The Evaluation of the Effectiveness of Adsorbents Based on Acrylamide Hydrogels and Cryogels for Water Purification from Radioactive Contaminants
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of Gels
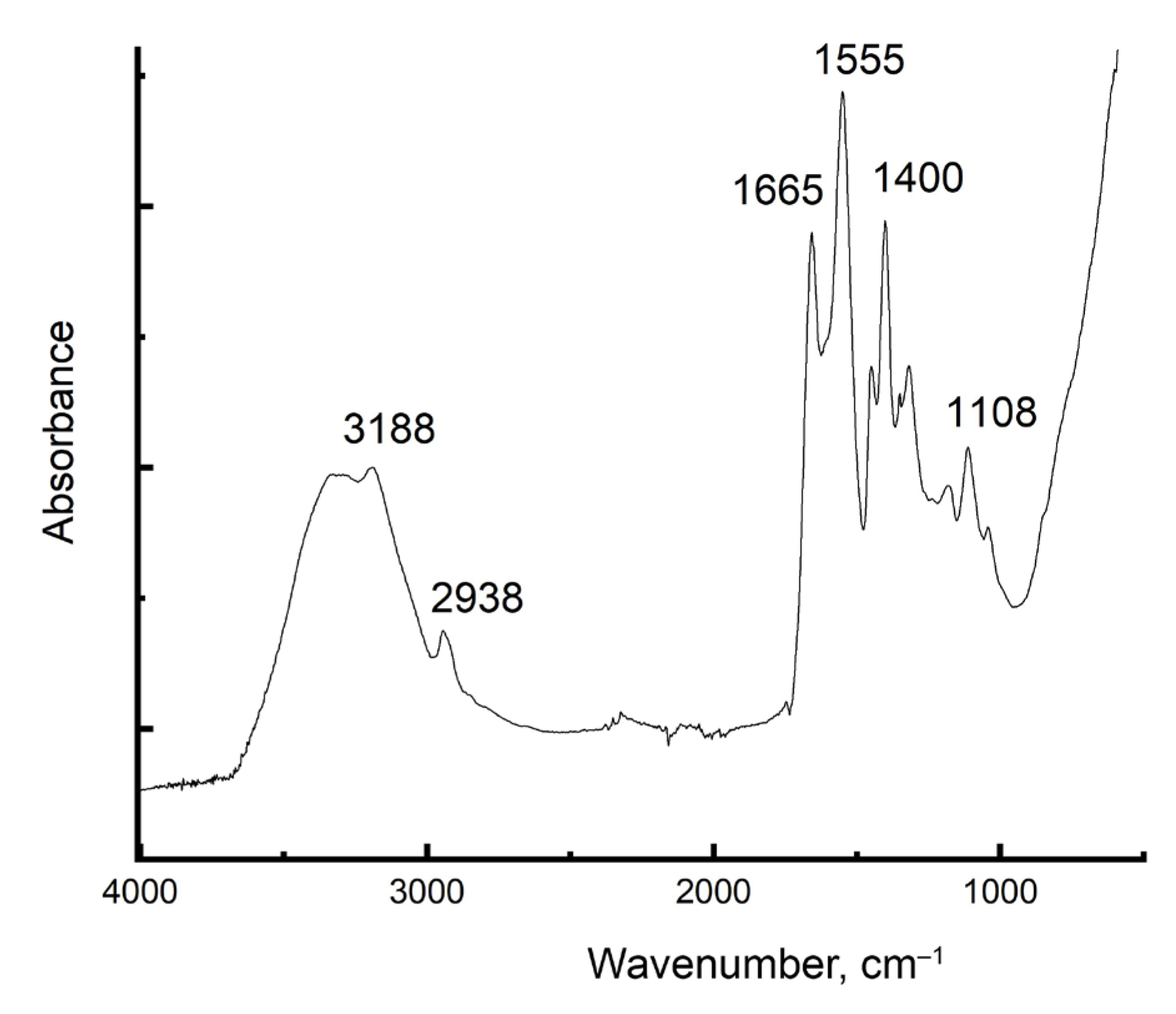
2.2. FTIR Spectroscopy Results
2.3. Swelling Kinetics of Hydrogels
2.4. Mechanical Properties of Hydrogels and Cryogels
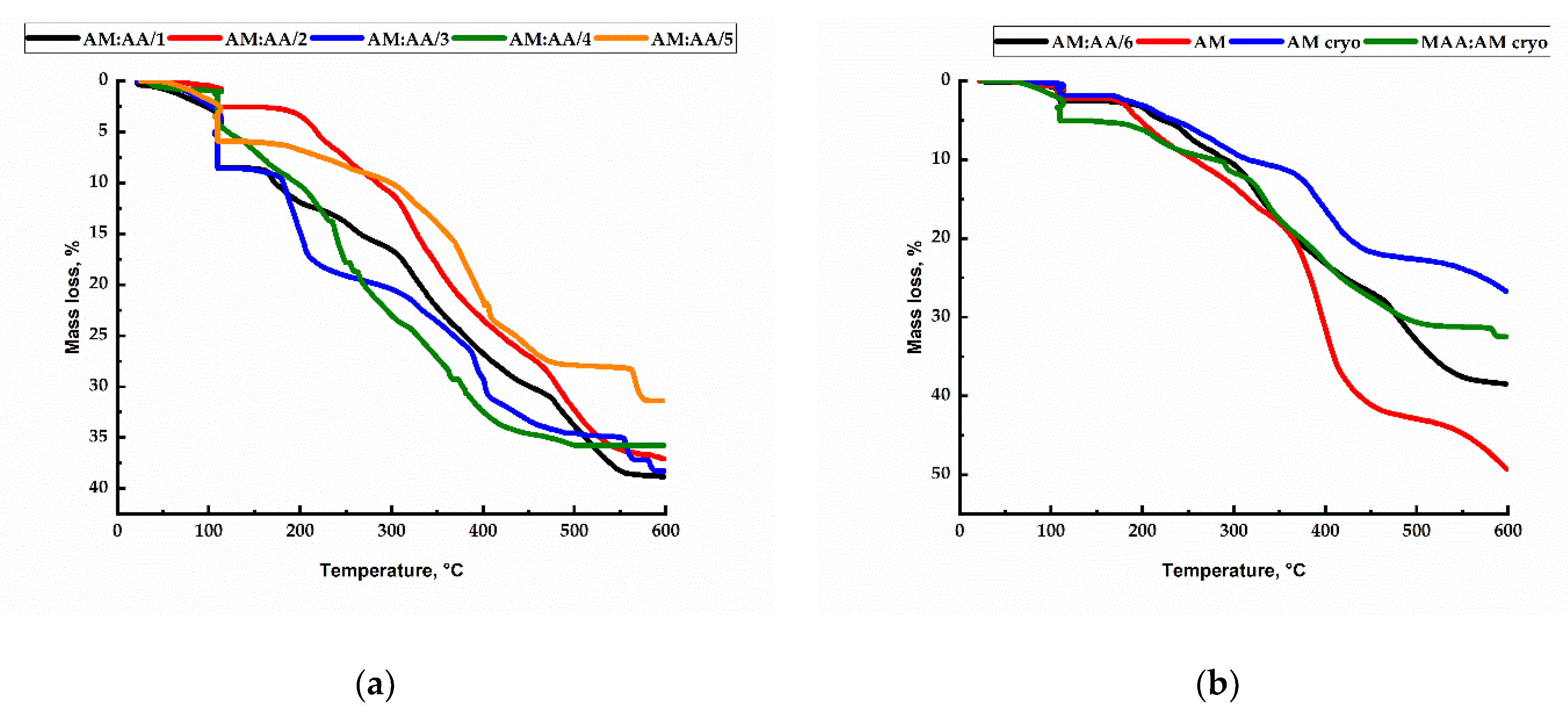
2.5. TGA Analysis of Hydrogels and Cryogels
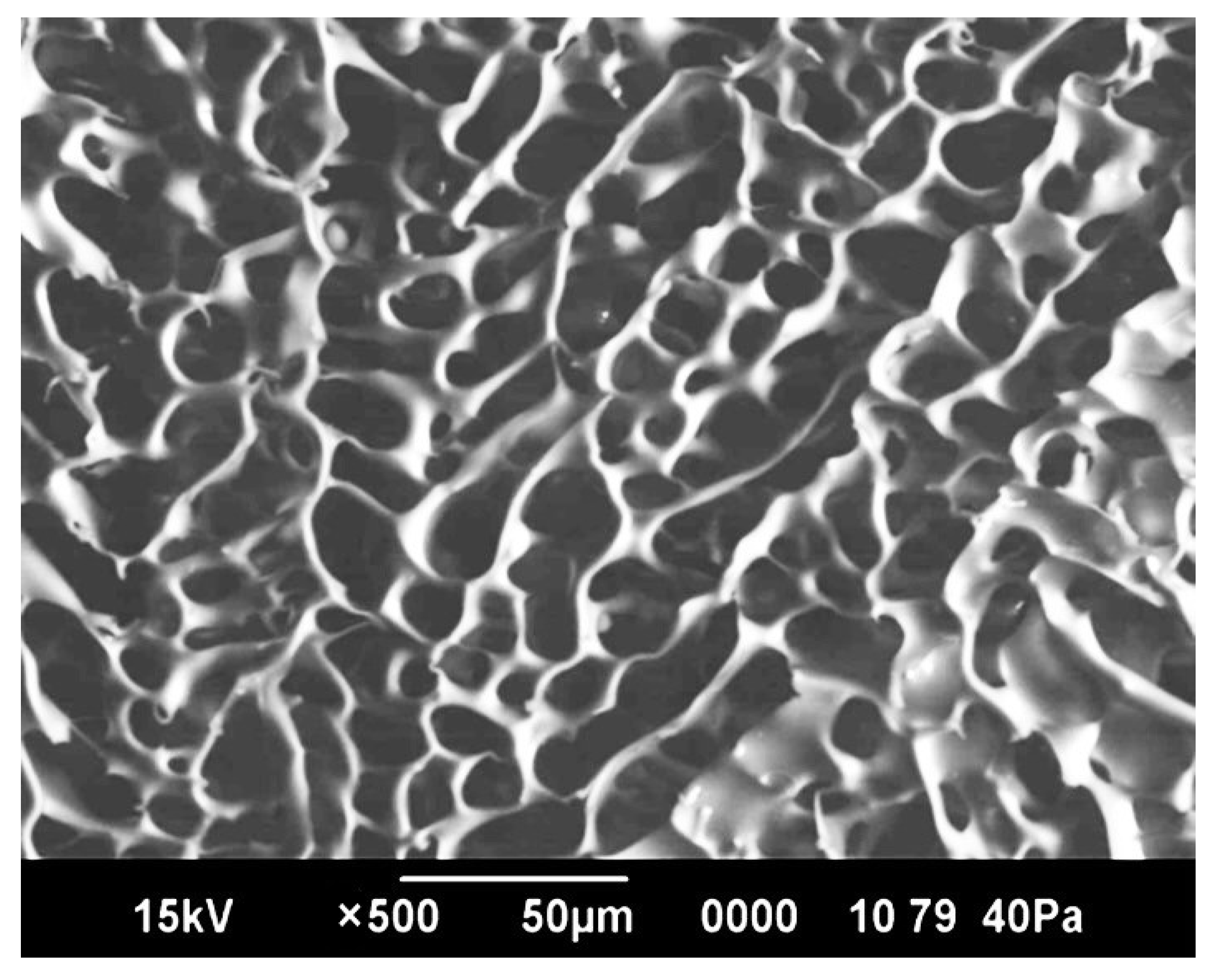
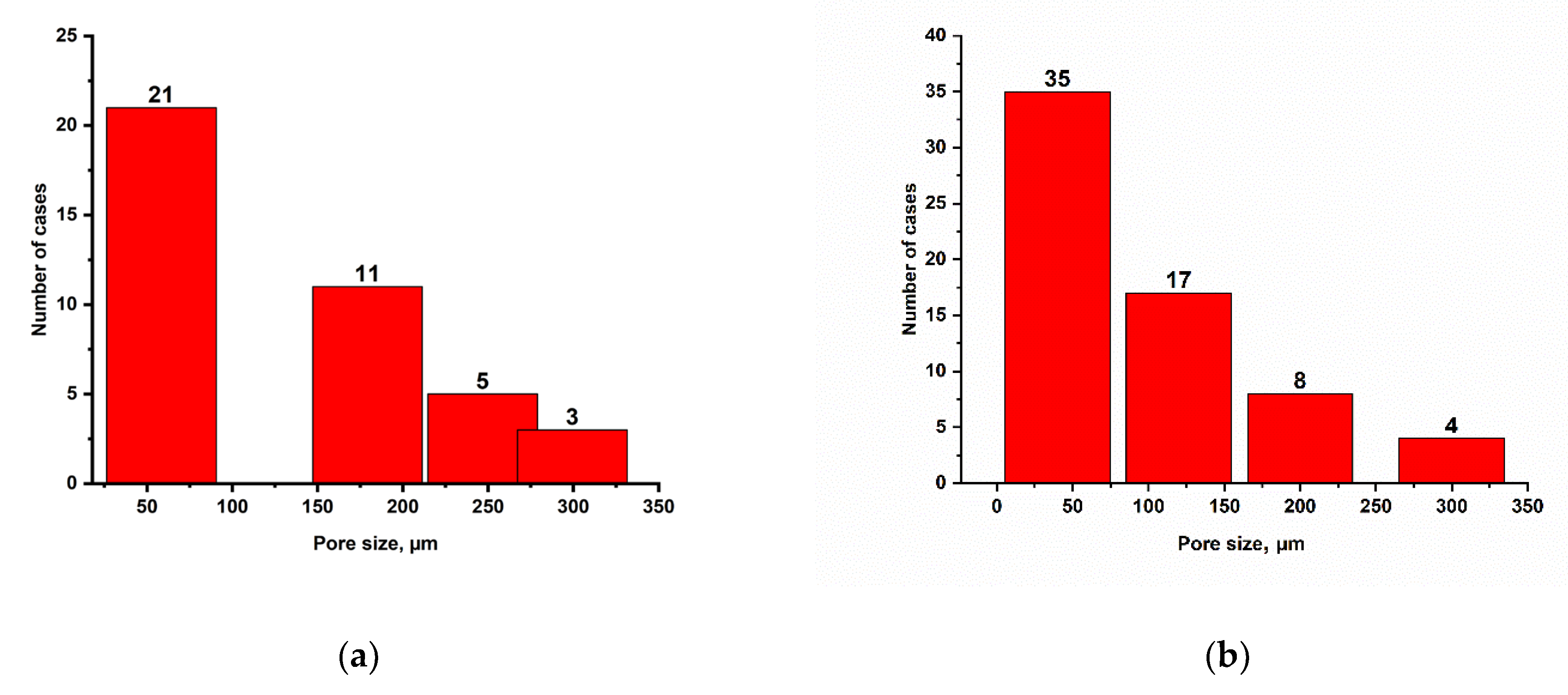
2.6. Study of Cryogel Morphology
2.7. Study of Radionuclide Adsorption
3. Conclusions
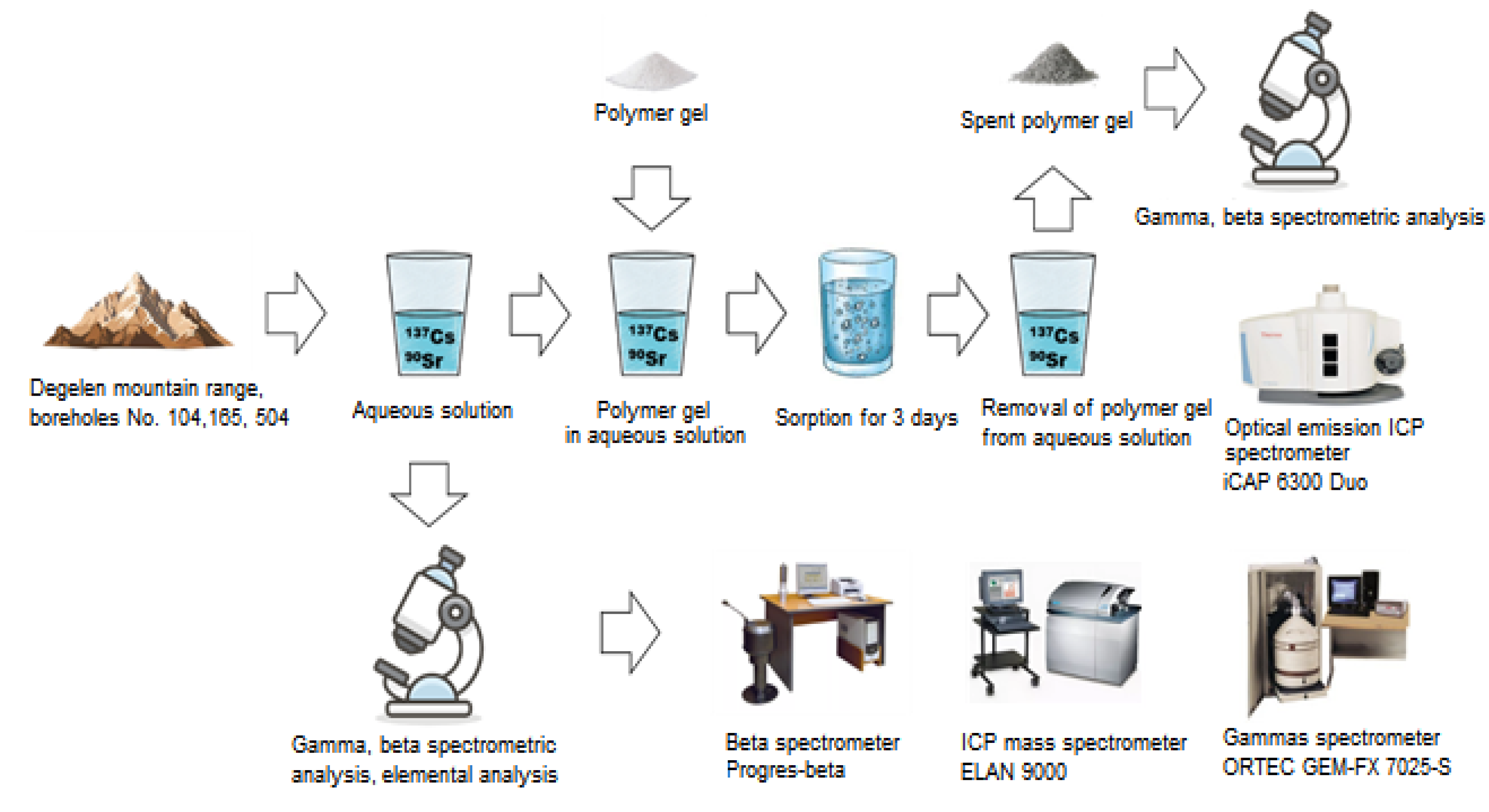
4. Materials and Methods
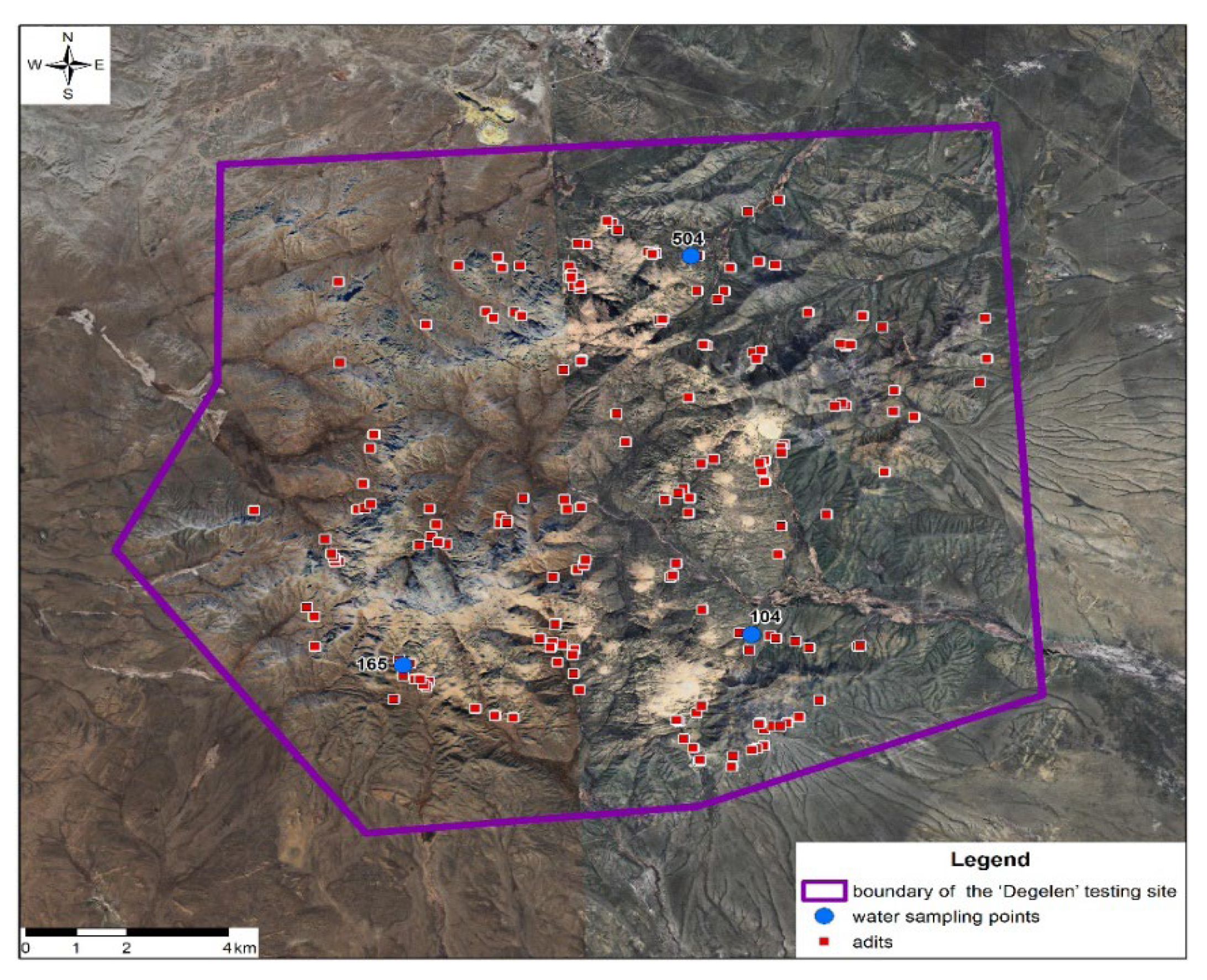
4.1. Materials
4.2. Methods
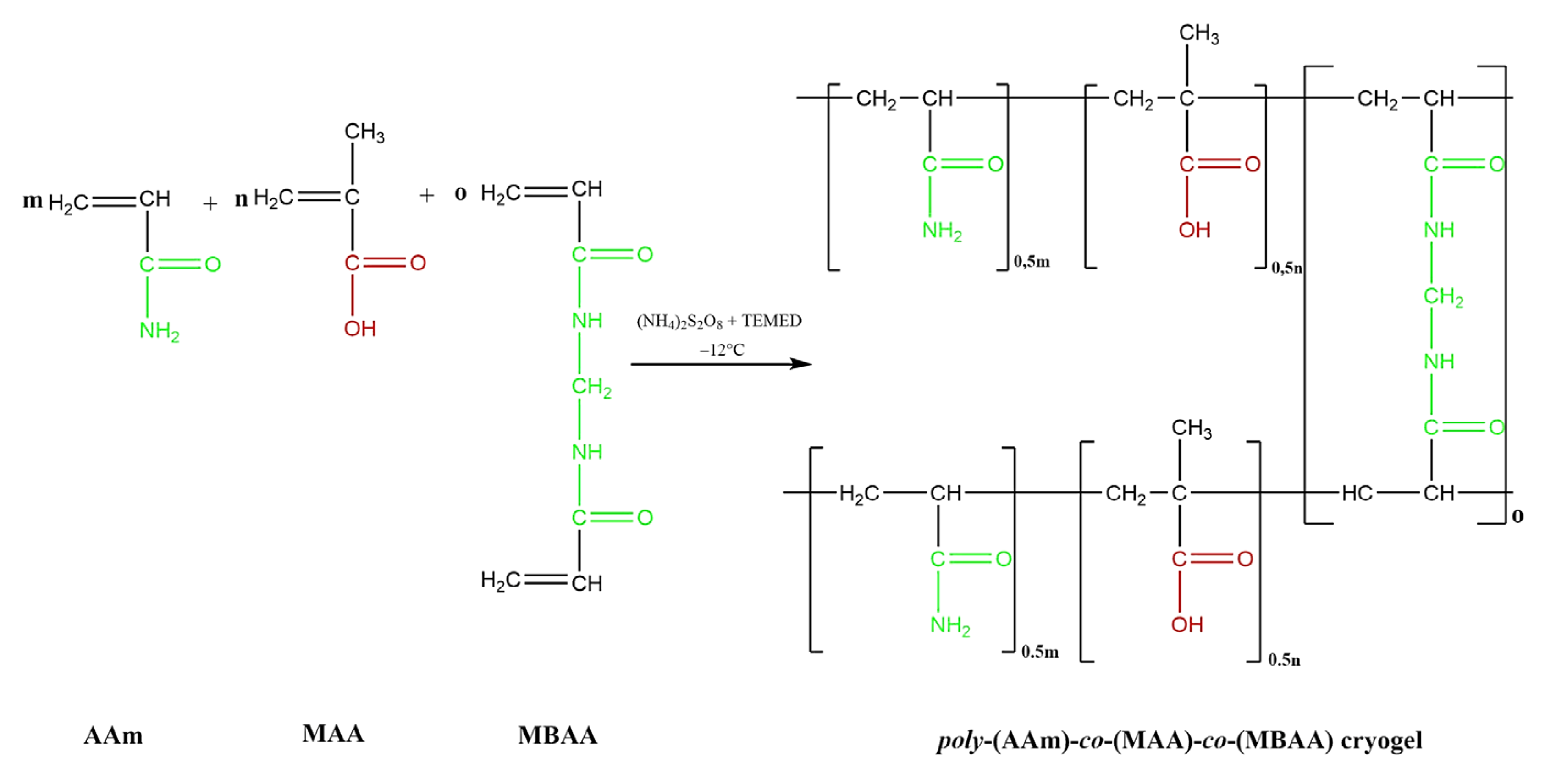
4.2.1. Preparation of Polymer Hydrogels and Cryogels
4.2.2. Swelling Kinetics
4.2.3. Mechanical Strength of Polymer Gels
4.2.4. Thermogravimetric Analysis of Hydrogels
4.2.5. SEM of Cryogels
4.2.6. Adsorption Experiments
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Acrylomide |
| AA | Acrylic acid |
| MAA | Methacrylic acid |
| MBAA | Methylenebisacrylamide |
| PSA | Ammonium persulfate |
| TEMED | Tetramethylethylenediamine |
| SD | Swelling degree |
References
- Aidarkhanova, A.; Larionova, N.; Tashekova, A.; Dyussembayeva, M.; Mamyrbayeva, A.; Timonova, L.; Shakenov, Y.; Mulikova, A.; Aidarkhanov, A. Assessment of the radionuclide and chemical composition of the Irtysh River water at the Republic of Kazakhstan territory. RSC Adv. 2024, 14, 26208–26218. [Google Scholar] [CrossRef] [PubMed]
- Mukhamediyarov, N.; Makarychev, S.; Umarov, M.; Dyuisembaeva, M.; Shakenov, Y.; Kolbin, V.; Yesilkanov, G.; Temirzhanova, A.; Tashekova, A.; Zhamaldinov, F.; et al. Uranium speciation and spatial distribution in the bottom sediments along the Uzynbulak creek at the Semipalatinsk test site. J. Radioanal. Nucl. Chem. 2023, 333, 2547–2556. [Google Scholar] [CrossRef]
- Mukhamediyarov, N.; Makarychev, S.; Kolbin, V.; Dyuisembaeva, M.; Shakenov, Y.; Yesilkanov, G.; Temirzhanova, A.; Tashekova, A.; Umarov, M. Study of spatial distribution of elements in the system “water—Bottom sediments” of Uzynbulak creek of Semipalatinsk test site. Izv. Saratov Univ. Earth Sci. 2022, 22, 235–242. [Google Scholar] [CrossRef]
