluation. Int. J. Appl. Pharm. 2019, 11, 189–198. [Google Scholar] [CrossRef]
- Williams, E.C.; Toomey, R.; Alcantar, N. Controlled release niosome embedded chitosan system: Effect of crosslink mesh dimensions on drug release. J. Biomed. Mater. Res. Part A 2012, 100, 3296–3303. [Google Scholar] [CrossRef]
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.J.; Lee, K.J.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Healthc. Mater. 2019, 8, 1801363. [Google Scholar] [CrossRef]
- Ye, S.; Cao, Q.; Ni, P.; Xiong, S.; Zhong, M.; Yuan, T.; Shan, J.; Liang, J.; Fan, Y.; Zhang, X. A ceramic microbridge microfluidic chip to study osteogenic differentiation of mesenchymal stem cells in bioactive ceramic immune microenvironment. Bioact. Mater. 2025, 45, 520–533. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, X.; Wang, J.; Tian, H.; Zhao, P.; Tian, Y.; Gu, Y.; Wang, L.; Wang, C. Droplet microfluidics for the production of microparticles and nanoparticles. Micromachines 2017, 8, 22. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Han, H.; Santos, H.A.; Zhang, Y.; Zhang, H.; Wei, J.; Cai, Z. Fabricated technology of biomedical micro-nano hydrogel. Biomed. Technol. 2023, 2, 31–48. [Google Scholar] [CrossRef]
- Mellott, M.B.; Searcy, K.; Pishko, M.V. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 2001, 22, 929–941. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2005, 236, 188–194. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications, 1st ed.; Carpi, A., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 117–150. [Google Scholar]
- Martín-Banderas, L.; Sáez-Fernández, E.; Holgado, M.Á.; Durán-Lobato, M.M.; Prados, J.C.; Melguizo, C.; Arias, J.L. Biocompatible gemcitabine-based nanomedicine engineered by Flow Focusing® for efficient antitumor activity. Int. J. Pharm. 2013, 443, 103–109. [Google Scholar] [CrossRef]
- Roces, C.B.; Christensen, D.; Perrie, Y. Translating the fabrication of protein-loaded poly(lactic-co-glycolic acid) nanoparticles from bench to scale-independent production using microfluidics. Drug Deliv. Transl. Res. 2020, 10, 582–593. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Mäkilä, E.; Fan, J.; Herranz-Blanco, B.; Wang, C.F.; Rosa, R.; Ribeiro, A.J.; Salonen, J.; Hirvonen, J.; et al. Microfluidic assisted one-step fabrication of porous silicon@acetalated dextran nanocomposites for precisely controlled combination chemotherapy. Biomaterials 2014, 39, 249–259. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harbotrth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-mediated gene silencing in non-human primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef]
- Abrams, M.T.; Koser, M.L.; Seitzer, J.; Williams, S.C.; DiPietro, M.A.; Wang, W.; Shaw, A.W.; Mao, X.; Jadhav, V.; Davide, J.P.; et al. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: Effect of dexamethasone co-treatment. Mol. Ther. 2009, 18, 171–180. [Google Scholar] [CrossRef]
- Ran, R.; Middelberg, A.P.J.; Zhao, C.X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef]
