Evaluation of Carboxymethyl Chitosan–Genipin Hydrogels as Reservoir Systems for Suramin Delivery in Epithelial Tissues
Abstract
1. Introduction
2. Results and Discussion
2.1. Scanning Electron Microscopy (SEM)
2.2. Swelling Ratio
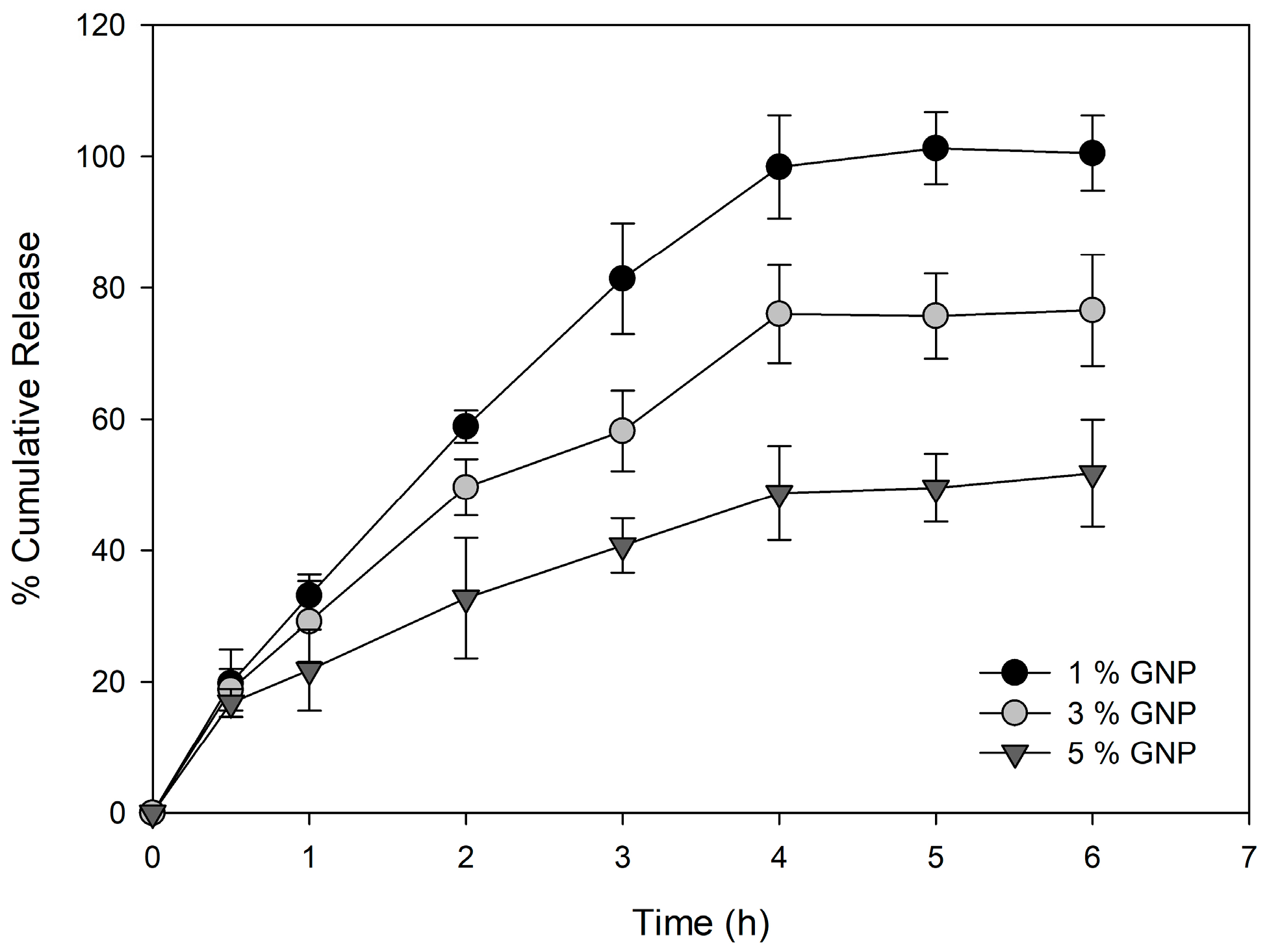
2.3. In Vitro Drug Release
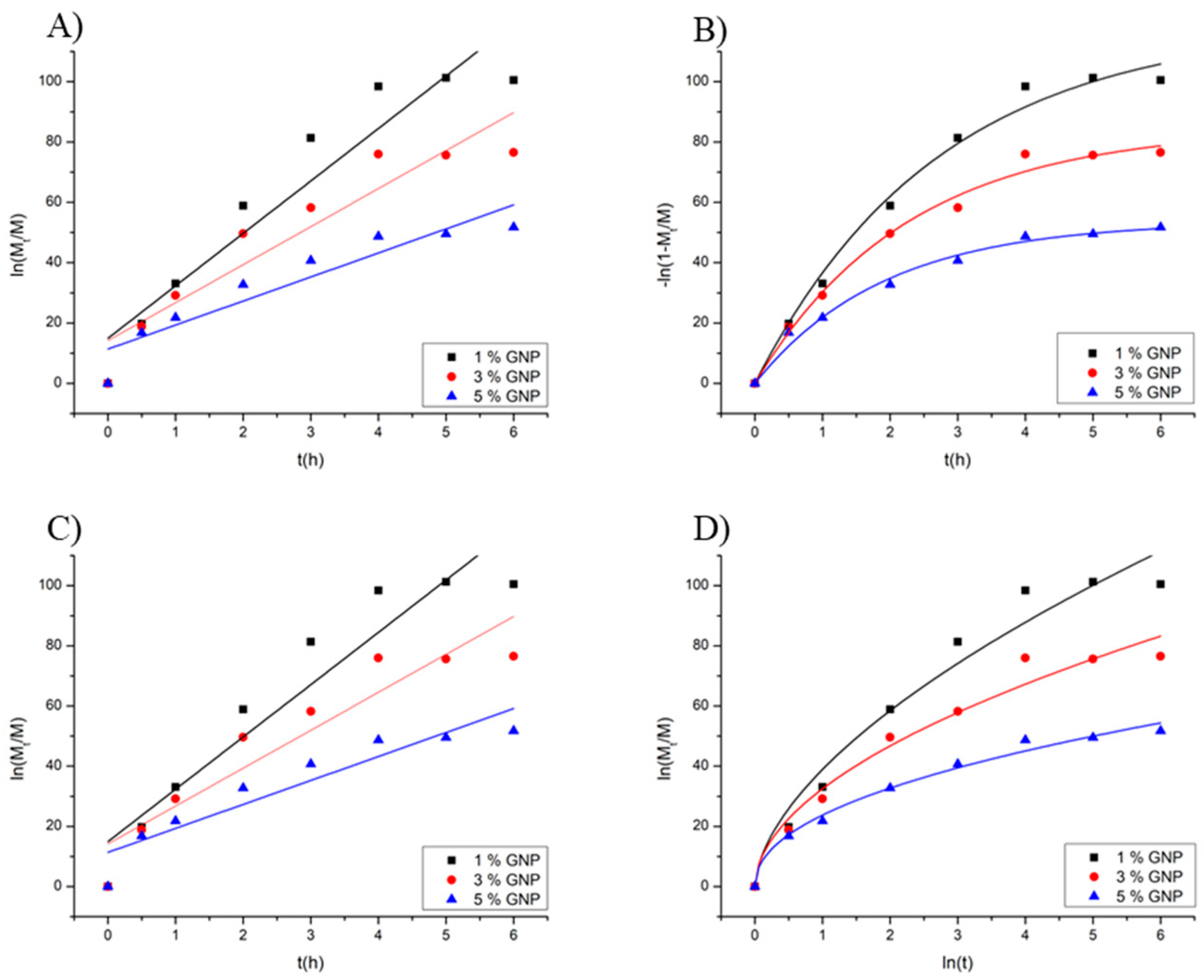
2.4. Drug Release Kinetics
2.5. In Vitro Cell Viability of 2D Cell Culture of a Human Skin Cell Line and Human Primary Skin Cells
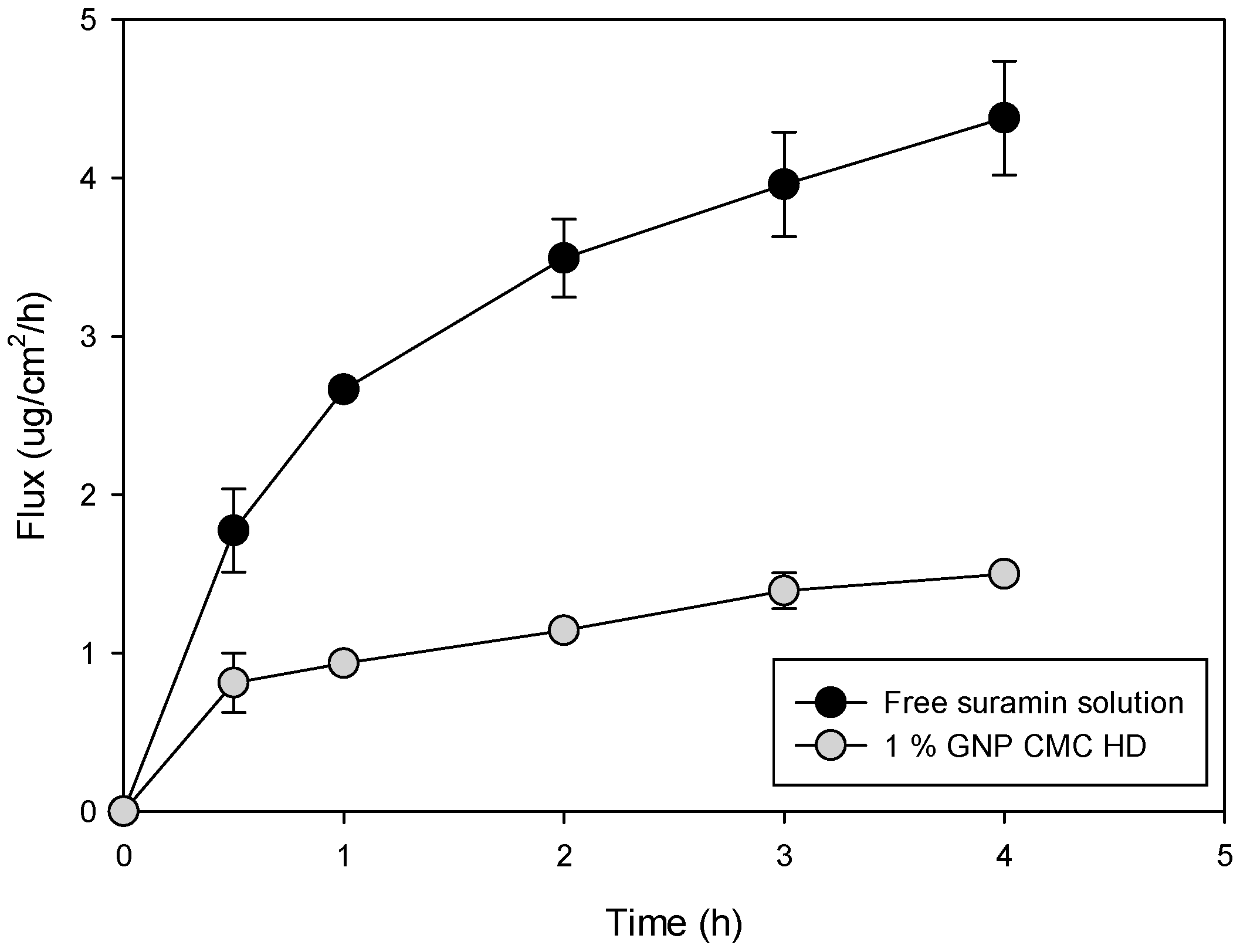
2.6. In Vitro Permeation of Suramin Using Strat-M® Synthetic Biomimetic Membrane and Franz Cell Diffusion System
2.7. In Vitro Permeation of Suramin Using 3D Normal Human-Derived Epidermal Keratinocytes (EpiDerm™)
3. Conclusions
4. Materials and Methods
4.1. Production of CMC HD
4.2. Scanning Electron Microscopy (SEM)
4.3. CMC HD Swelling Ratio
4.4. In Vitro Suramin Release
4.5. Kinetic Analysis of Suramin Release
4.6. High-Performance Liquid Chromatography (HPLC) Analysis
4.7. In Vitro Cell Viability of Human Primary Skin Cells and a Human Skin Cell Line Cultured in 2D
4.8. In Vitro Permeation of Suramin Using Strat-M® Synthetic Biomimetic Membrane and Franz Cell Diffusion System
4.9. Utilizing 3D Normal Human-Derived Epidermal Keratinocytes (EpiDerm™) for Suramin Permeation In Vitro
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMC | Carboxymethyl chitosan |
| SR | Swelling ratio |
| HDF | Human dermal fibroblasts |
| SEM | Scanning electron microscopy |
| Wd | Weighed |
| Ws | Immediately weighed |
References
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why venous leg ulcers have difficulty healing: Overview on pathophysiology, clinical consequences, and treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Samiraninezhad, N.; Asadi, K.; Rezazadeh, H.; Gholami, A. Using chitosan, hyaluronic acid, alginate, and gelatin-based smart biological hydrogels for drug delivery in oral mucosal lesions: A review. Int. J. Biol. Macromol. 