- Aidarkhanova, A.; Lukashenko, S.; Larionova, N.; Polevik, V. Radionuclide transport in the “sediments—Water—Plants” system of the water bodies at the Semipalatinsk test site. J. Environ. Radioact. 2018, 184–185, 122–126. [Google Scholar] [CrossRef]
- Panitskiy, A.; Bazarbaeva, A.; Baigazy, S.; Polivkina, Y.; Alexandrovich, I.; Abisheva, M. Bioaccumulation of radionuclides in hoofed animals inhabiting the Semipalatinsk Test Site. PLoS ONE 2023, 18, e0294632. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.; Kim, Y.; Bae, K.; Harbottle, D.; Lee, J.W. Facile one-pot synthesis of dual-cation incorporated titanosilicate and its deposition to membrane surfaces for simultaneous removal of Cs+ and Sr2+. Appl. Surf. Sci. 2019, 493, 165–176. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.K.; Kim, J.H.; Yim, M.S.; Harbottle, D.; Lee, J.W. Synthesis of functionalized porous montmorillonite via solid-state NaOH treatment for efficient removal of cesium and strontium ions. Appl. Surf. Sci. 2018, 450, 404–412. [Google Scholar] [CrossRef]
- Abidli, A.; Rejeb, Z.; Zaoui, A.; Naguib, N.; Park, C. Comprehensive insights into the application of graphene-based aerogels for metals removal from aqueous media: Surface chemistry, mechanisms, and key features. Adv. Colloid Interface Sci. 2025, 335, 103338. [Google Scholar] [CrossRef]
- Badran, A.M.; Utra, U.; Yussof, N.S.; Bashir, M.J.K. Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal. Separations 2023, 10, 565. [Google Scholar] [CrossRef]
- Yu, K.J.; Yang, L.N.; Zhang, S.Y.; Zhang, N. Nanocellulose-based aerogels for the adsorption and removal of heavy-metal ions from wastewater: A review. Mater. Today Commun. 2025, 43, 111744. [Google Scholar] [CrossRef]
- Gupta, A.D.; Kirti, N.; Katiyar, P.; Singh, H. A critical review on three-dimensional cellulose-based aerogels: Synthesis, physico-chemical characterizations and applications as adsorbents for heavy metals removal from water. Cellulose 2023, 30, 3397–3427. [Google Scholar] [CrossRef]
- Paul, J.; Ahankari, S.S. Nanocellulose-based aerogels for water purification: A review. Carbohydr. Polym. 2023, 309, 120677. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]