- Krzysztoń, R.; Salem, B.; Lee, D.J.; Schwake, G.; Wagner, E.; Rädler, J.O. Microfluidic self-assembly of folate-targeted monomolecular siRNA-lipid nanoparticles. Nanoscale 2017, 9, 7442–7453. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-Step assembly of homogenous Lipid−Polymeric and Lipid−Quantum DoT nanoparticles enabled by microfluidic rapid mixing. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release 2016, 235, 236–244. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of sIRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef]
- Pareja Tello, R.; Wang, S.; Fontana, F.; Correia, A.; Molinaro, G.; López Cerdà, S.; Hietala, S.; Hirvonen, J.; Barreto, G.; Santos, H.A. Fabrication of hydrogel microspheres via microfluidics using inverse electron demand Diels-Alder click chemistry-based tetrazine-norbornene for drug delivery and cell encapsulation applications. Biomater. Sci. 2023, 11, 4972–4984. [Google Scholar] [CrossRef]
- Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Park, J.; Park, H. Cell encapsulation within alginate hydrogels prepared using microfluidics for improved cell viability and functionality. Adv. Healthc. Mater. 2018, 7, 1800770. [Google Scholar]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.S.; Gupta, I.; Singhal, R. UV irradiation-induced crosslinking of polymeric hydrogels: A study on swelling and mechanical properties. J. Appl. Polym. Sci. 2007, 103, 1743–1751. [Google Scholar]
- Forigua, A.; Kirsch, R.L.; Willerth, S.M.; Elvira, K.S. Recent advances in the design of microfluidic technologies for the manufacture of drug releasing particles. J. Control. Release 2021, 333, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Dendukuri, D.; Doyle, P.S. Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab Chip 2008, 8, 1640–1647. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.Y.; Yi, C.; Li, C.W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications-a review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef]
- Kim, Y.; Lee Chung, B.; Ma, M.; Mulder, W.J.M.; Fayad, Z.A.; Farokhzad, O.C.; Langer, R. Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012, 12, 3587–3591. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Dutta, D.; Dutta, S.; Dey, B.K. Recent advances in microfluidic systems for biomedical applications. Biosens. Bioelectron. 2019, 138, 111–130. [Google Scholar]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled Vesicle Self-Assembly in Microfluidic Channels with Hydrodynamic Focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int. J. Pharm. 2020, 584. [Google Scholar] [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef]
- Han, S.; Hwang, D.K. No more bonding, no more clamping, magnetically assisted membrane integration in microfluidic devices. Microfluid. Nanofluidics 2018, 22, 107. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Liao, S.; He, Y.; Chu, Y.; Liao, H.; Wang, Y. Solvent-resistant and fully recyclable perfluoropolyether-based elastomer for microfluidic chip fabrication. J. Mater. Chem. A 2019, 7, 16249–16286. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef]