2023, 252, 126573. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.P.; Pillay, V. Bioplatform fabrication approaches affecting chitosan-based interpolymer complex properties and performance as wound dressings. Molecules 2020, 25, 222. [Google Scholar] [CrossRef] [PubMed]
- Fiamingo, A.; Campana-Filho, S.P. Structure, morphology and properties of genipin-crosslinked carboxymethylchitosan porous membranes. Carbohydr. Polym. 2016, 143, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, W.; Sun, W.; Li, X.; Duan, J.; Cui, J.; Feng, Z.; Mansour, H.M. Influence of the carboxymethyl chitosan anti-adhesion solution on the TGF-β1 in a postoperative peritoneal adhesion rat. J. Mater. Sci. Mater. Med. 2013, 24, 2549–2559. [Google Scholar] [CrossRef]
- de Lacerda Bukzem, A.; Dos Santos, D.M.; Leite, I.S.; Inada, N.M.; Campana-Filho, S.P. Tuning the properties of carboxymethylchitosan-based porous membranes for potential application as wound dressing. Int. J. Biol. Macromol. 2021, 166, 459–470. [Google Scholar] [CrossRef]
- Zhuang, S.; Schnellmann, R.G. Suramin promotes proliferation and scattering of renal epithelial cells. J. Pharmacol. Exp. Ther. 2005, 314, 383–390. [Google Scholar] [CrossRef]
- Zhuang, S.; Lu, B.; Daubert, R.A.; Chavin, K.D.; Wang, L.; Schnellmann, R.G. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int. 2009, 75, 304–311. [Google Scholar] [CrossRef]
- Korrapati, M.C.; Shaner, B.E.; Schnellmann, R.G. Recovery from glycerol-induced acute kidney injury is accelerated by suramin. J. Pharmacol. Exp. Ther. 2012, 341, 126–136. [Google Scholar] [CrossRef]