- Hugar, A.; Hanagadakar, M.; Hiremath, J.; Merava, K.; Rayawgol, S. Sorption of divalent metal ions by modified carbopol-based adsorbent nanocomposite hydrogel. Desalination Water Treat. 2024, 320, 100590. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Liu, L.; Gao, Y.; Zheng, L.; Liu, M. Modified kaolin hydrogel for Cu2+ adsorption. e-Polymers 2022, 22, 986–996. [Google Scholar] [CrossRef]
- Shan, S.; Sun, X.; Xie, Y.; Li, W.; Ji, T. High-Performance Hydrogel Adsorbent Based on Cellulose, Hemicellulose, and Lignin for Copper(II) Ion Removal. Polymers 2021, 13, 3063. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Xie, W.; Chen, Q.; Jiang, Y.; Wu, X.; Yang, L. Realizing efficient solar evaporation and rapid lithium sorption by the halloysite nanotubes-based hydrogel. J. Clean. Prod. 2024, 437, 140523. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Zhang, X.; Guo, H.; Cao, X.; Lou, Z.; Zhang, W.; Wang, C.A. Novel lignin hydrogel supported Zn(VI) for efficient removal of Cr(VI). Chemosphere 2022, 301, 134781. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Tan, Z.; Zeng, J.; Zhang, D.; Dong, H.; He, Y. Facile separation of Th(IV) from aqueous solution by graphene hydrogel. J. Radioanal. Nucl. Chem. 2020, 326, 379–386. [Google Scholar] [CrossRef]
- Luo, Q.; Yuan, H.; Zhang, M.; Jiang, P.; Liu, M.; Xu, D.; Guo, X.; Wu, Y. A 3D porous fluorescent hydrogel based on amino-modified carbon dots with excellent sorption and sensing abilities for environmentally hazardous Cr(VI). J. Hazard. Mater. 2021, 401, 123432. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, I.; Privar, Y.; Parotkina, Y.; Mironenko, A.; Eliseikina, M.; Balatskiy, D.; Golikov, A.; Bratskaya, S. Rational Design of Polyamine-Based Cryogels for Metal Ion Sorption. Molecules 2020, 25, 4801. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Dinu, I.; Dinu, M. Synthesis of Ethylenediaminetetraacetic Acid-Functionalized Chitosan Cryogels as Potential Sorbents of Heavy Metal Ions. Mater. Plast. 2021, 58, 155–166. [Google Scholar] [CrossRef]
- Chen, J.; Liao, C.; Guo, X.; Hou, S.; He, W. PAAO cryogels from amidoximated P(acrylic acid-co-acrylonitrile) for the adsorption of lead ion. Eur. Polym. J. 2022, 171, 111192. [Google Scholar] [CrossRef]
- Yan, J.; Yang, H.; da Silva, J.; Rojas, O. Loading of Iron (II, III) Oxide Nanoparticles in Cryogels Based on Microfibrillar Cellulose for Heavy Metal Ion Separation. Adv. Polym. Technol. 2020, 2020, 9261378. [Google Scholar] [CrossRef]