- Lai, X.; Lu, B.; Zhang, P.; Zhang, X.; Pu, Z.; Yu, H.; Li, D. Sticker Microfluidics: A Method for Fabrication of Customized Monolithic Microfluidics. ACS Biomater. Sci. Eng. 2019, 5, 6801–6810. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Sengupta, P.; Khanra, K.; Chowdhury, A.R.; Datta, P. Lab-on-a-chip sensing devices for biomedical applications. In Bioelectronics and Medical Devices: From Materials to Devices—Fabrication, Applications and Reliability, 1st ed.; Kunal, P., Heinz-Bernhard, K., Anwesha, K., Sandip, B., Indranil, B., Usha, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Shi, H.; Nie, K.; Dong, B.; Long, M.; Xu, H.; Liu, Z. Recent progress of microfluidic reactors for biomedical applications. Chem. Eng. J. 2019, 361, 635–650. [Google Scholar] [CrossRef]
- Barata, D.; Provaggi, E.; Van Blitterswijk, C.; Habibovic, P. Development of a microfluidic platform integrating high-resolution microstructured biomaterials to study cell-material interactions. Lab Chip 2017, 17, 4134–4147. [Google Scholar] [CrossRef]
- Collins, F.S.; Morgan, M.; Patrinos, A. The Human Genome Project: Lessons from large-scale biology. Science 2003, 300, 286–290. [Google Scholar] [CrossRef]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- McIntyre, D.; Lashkaripour, A.; Fordyce, P.; Densmore, D. Machine learning for microfluidic design and control. Lab Chip 2022, 22, 2925–2937. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A Gas Chromatographic Air Analyzer Fabricated on a Silicon Wafer. IEEE Trans. Electron. Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Warner, R.M. Microelectronics: Its Unusual Origin and Personality. IEEE Trans. Electro Devices 2001, 48, 2457–2467. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Xia, T.; Jiang, R.; Fu, Y.; Jin, N. Automated Blood Cell Detection and Counting via Deep Learning for Microfluidic Point-of-Care Medical Devices. IOP Conf. Ser Mater. Sci. Eng. 2019, 646, 012048. [Google Scholar] [CrossRef]
- Xu, G.; Nolder, D.; Reboud, J.; Oguike, M.C.; Van Schalkwyk, D.A.; Sutherland, C.J.; Cooper, J.M. Paper-Origami-Based Multiplexed Malaria Diagnostics from Whole Blood. Angew. Chem. 2016, 128, 15476–15479. [Google Scholar] [CrossRef]
- Nazari, M.; Sani, H.M.; Kayhani, M.H.; Daghighi, Y. Different stages of liquid film growth in a microchannel: Two-phase lattice boltzmann study. Braz. J. Chem. Eng. 2018, 35, 977–994. [Google Scholar] [CrossRef]
- Nooranidoost, M.; Kumar, R. Geometry effects of axisymmetric flow-focusing microchannels for single cell encapsulation. Materials 2019, 12, 2811. [Google Scholar] [CrossRef]
- Wiedemeier, S.; Römer, R.; Wächter, S.; Staps, U.; Kolbe, C.; Gastrock, G. Precision moulding of biomimetic disposable chips for droplet-based applications. Microfluid. Nanofluidics 2017, 21, 167. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Azimi-Boulali, J.; Madadelahi, M.; Madou, M.J.; Martinez-Chapa, S.O. Droplet and particle generation on centrifugal microfluidic platforms: A review. Micromachines 2020, 11, 603. [Google Scholar] [CrossRef]
- Sivasamy, J.; Wong, T.N.; Nguyen, N.T.; Kao, L.T.H. An investigation on the mechanism of droplet formation in a microfluidic T-junction. Microfluid. Nanofluidics 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, S.W.; Tan, J.; Luo, G.S. Correlations of droplet formation in T-junction microfluidic devices: From squeezing to dripping. Microfluid. Nanofluidics 2008, 5, 711–717. [Google Scholar] [CrossRef]