- Korrapati, M.C.; Shaner, B.E.; Neely, B.A.; Alge, J.L.; Arthur, J.M.; Schnellmann, R.G. Diabetes-induced renal injury in rats is attenuated by suramin. J. Pharmacol. Exp. Ther. 2012, 343, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Dupre, T.V.; Doll, M.A.; Shah, P.P.; Sharp, C.N.; Kiefer, A.; Scherzer, M.T.; Saurabh, K.; Saforo, D.; Siow, D.; Casson, L.; et al. Suramin protects from cisplatin-induced acute kidney injury. Am. J. Physiol.-Ren. Physiol. 2016, 310, F248–F258. [Google Scholar] [CrossRef]
- Korrapati, M.C.; Howell, L.H.; Shaner, B.E.; Megyesi, J.K.; Siskind, L.J.; Schnellmann, R.G. Suramin: A potential therapy for diabetic nephropathy. PLoS ONE 2013, 8, e73655. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Rehman, H.; Shi, Y.; Krishnasamy, Y.; Lemasters, J.J.; Schnellmann, R.G.; Zhong, Z. Suramin decreases injury and improves regeneration of ethanol-induced steatotic partial liver grafts. J. Pharmacol. Exp. Ther. 2013, 344, 417–425. [Google Scholar] [CrossRef]
- Rieck, P.; Denis, J.; Peters, D.; Hartmann, C.; Pouliquen, Y.; Courtois, Y. Fibroblast growth factor 2, heparin and suramin reduce epithelial ulcer development in experimental HSV-1 keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 733–740. [Google Scholar] [CrossRef]
- Mietz, H.; Chévez-Barrios, P.; Feldman, R.M.; Lieberman, M.W. Suramin inhibits wound healing following filtering procedures for glaucoma. Br. J. Ophthalmol. 1998, 82, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Shyu, S.S.; Peng, C.K. Characterization of ring-opening polymerization of genipin and pH-dependent cross-linking reactions between chitosan and genipin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Lan, Y.-Q.; Guo, H.; Cheng, L.-Z.; Fan, J.-Z.; Cai, X.; Zhang, L.-M.; Chen, R.-F.; Zhou, H.-S. Ophthalmic drug-loaded N, O-carboxymethyl chitosan hydrogels: Synthesis, in vitro and in vivo evaluation. Acta Pharmacol. Sin. 2010, 31, 1625–1634. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhao, J.; Guo, F.; Wang, Y. Enhanced gelling properties and hydration capacity of ginkgo seed proteins by genipin cross-linking. Food Chem. 2023, 399, 133924. [Google Scholar] [CrossRef]