- Humelnicu, D.; Dragan, E.; Ignat, M.; Dinu, M.A. Comparative Study on Cu2+, Zn2+, Ni2+, Fe3+, and Cr3+ Metal Ions Removal from Industrial Wastewaters by Chitosan-Based Composite Cryogels. Molecules 2020, 25, 2664. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) ions from wastewater using polyethyleneimine (PEI) cryogels. J. Environ. Manag. 2022, 329, 117002. [Google Scholar] [CrossRef]
- Mecca, T.; Ussia, M.; Caretti, D.; Cunsolo, F.; Dattilo, S.; Scurti, S.; Privitera, V.; Carroccio, S. N-methyl-D-glucamine based cryogels as reusable sponges to enhance heavy metals removal from water. Chem. Eng. J. 2020, 399, 125753. [Google Scholar] [CrossRef]
- Privar, Y.; Malakhova, I.; Pestov, A.; Fedorets, A.; Azarova, Y.; Schwarz, S.; Bratskaya, S. Polyethyleneimine cryogels for metal ions sorption. Chem. Eng. J. 2018, 334, 1392–1398. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.; Azat, S.; Nurgozhin, T.; Inglezakis, V. Removal of Cd2+ from Water by Use of Super-Macroporous Cryogels and Comparison to Commercial Adsorbents. Polymers 2020, 12, 2405. [Google Scholar] [CrossRef]
- Jin, H.; Xu, X.; Yu, X.; Yu, S.; Wang, S.; Qu, X. Bimetallic Organic Gel for Effective Methyl Orange Dye Adsorption. Gels 2024, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Z.; Chen, R.; Hou, Y.; Lu, R.; Li, S.; Ren, S.; Gao, Z. A multifunctional polyacrylamide/chitosan hydrogel for dyes adsorption and metal ions detection in water. Int. J. Biol. Macromol. 2023, 246, 125613. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, A.; Kakanejadifard, A. Synthesis and characterization of grapheme-chitosan hydrogel as adsorbent for methyl orange. Polym. Bull. 2024, 81, 8607–8625. [Google Scholar] [CrossRef]
- Jabeen, S.; Alam, S.; Shah, L.A.; Zahoor, M.; Umar, M.N.; Ullah, R. Novel hydrogel poly (GG-co-acrylic acid) for the sorptive removal of the color Rhodamine-B from contaminated water. Heliyon 2023, 9, 19780. [Google Scholar] [CrossRef]
- Chaurasiya, A.; Pande, P.P.; Shankar, R.; Dey, K.K.; Singh, D.; Kashaudhan, K.; Kumar, P. Allyl sorbitol crosslinker-based novel hydrogel for the removal of toxic metal ions from water. Int. J. Environ. Anal. Chem. 2025, 1–26. [Google Scholar] [CrossRef]