- Sontti, S.G.; Atta, A. Numerical Insights on Controlled Droplet Formation in a Microfluidic Flow-Focusing Device. Ind. Eng. Chem. Res. 2020, 59, 3702–3716. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Roy, E.; Pallandre, A.; Zribi, B.; Horny, M.C.; Delapierre, F.D.; Cattoni, A.; Gamby, J.; Haghiri-Gosnet, A.M. Overview of Materials for Microfluidic Applications. In Advances in Microfluidics—New Applications in Biology, Energy, and Materials Sciences, 1st ed.; Yu, X., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Elvira, K.S.; Gielen, F.; Tsai, S.S.H.; Nightingale, A.M. Materials and methods for droplet microfluidic device fabrication. Lab Chip 2022, 22, 859–875. [Google Scholar] [CrossRef]
- Ryzhkov, V.V.; Echeistov, V.V.; Zverev, A.V.; Baklykov, D.A.; Konstantinova, T.; Lotkov, E.S.; Ryazantcev, P.G.; Alibekov, R.S.; Kuguk, A.K.; Aleksandrov, A.R.; et al. Integrated membrane-free thermal flow sensor for silicon-on-glass microfluidics. Lab Chip 2023, 23, 2789–2797. [Google Scholar] [CrossRef]
- Zhelev, N.; Abhilash, T.S.; Bennett, R.G.; Smith, E.N.; Ilic, B.; Parpia, J.M.; Levitin, L.V.; Rojas, X.; Casey, A.; Saunders, J. Fabrication of micro fluidic cavities using Si-to-glass anodic bonding. Rev. Sci. Instrum. 2018, 89, 073902. [Google Scholar] [CrossRef]
- Mayo, J.A. Dispositivos Microfluídicos Impresos en 3D un Enfoque Alterno para una Variedad de Aplicaciones. Universidad Simón Bolívar, 2023, 1–7. Available online: https://www.researchgate.net/publication/372394373_Dispositivos_microfluidicos_impresos_en_3D_un_enfoque_alterno_para_una_variedad_de_aplicaciones (accessed on 16 March 2025). [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Luengo, J.; Minazzoli, C.; D’Angelo, M.V.; Cachile, M.A.; Freytes, V.M. Study of wettability and charactrization of the PDMS-Glass system in microfluidic devices. An. Asoc. Fis. Argent. 2017, 28, 10–14. [Google Scholar]
- Lin, Z.; Xu, J.; Song, Y.; Li, X.; Wang, P.; Chu, W.; Wang, Z.; Cheng, Y. Freeform microfluidic networks encapsulated in laser printed three-dimensional macro-scale glass objects. Adv. Mater. Technol. 2020, 5, 1900989. [Google Scholar] [CrossRef]
- Zhou, C.; Liang, S.; Qi, B.; Liu, C.; Cho, N.J. One-pot microfluidic fabrication of micro ceramic particles. Nat. Commun. 2024, 15, 8862. [Google Scholar] [CrossRef]
- Shan, X.; Ling, S.H.; Maw, H.P.; Lu, C.W.; Lam, Y.C. Micro embossing of ceramic green substrates for micro devices. In Proceedings of the 2008 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS, Nice, France, 9–11 April 2008; IEEE: New York, NY, USA; pp. 355–359. [Google Scholar] [CrossRef]
- Gencturk, E.; Mutlu, S.; Ulgen, K.O. Advances in microfluidic devices made from thermoplastics used in cell biology and analyses. Biomicrofluidics 2017, 11, 051502. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Liu, S.; Liu, J.; Gao, K.; Zhang, Y. Rapid prototyping of shrinkable BOPS-based microfluidic devices. Microfluid. Nanofluidics 2018, 22, 136. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Jarad, N.A.; Didar, T.F. The Fabrication and Bonding of Thermoplastic Microfluidics: A Review. Materials 2022, 15, 6478. [Google Scholar] [CrossRef]