- Del Olmo, J.A.; Pérez-Álvarez, L.; Sáez-Martínez, V.; Benito-Cid, S.; Ruiz-Rubio, L.; Pérez-González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Wound healing and antibacterial chitosan-genipin hydrogels with controlled drug delivery for synergistic anti-inflammatory activity. Int. J. Biol. Macromol. 2022, 203, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and Characterization of Chemically Cross-Linked Acrylic Acid/Gelatin Hydrogels: Effect of pH and Composition on Swelling and Drug Release. Int. J. Polym. Sci. 2015, 2015, 187961. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Shive, M.; Bichara, A.; Berrada, M.; Le Garrec, D.; Chenite, A.; Leroux, J.-C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef]
- Craciun, A.M.; Tartau, L.M.; Pinteala, M.; Marin, L. Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy. J. Colloid Interface Sci. 2019, 536, 196–207. [Google Scholar] [CrossRef]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Patel, R.R.; Patel, J.K. Chitosan mediated targeted drug delivery system: A review. J. Pharm. Pharm. Sci. 2010, 13, 536–557. [Google Scholar] [CrossRef]
- Chen, J.; Luo, J.; Feng, J.; Wang, Y.; Lv, H.; Zhou, Y. Spatiotemporal controlled released hydrogels for multi-system regulated bone regeneration. J. Control. Release 2024, 372, 846–861. [Google Scholar] [CrossRef]
- Korsmeyer, R.W. Diffusion controlled systems: Hydrogels. In Polymers for Controlled Drug Delivery; CRC Press: Boca Raton, FL, USA, 2023; pp. 15–37. [Google Scholar]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, C. Chitosan superporous hydrogel composite-based floating drug delivery system: A newer formulation approach. J. Pharm. Bioallied Sci. 2010, 2, 124–131. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Emani, S.; Vangala, A.; Buonocore, F.; Yarandi, N.; Calabrese, G. Chitosan hydrogels cross-linked with trimesic acid for the delivery of 5-fluorouracil in cancer therapy. Pharmaceutics 2023, 15, 1084. [Google Scholar] [CrossRef]