- Alraddadi, H.M.; Fagieh, T.M.; Bakhsh, E.M.; Akhtar, K.; Khan, S.B.; Khan, S.A.; Bahaidarah, E.A.; Homdi, T.A. Adsorptive removal of heavy metals and organic dyes by sodium alginate/ coffee waste composite hydrogel. Int. J. Biol. Macromol. 2023, 247, 125708. [Google Scholar] [CrossRef]
- Kamal, K.H.; Hassan, M.A.; Kamel, S.; El-Sayed, N.S. Efficient removal of Cd2+ ions and methylene blue from aqueous solutions by polyanionic sodium alginate-derived hydrogel. Surf. Interfaces 2024, 51, 104596. [Google Scholar] [CrossRef]
- Taye, A.; Yifru, A.; Getachew, N.; Mehretie, S.; Admassie, S. Adsorption of hexavalent chromium using Water Hyacinth Leaf Protein Concentrate/Graphene Oxide hydrogel. Environ. Monit. Assess. 2023, 195, 1342. [Google Scholar] [CrossRef]
- Wang, B.; Feng, S.; Wang, C.; Liu, X.; Chen, L.; Yan, D. Nanostructure-Based Oil–Water Separation: Mechanism and Status. Separations 2023, 10, 569. [Google Scholar] [CrossRef]
- Wang, H.; Xue, J.Y.; Ju, Y.Y.; Gao, Y.J.; Cheng, X.W.; Guan, J.P. Multifunctional nanofiber aerogels for environmental applications: Oil-water separation, thermal insulation, fire-proofing. J. Environ. Chem. Eng. 2025, 13, 115541. [Google Scholar] [CrossRef]
- Cao, M.; Peng, Q.; Wang, Y.; Luo, G.S.; Feng, L.J.; Zhao, S.L.; Yuan, Y.H.; Wang, N. High-efficiency uranium extraction from seawater by low-cost natural protein hydrogel. Int. J. Biol. Macromol. 2023, 242, 124792. [Google Scholar] [CrossRef]
- Gao, F.X.; Bai, J.W.; Yan, H.J.; Zheng, S.Y.; Su, S.Z.; Li, J.Q.; Zhang, C.H.; Wang, J. Gelatin-polyacrylamide double network enhanced polyamidoxime magnetic photothermal composite hydrogel for highly efficient and selective uranium extraction from seawater. Chem. Eng. J. 2024, 485, 149822. [Google Scholar] [CrossRef]
- Gong, T.; Liang, H.Y.; Li, X.; Li, G.Q.; Chen, F.Y.; Wang, H.Q. High-adsorption-capacity and recyclable double-network hydrogel nanofibers for dynamic uranium sorption. Sep. Purif. Technol. 2024, 342, 126963. [Google Scholar] [CrossRef]
- Panitskiy, A.; Bazarbaeva, A.; Baigazy, S.; Alexandrovich, I.; Larionova, N. Radioecological characteristics of Siberian roe deer (Capreolus pygargus Pal., 1771) inhabiting locations of nuclear weapon tests. PLoS ONE 2024, 19, e0308518. [Google Scholar] [CrossRef]