- Ahmadianyazdi, A.; Miller, I.J.; Folch, A. Tunable resins with PDMS-like elastic modulus for stereolithographic 3D-printing of multimaterial microfluidic actuators. Lab Chip 2023, 23, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.; Allen, W.S.; Arroyo-Currás, N.; Hur, S.C. Rapid prototyping of thermoplastic microfluidic devices via SLA 3D printing. Sci. Rep. 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.T.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Clancy, A.; Chen, D.; Bruns, J.; Nadella, J.; Stealey, S.; Zhang, Y.; Timperman, A.; Zustiak, S.P. Hydrogel-based microfluidic device with multiplexed 3D in vitro cell culture. Sci. Rep. 2022, 12, 17781. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, S.; Hirvonen, J.; Santos, H.A.; Li, W. Microfluidics Fabrication of Micrometer-Sized Hydrogels with Precisely Controlled Geometries for Biomedical Applications. Adv. Healthc. Mater. 2022, 11, 2200846. [Google Scholar] [CrossRef]
- Shahriari, S.; Selvaganapathy, P.R. Integration of hydrogels into microfluidic devices with porous membranes as scaffolds enables their drying and reconstitution. Biomicrofluidics 2022, 16, 054108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, F.; Yang, Y.; Zhao, G.; Zhang, C.; Wang, R.; Huang, X. Research Progress and Future Trends of Microfluidic Paper-Based Analytical Devices in In-Vitro Diagnosis. Biosensors 2022, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Anushka; Bandopadhyay, A.; Das, P.K. Paper based microfluidic devices: A review of fabrication techniques and applications. Eur. Phys. J. Spec. Top. 2023, 232, 781–815. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef]
- Meka, L.; Kesavan, B.; Kalamata, V.N.; Eaga, C.M.; Bandari, S.; Vobalaboina, V.; Yamsani, M.R. Design and evaluation of polymeric coated minitablets as multiple unit gastroretentive floating drug delivery systems for furosemide. J. Pharm. Sci. 2009, 98, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; Devoe, D.L.; Gaitan, M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef]
- Rhee, M.; Valencia, P.M.; Rodriguez, M.I.; Langer, R.; Farokhzad, O.C.; Karnik, R. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv. Mater. 2011, 23, H79–H83. [Google Scholar] [CrossRef]
- Bicudo, R.C.S.; Santana, M.H.A. Production of hyaluronic acid (HA) nanoparticles by a continuous process inside microchannels: Effects of non-solvents, organic phase flow rate, and HA concentration. Chem. Eng. Sci. 2012, 84, 134–141. [Google Scholar] [CrossRef]
- Kumar, K.; Nightingale, A.M.; Krishnadasan, S.H.; Kamaly, N.; Wylenzinska-Arridge, M.; Zeissler, K.; Branford, W.R.; Ware, E.; Demello, A.J.; Demello, J.C. Direct synthesis of dextran-coated superparamagnetic iron oxide nanoparticles in a capillary-based droplet reactor. J. Mater. Chem. 2012, 22, 4704–4708. [Google Scholar] [CrossRef]
- Lazarus, L.L.; Riche, C.T.; Marin, B.C.; Gupta, M.; Malmstadt, N.; Brutchey, R.L. Two-phase microfluidic droplet flows of ionic liquids for the synthesis of gold and silver nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 3077–3083. [Google Scholar] [CrossRef]
- Sebastian Cabeza, V.; Kuhn, S.; Kulkarni, A.A.; Jensen, K.F. Size-controlled flow synthesis of gold nanoparticles using a segmented flow microfluidic platform. Langmuir 2012, 28, 7007–7013. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef]
- Kim, Y.; Fay, F.; Cormode, D.P.; Sanchez-Gaytan, B.L.; Tang, J.; Hennessy, E.J.; Ma, M.; Moore, K.; Farokhzad, O.C.; Fisher, E.A.; et al. Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics. ACS Nano 2013, 7, 9975–9983. [Google Scholar] [CrossRef] [PubMed]
- Pustulka, K.M.; Wohl, A.R.; Lee, H.S.; Michel, A.R.; Han, J.; Hoye, T.R.; McCormick, A.V.; Panyam, J.; Macosko, C.W. Flash nanoprecipitation: Particle structure and stability. Mol. Pharm. 2013, 10, 4367–4377. [Google Scholar] [CrossRef]
- Hood, R.R.; Devoe, D.L.; Atencia, J.; Vreeland, W.N.; Omiatek, D.M. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip 2014, 14, 2403–2409. [Google Scholar] [CrossRef]
- Lim, J.M.; Bertrand, N.; Valencia, P.M.; Rhee, M.; Langer, R.; Jon, S.; Farokhzad, O.C.; Karnik, R. Parallel microfluidic synthesis of size-tunable polymeric nanoparticles using 3D flow focusing towards in vivo study. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 401–409. [Google Scholar] [CrossRef]
- Saad, W.S.; Prud’Homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Baby, T.; Liu, Y.; Middelberg, A.P.J.; Zhao, C.X. Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles. Chem. Eng. Sci. 2017, 169, 128–139. [Google Scholar] [CrossRef]
- Wilson, D.R.; Mosenia, A.; Suprenant, M.P.; Upadhya, R.; Routkevitch, D.; Meyer, R.A.; Quinones-Hinojosa, A.; Green, J.J. Continuous microfluidic assembly of biodegradable poly(Beta-amino ester)/DNA nanoparticles for enhanced gene delivery. J. Biomed. Mater. Res. A 2017, 105, 1813–1825. [Google Scholar] [CrossRef]
- Ran, R.; Wang, H.; Liu, Y.; Hui, Y.; Sun, Q.; Seth, A.; Wibowo, D.; Chen, D.; Zhao, C.X. Microfluidic self-assembly of a combinatorial library of single- and dual-ligand liposomes for in vitro and in vivo tumor targeting. Eur. J. Pharm. Biopharm. 2018, 130, 1–10. [Google Scholar] [CrossRef]
- Leung, M.H.M.; Shen, A.Q. Microfluidic Assisted Nanoprecipitation of PLGA Nanoparticles for Curcumin Delivery to Leukemia Jurkat Cells. Langmuir 2018, 34, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Abalde-Cela, S.; Taladriz-Blanco, P.; De Oliveira, M.G.; Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 2018, 8, 2440. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, O.; Koyuncu, A.H.; Pittermannová, A.; Ulbrich, P.; Tokárová, V. Governing factors for preparation of silver nanoparticles using droplet-based microfluidic device. Biomed. Microdevices 2019, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Aluunmani, R.; Bolognesi, G.; Vladisavljević, G.T. Facile Microfluidic Fabrication of Biocompatible Hydrogel Microspheres in a Novel Microfluidic Device. Molecules 2022, 27, 4013. [Google Scholar] [CrossRef]
- Hanna, K.; Yasar-inceoglu, O.; Yasar, O. Drug Delivered Poly (ethylene glycol) Diacrylate (PEGDA) Hydrogels and Their Mechanical Characterization Tests for Tissue Engineering Applications. MRS Adv. 2018, 3, 1697–1702. [Google Scholar] [CrossRef]
- Ambebila, E.N.; Santamaría, E.; Maestro, A.; Gutiérrez, J.M.; González, C. Gellan Hydrogels: Preparation, Rheological Characterization and Application in Encapsulation of Curcumin. Food Biophys. 2019, 14, 154–163. [Google Scholar] [CrossRef]
- Homyak, C.C.; Fernandez, A.; Touve, M.A.; Zhao, B.; Anson, F.; Hardy, J.A.; Vachet, R.W.; Gianneschi, N.C.; Ross, J.L.; Thayumanavan, S. Lipogels for Encapsulation of Hydrophilic Proteins and Hydrophobic Small Molecules. Biomacromolecules 2018, 19, 132–140. [Google Scholar] [CrossRef]