- Aidarkhanova, A.; Larionova, N.; Tleukanova, Z.; Mamyrbaeva, A.; Ermakova, R.; Svetacheva, Y.; Aktayev, M.; Panitskiy, A. The character of radionuclide contamination of natural lakes at the territory of the Semipalatinsk test site. J. Environ. Radioact. 2022, 255, 107041. [Google Scholar] [CrossRef]
- Aidarkhanova, A.; Timonova, L.; Aidarkhanov, A.; Monayenko, V.; Iskenov, A.; Subbotin, S.; Pronin, S.; Belykh, N. The character of Lake Kishkensor contamination in the area of underground nuclear explosions at the Semipalatinsk Test Site territory. Environ. Monit. Assess. 2024, 196, 1068. [Google Scholar] [CrossRef]
- Aktayev, M.; Subbotin, S.; Aidarkhanov, A.; Aidarkhanova, A.; Timonova, L.; Larionova, N. Characterization of geological and lithological features in the area proximal to tritium-contaminated groundwater at the Semipalatinsk test site. PLoS ONE 2024, 19, 0300971. [Google Scholar] [CrossRef]
- Gorlachev, I.; Kharkin, P.; Dyussembayeva, M.; Lukashenko, S.; Gluchshenko, G.; Matiyenko, L.; Zheltov, D.; Kitamura, A.; Khlebnikov, N. Comparative analysis of water contamination of the Shagan river at the Semipalatinsk test site with heavy metals and artificial radionuclides. J. Environ. Radioact. 2020, 213, 106110. [Google Scholar] [CrossRef]
- Yelemessova, G.; Gussenov, I.; Ayazbayeva, A.; Shakhvorostov, A.; Orazzhanova, L.; Klivenko, A.; Kudaibergenov, S. Preparation and Characterization of Preformed Polyelectrolyte and Polyampholyte Gel Particles for Plugging of High-Permeability Porous Media. Gels 2024, 10, 562. [Google Scholar] [CrossRef]
- Kutergin, A.; Nedobukh, T.; Nikiforov, A.; Zenkova, K.; Tarasovskikh, T. Sorption extraction of strontium radionuclides from surface waters with natural aluminosilicate. Water Manag. Russ. Probl. Technol. Manag. 2021, 4, 118–134. (In Russian) [Google Scholar] [CrossRef]
- Voronina, A.; Noskova, A.; Semenishchev, V.; Blinova, M.; Nikiforov, A. Purification of seawater from cesium and strontium radionuclides. Water Manag. Russ. 2019, 6, 102–120. (In Russian) [Google Scholar]
- Gussenov, I.; Shakhvorostov, A.; Ayazbayeva, A.; Gizatullina, N.; Klivenko, A.; Kudaibergenov, S. Preparation and Characterization of a Preformed Polyampholyte Particle Gel Composite for Conformance Control in Oil Recovery. Polymers 2023, 15, 4095. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.; Tatykhanova, G.; Klivenko, A. Complexation of macroporous amphoteric cryogels based on N,N-dimethylaminoethyl methacrylate and methacrylic acid with dyes, surfactant, and protein. J. Appl. Polym. Sci. 2016, 133, 43784. [Google Scholar] [CrossRef]