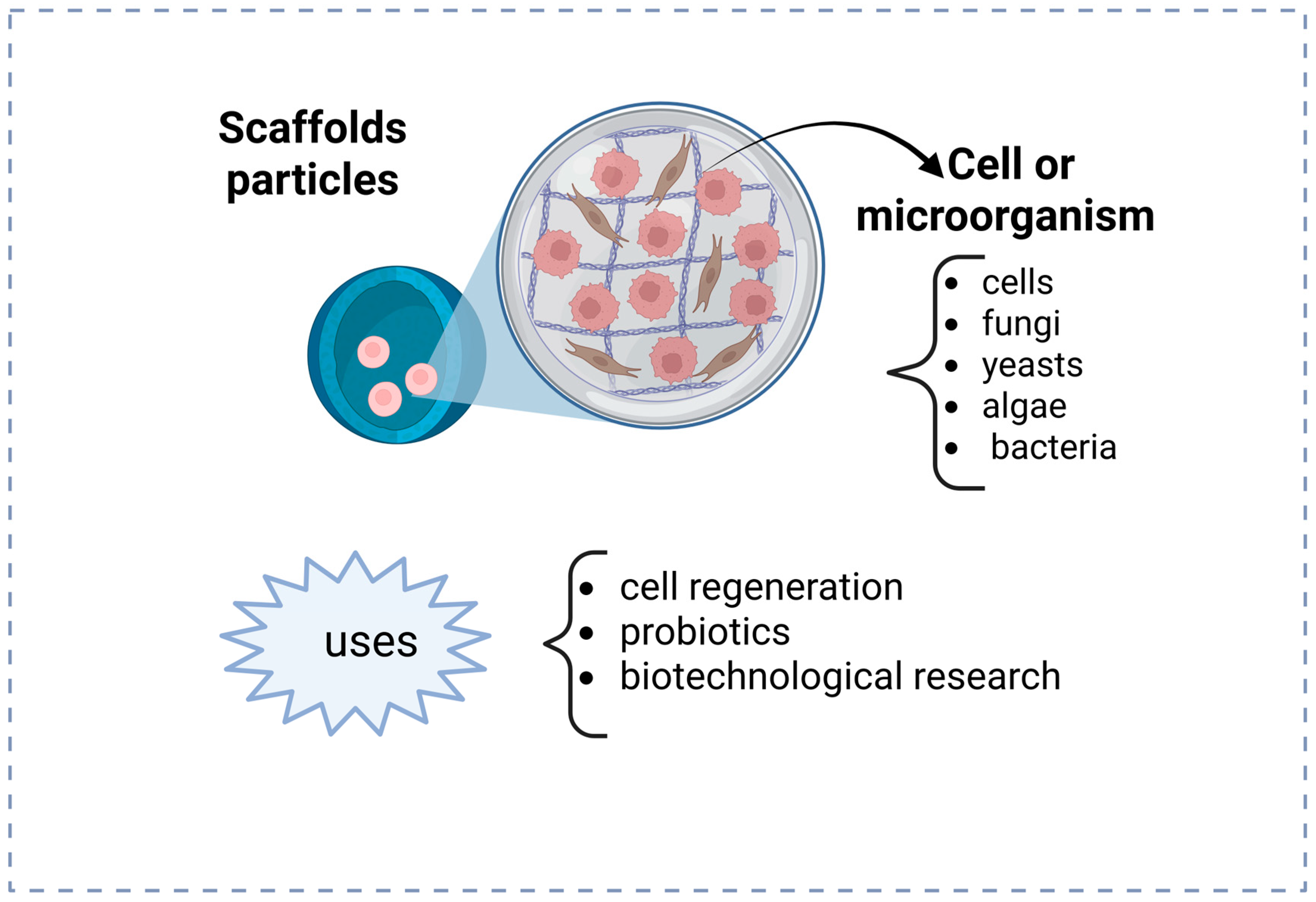
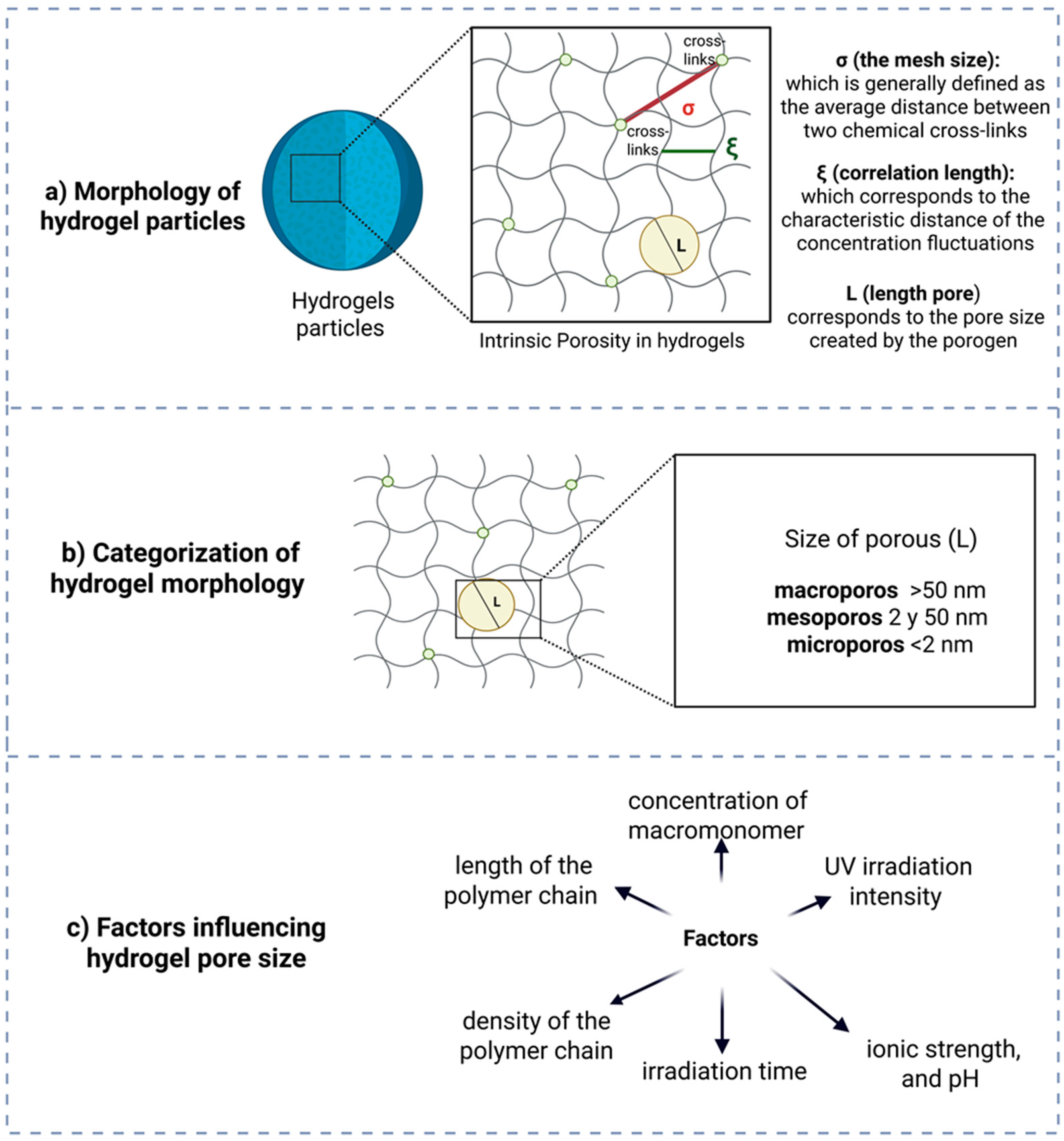

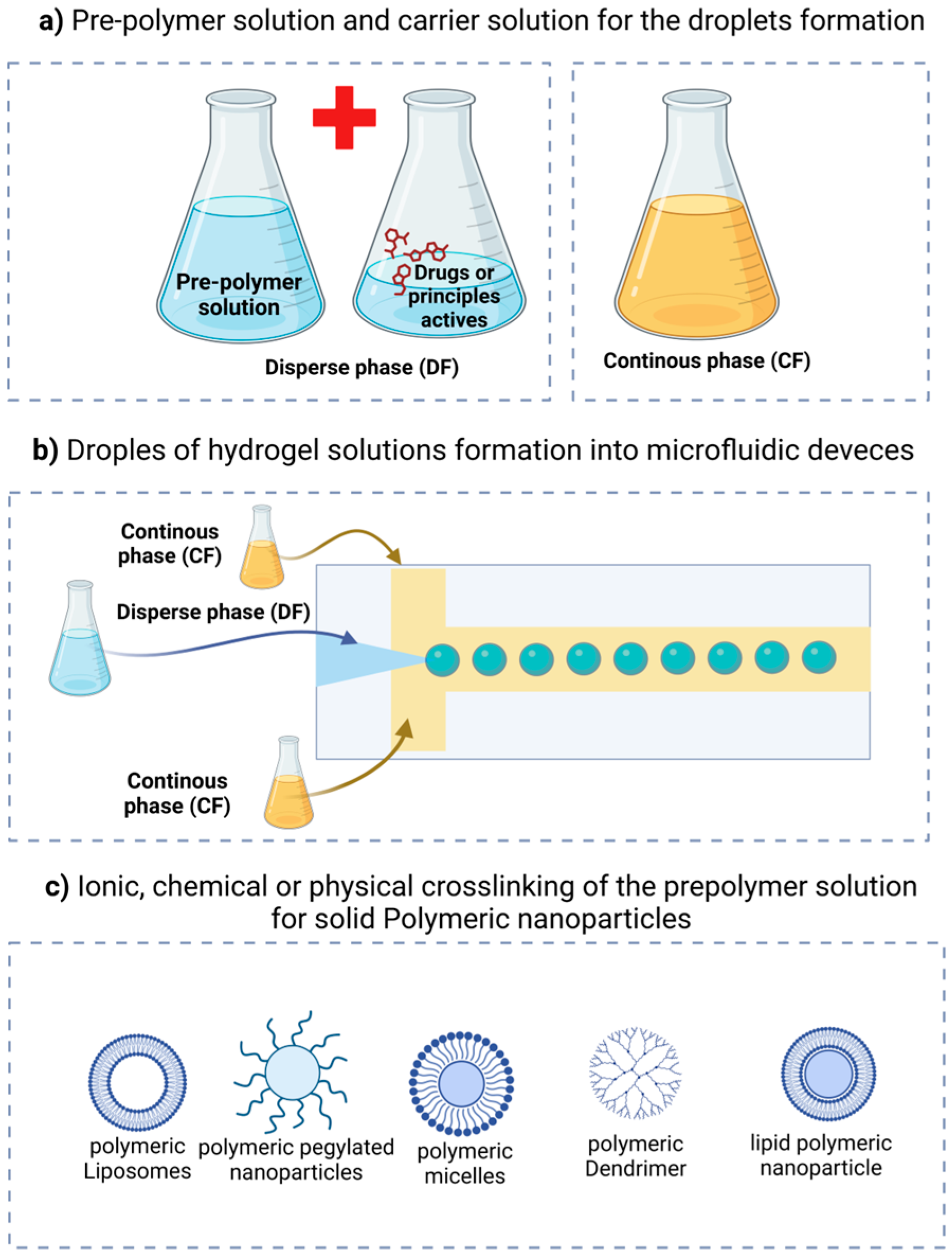
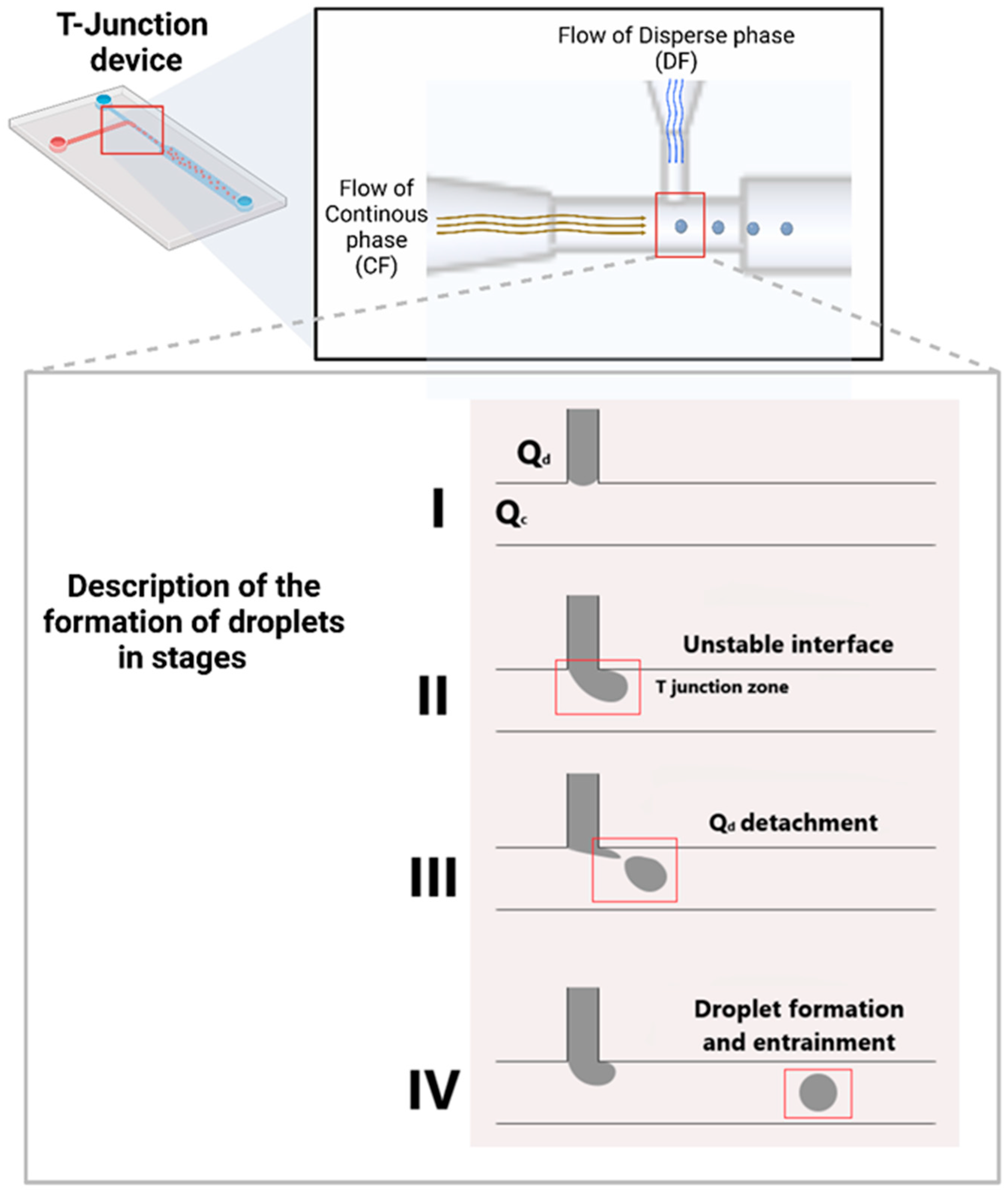
| Year | Device | Material of Device | Device Manufacturing Technology | Author/References |
|---|---|---|---|---|
| 2004 | 2D HFF | Silicon/glass; PDMS | Photolithography on silicon | [65] |
| 2008 | 2D HFF | Silicon/glass; PDMS | Soft lithography on PDMS (SU-8) | [121] |
| 2009 | SHM | PDMS; Cyclicolefin copolymer (COC) | Floating coating drug delivery system | [122] |
| 2010 | 2D HFF | Silicon/glass; PDMS | Photolithography and deep reactive ion etching (DRIE) on silicon | [123] |
| 2011 | 3D HFF | Glass capillaries; PDMS | Soft lithography on PDMS | [124] |
| 2012 | 2D HFF, T-junction, flow focusing, and co-flowing | Silicon/glass; PDMS | Photolithography process on glass | [125] |
| 2012 | 3D HFF variants | PDMS; glass capillaries | Photo- and soft lithography on PDMS (SU-8) | [61] |
| 2012 | Droplet mixer | PDMS; glass capillaries | Capillary droplet reactor and silicone tubing | [126] |
| 2012 | Droplet mixer | PDMS; glass capillaries | Photo- and soft lithography on PDMS (SU-8) | [127] |
| 2012 | Jet mixers (MIVM | Polycarbonate and PTFE tubing; Teflon tubing | Silicon/Pyrex microfluidic device | [128] |
| 2013 | 2D HFF | Silicon/glass; PDMS | Flow-focusing application camera | [40] |
| 2013 | 3D HFF variants | PDMS; glass capillaries | Photo- and soft lithography on PDMS (SU-8) | [129] |
| 2013 | 3D HFF variants | PDMS; glass | Photo- and soft lithography on PDMS (SU-8) | [130] |
| 2013 | Jet mixers (MIVM and CIJ) | Polycarbonate and PTFE tubing; Teflon tubing | Multi-inlet vortex and confined impact jet mixer | [131] |
| 2014 | 3D HFF | Glass capillaries; | Multi-capillary glass matrix design | [132] |
| 2014 | 3D HFF | PDMS; glass | Photo- and soft lithography on PDMS (SU-8) | [133] |
| 2014 | Jet mixers (MIVM and CIJ) | Polycarbonate and PTFE tubing; Teflon tubing | Coaxial turbulent jet mixer with clear polycarbonate tubes and clear probe | [42] |
| 2015 | 3D HFF variants | PDMS; glass capillaries | Borosilicate capillary assembly (glass) | [42] |
| 2015 | Baffle mixer | PDMS; glass | 3D glass capillary device | [42] |
| 2015 | SHM | PDMS; Cyclicolefin copolymer (COC) | Microfluidics chip device | [4] |
| 2016 | 2D HFF | Silicon/glass; PDMS | Photo- and soft lithography on PDMS (SU-8) | [45] |
| 2016 | SHM | PDMS; Cyclicolefin copolymer (COC) | Multi-inlet vortex and confined impact jet mixer | [134] |
| 2016 | SHM | PDMS; Cyclicolefin copolymer (COC) | Microfluidic mixing system. | [49] |
| 2017 | 2D HFF | Silicon/glass; PDMS | Soft lithography on PDMS (SU-8) | [46] |
| 2017 | 2D HFF | Silicon/glass; PDMS | Soft lithography on PDMS (SU-8) | [135] |
| 2017 | 3D HFF variants | PDMS; glass capillaries | Photo- and soft lithography on PDMS (SU-8) | [136] |
| 2018 | 2D HFF | Silicon/glass; PDMS | Injection molded propylene | [137] |
| 2018 | 2D HFF/cross-slot microfluidic | Silicon/glass; PDMS | Soft lithography on PDMS (SU-8) | [138] |
| 2018 | Droplet mixer | PDMS; Glass capillaries | Photolithography on PDMS (SU-8) | [48] |
| 2018 | Jet mixers (MIVM and CIJ) | Polycarbonate and PTFE tubing; Teflon tubing | Photo- and soft lithography on PDMS (SU-8) | [139] |