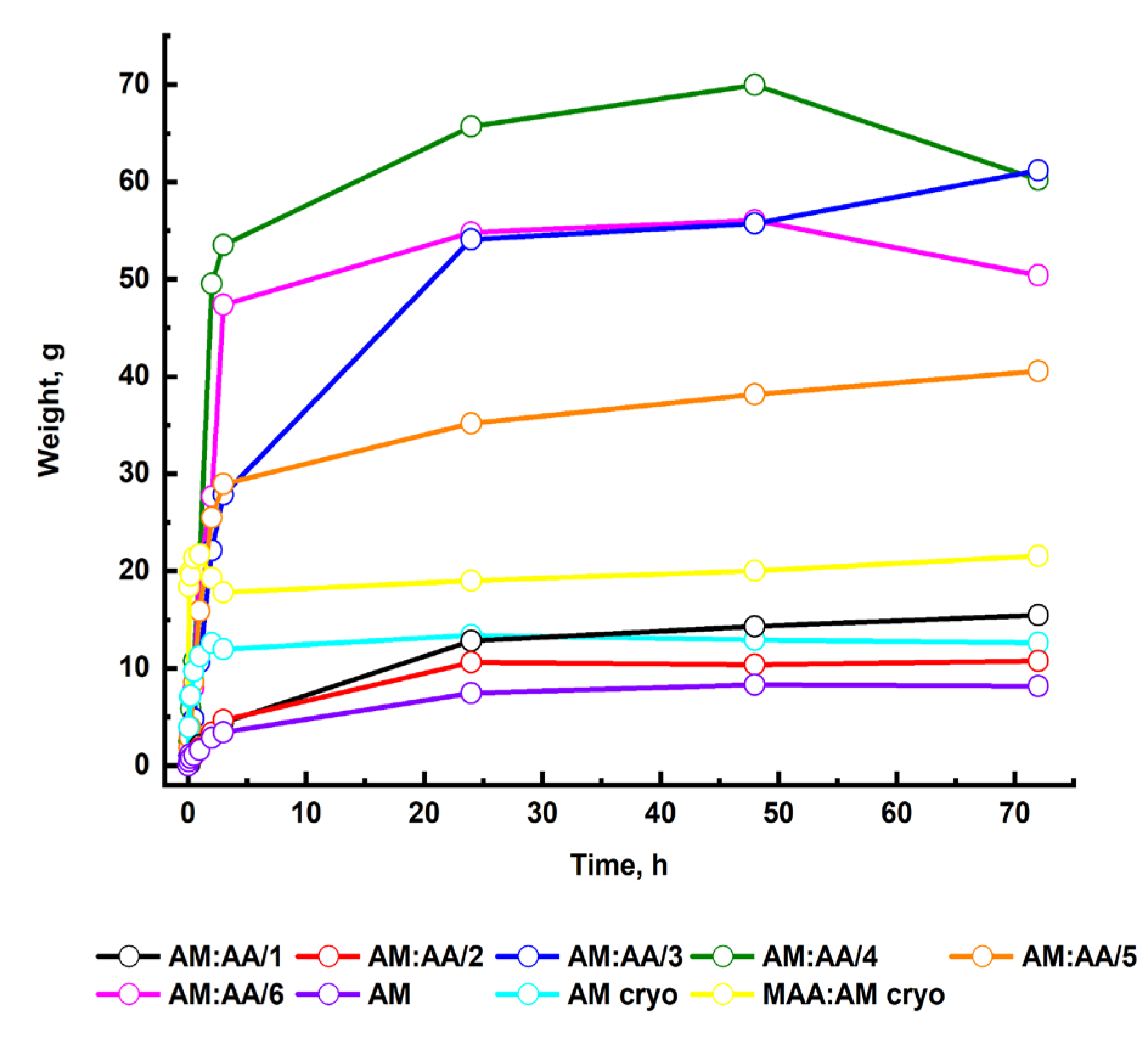
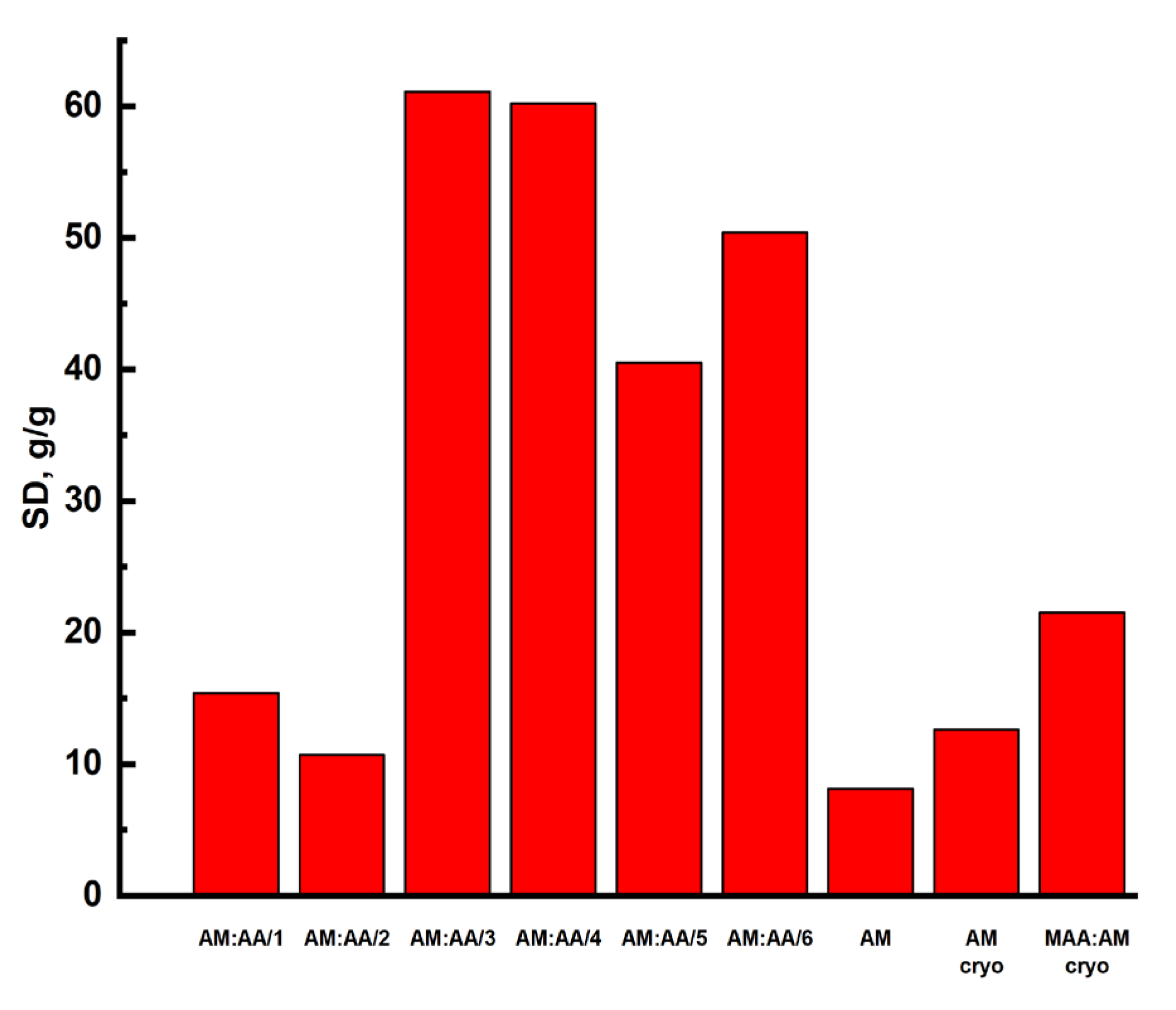

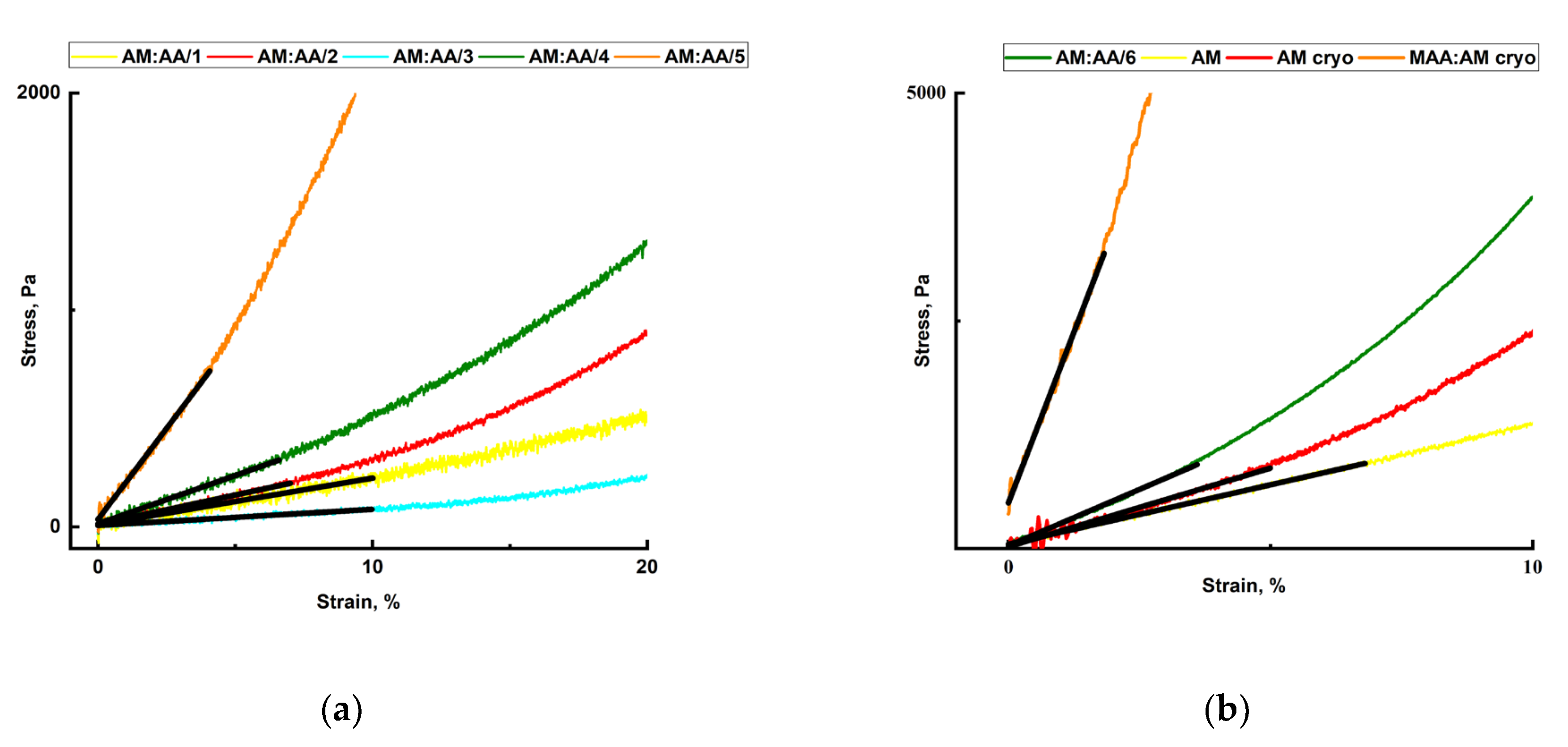



| Sample | Se | k1 | k2 | R2 | Primary Process |
|---|---|---|---|---|---|
| AM:AA/1 | 15.28 | 9.19 | 0.012 | 0.99 | Relaxation |
| AM:AA/2 | 10.62 | 0.002 | 0.003 | 0.99 | Relaxation and diffusion |
| AM:AA/3 | 5.7 | 0.004 | −0.004 | 0.99 | Relaxation and diffusion |
| AM:AA/4 | 6.55 | 0.011 | −0.024 | 0.98 | Relaxation and diffusion |
| AM:AA /5 | 3.78 | 0.009 | −0.003 | 0.99 | Relaxation and diffusion |
| AM:AA /6 | 5.41 | 0.009 | −0.018 | 0.98 | Relaxation and diffusion |
| AM | 8.19 | 0.001 | 0.021 | 0.99 | Relaxation and diffusion |
| Sample Concentration | AA:AM/1 | AA:AM/2 | AA:AM/3 | AA:AM/4 | AA:AM/5 | AA:AM/6 | AM | AM Cryo | MAA:AM Cryo |
|---|---|---|---|---|---|---|---|---|---|
| Aqueous solution activity, Bq/L | 260 | 165 | 250 | 260 | 115 | 120 | 165 | 170 | 170 |
| Polymer gel activity after adsorption, Bq/g | 7.6 | 13.3 | 77.3 | 8.5 | 12 | 3.8 | 2.6 | 2.9 | 6.4 |
| Concentration of 137Cs in aqueous solution, mg/L | 8.1 × 10−8 | 5.1 × 10−8 | 7.8 × 10−8 | 8.1 × 10−8 | 3.6 × 10−8 | 3.7 × 10−8 | 5.1 × 10−8 | 5.2 × 10−8 | 5.3 × 10−8 |
| Concentration of 137Cs in polymer gel after adsorption, mg/g | 2.3 × 10−9 | 4.1 × 10−9 | 2.4 × 10−8 | 2.6 × 10−9 | 3.7 × 10−9 | 1.1 × 10−9 | 8.1 × 10−10 | 9.0 × 10−10 | 1.9 × 10−9 |
| Sample Concentration | AA:AM/1 | AA:AM/2 | AA:AM/3 | AA:AM/4 | AA:AM/5 | AA:AM/6 | AM | AM Cryo | MAA:AM Cryo |
|---|---|---|---|---|---|---|---|---|---|
| Aqueous solution activity, Bq/L | 470 | 545 | 448 | 470 | 250 | 277 | 545 | 585 | 585 |
| Polymer gel activity after adsorption, Bq/g | 24 | 260 | 63 | 50 | 80 | 60 | 10 | 35 | 360 |
| Concentration of 90Sr in aqueous solution, mg/L | 9.2 × 10−8 | 1.0 × 10−7 | 8.8 × 10−8 | 9.2 × 10−8 | 4.9 × 10−8 | 5.4 × 10−8 | 1.0 × 10−7 | 1.1 × 10−7 | 1.1 × 10−7 |
| Concentration of 90Sr in polymer gel after adsorption, mg/g | 4.7 × 10−9 | 5.1 × 10−8 | 1.2 × 10−8 | 9.8 × 10−9 | 1.5 × 10−8 | 1.1 × 10−8 | 1.9 × 10−9 | 6.9 × 10−9 | 7.0 × 10−8 |