| bezel 2019 | 3D HFF variants | Glass capillaries | Insertion of conical cylindrical capillaries in a square capillary | [5] |
| 2019 | Droplet mixer | PDMS; glass capillaries | Soft lithography on PDMS (SU-8) | [140] |
| Year | Device Geometry | Polymer | Drugs | Applications | Author, Year |
|---|---|---|---|---|---|
| 2008 | 2D HFF | PLGA-PEG | --- | Drug delivery | [121] |
| 2010 | 2D HFF | PLGA-PEG | Lecithin | Sustained release drug delivery | [47] |
| 2011 | 3D HFF | PLGA-PEG | --- | Drug delivery | [124] |
| 2012 | 2D HFF, T-junction, flow focusing, and co-flowing | Hyaluronic acid (HA) | --- | Drug delivery and cosmetic field | [125] |
| 2012 | 3D HFF variants | PLGA + LIPIDS + PEG | --- | Controlled release | [61] |
| 2013 | 2D HFF | PLGA | Gemcitabine | Drug delivery in cancer | [40] |
| 2013 | 3D HFF variants | PLGA-PEG | Docetaxel | Prostate cancer | [129] |
| 2014 | 2D HFF | PLGA-PEG | --- | Drug delivery | [142] |
| 2014 | 3D HFF | PLGA-PEG | Docetaxel | Drug delivery | [133] |
| 2015 | SHM | LNPs: phospholipids, cholesterol and polyethylene glycol (PEG) | --- | RNA delivery | [4] |
| 2016 | SHM | PCL-b-PEG in THF. | Paclitaxel-VES combined/VES | Enhanced nanoparticle formation | [134] |
| 2016 | SHM | polyethylene glycol lipid (PEG) | Ionizable amino-lipid, diaryl-noleoylme-thyl-4-dimethylaminobutyrate (DLin-MC3-DMA) | LNP-siRNA for RNA delivery | [49] |
| 2017 | 2D HFF | DOPE, DOTAP, DOPC and DSPE-PEG (2000) | mNALPs with folate-conjugated | Gene tumor targeting | [46] |
| 2017 | 3D HFF variants | Pobi(beta-amino ester) PBAE | Plasmid DN | Gene delivery for gene therapy | [136] |
| 2018 | 2D HFF/cross-slot microfluidic | PLGA | Curcumin | Drug delivery | [143] |
| 2018 | Droplet mixer | lipid/alcohol | Interfering RNA (siRNA) DNA or RNA/buffer | Nanomedicine drug delivery systems | [48] |
| 2018 | 2D HFF | PLGA | Model protein (OVA) | Vaccine adjuvants | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinojosa-Ventura, G.; Acosta-Cuevas, J.M.; Velázquez-Carriles, C.A.; Navarro-López, D.E.; López-Alvarez, M.Á.; Ortega-de la Rosa, N.D.; Silva-Jara, J.M. From Basic to Breakthroughs: The Journey of Microfluidic Devices in Hydrogel Droplet Generation. Gels 2025, 11, 309. https://doi.org/10.3390/gels11050309
Hinojosa-Ventura G, Acosta-Cuevas JM, Velázquez-Carriles CA, Navarro-López DE, López-Alvarez MÁ, Ortega-de la Rosa ND, Silva-Jara JM. From Basic to Breakthroughs: The Journey of Microfluidic Devices in Hydrogel Droplet Generation. Gels. 2025; 11(5):309. https://doi.org/10.3390/gels11050309
Chicago/Turabian StyleHinojosa-Ventura, Gabriela, José Manuel Acosta-Cuevas, Carlos Arnulfo Velázquez-Carriles, Diego E. Navarro-López, Miguel Ángel López-Alvarez, Néstor D. Ortega-de la Rosa, and Jorge Manuel Silva-Jara. 2025. "From Basic to Breakthroughs: The Journey of Microfluidic Devices in Hydrogel Droplet Generation" Gels 11, no. 5: 309. https://doi.org/10.3390/gels11050309
APA StyleHinojosa-Ventura, G., Acosta-Cuevas, J. M., Velázquez-Carriles, C. A., Navarro-López, D. E., López-Alvarez, M. Á., Ortega-de la Rosa, N. D., & Silva-Jara, J. M. (2025). From Basic to Breakthroughs: The Journey of Microfluidic Devices in Hydrogel Droplet Generation. Gels, 11(5), 309. https://doi.org/10.3390/gels11050309