| Gels | Element Concentration in the Adsorbent, mg/g | |||
|---|---|---|---|---|
| K | Fe | Ni | U | |
| AM:AA/1 | 2.5 × 10−3 | 0.04 × 10−3 | 0.04 × 10−3 | 0.2 × 10−3 |
| AM:AA/2 | 1.1 × 10−3 | 0.08 × 10−3 | 0.01 × 10−3 | 0.2 × 10−3 |
| AM:AA/3 | 71.4 × 10−3 | 1.3 × 10−3 | 0.7 × 10−3 | 6.2 × 10−3 |
| AM:AA/4 | 0.6 × 10−3 | 0.02 × 10−3 | 0.02 × 10−3 | 0.1 × 10−3 |
| AM:AA/5 | 0.8 × 10−3 | 0.03 × 10−3 | 0.01 × 10−3 | 0.2 × 10−3 |
| AM:AA/6 | 0.001 × 10−3 | 0.05 × 10−3 | 0.03 × 10−3 | 0.001 × 10−3 |
| AM | 0.6 × 10−3 | 0.04 × 10−3 | 0.01 × 10−3 | 0.001 × 10−3 |
| AM cryo | 1.2 × 10−3 | 0.08 × 10−3 | 0.01 × 10−3 | 0.02 × 10−3 |
| MAA-AM cryo | 2 × 10−3 | 0.1 × 10−3 | 0.02 × 10−3 | 0.001 × 10−3 |
| Polymer Gel | Monomer, g | |||
|---|---|---|---|---|
| AM | AA | MAA | MBAA | |
| AM:AA/1 | 0.6 | 1.2 | - | 0.075 |
| AM:AA/2 | 1.1 | 2 | - | 0.1 |
| AM:AA/3 | 0.6 | 0.6 | - | 0.075 |
| AM:AA/4 | 1 | 1 | - | 0.1 |
| AM:AA/5 | 1 | 1 | - | 0.2 |
| AM:AA/6 | 1.8 | 0.2 | - | 0.1 |
| AM | 2 | - | - | 0.1 |
| AM cryo | 1.2 | - | - | 0.075 |
| MAA-AM cryo | 1.8 | - | 0.2 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artamonov, Y.; Krivitskiy, P.; Zhamaldinov, F.; Aseyev, V.; Klivenko, A. The Evaluation of the Effectiveness of Adsorbents Based on Acrylamide Hydrogels and Cryogels for Water Purification from Radioactive Contaminants. Gels 2025, 11, 311. https://doi.org/10.3390/gels11050311
Artamonov Y, Krivitskiy P, Zhamaldinov F, Aseyev V, Klivenko A. The Evaluation of the Effectiveness of Adsorbents Based on Acrylamide Hydrogels and Cryogels for Water Purification from Radioactive Contaminants. Gels. 2025; 11(5):311. https://doi.org/10.3390/gels11050311
Chicago/Turabian StyleArtamonov, Yuriy, Pavel Krivitskiy, Fail Zhamaldinov, Vladimir Aseyev, and Alexey Klivenko. 2025. "The Evaluation of the Effectiveness of Adsorbents Based on Acrylamide Hydrogels and Cryogels for Water Purification from Radioactive Contaminants" Gels 11, no. 5: 311. https://doi.org/10.3390/gels11050311
APA StyleArtamonov, Y., Krivitskiy, P., Zhamaldinov, F., Aseyev, V., & Klivenko, A. (2025). The Evaluation of the Effectiveness of Adsorbents Based on Acrylamide Hydrogels and Cryogels for Water Purification from Radioactive Contaminants. Gels, 11(5), 311. https://doi.org/10.3390/gels11050311