- Kildeeva, N.; Chalykh, A.; Belokon, M.; Petrova, T.; Matveev, V.; Svidchenko, E.; Surin, N.; Sazhnev, N. Influence of genipin crosslinking on the properties of chitosan-based films. Polymers 2020, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Lecumberri, E.; Heras, A. Chitosan-genipin microspheres for the controlled release of drugs: Clarithromycin, tramadol and heparin. Mar. Drugs 2010, 8, 1750–1762. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.-U. Insight into hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.Y.; Regdon, G.; Hamedelniel, E.I.; Sovány, T. Review of recently used techniques and materials to improve the efficiency of orally administered proteins/peptides. DARU J. Pharm. Sci. 2020, 28, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef]
- Sander, C.; Nielsen, H.M.; Jacobsen, J. Buccal delivery of metformin: TR146 cell culture model evaluating the use of bioadhesive chitosan discs for drug permeability enhancement. Int. J. Pharm. 2013, 458, 254–261. [Google Scholar] [CrossRef]
- Kichou, H.; Bonnier, F.; Dancik, Y.; Bakar, J.; Michael-Jubeli, R.; Caritá, A.C.; Perse, X.; Soucé, M.; Rapetti, L.; Tfayli, A. Strat-M® positioning for skin permeation studies: A comparative study including EpiSkin® RHE, and human skin. Int. J. Pharm. 2023, 647, 123488. [Google Scholar] [CrossRef]
- Mo, Y.-H.; Wang, H.; Jin, S.-H.; Peng, K.-L.; Yang, Z.-M.; Li, P.-W.; Chen, Y. Preparation and properties of a fast curing carboxymethyl chitosan hydrogel for skin care. Polym. Test. 2022, 113, 107667. [Google Scholar] [CrossRef]
- Shinoda, W. Permeability across lipid membranes. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2254–2265. [Google Scholar] [CrossRef]
- Hutson, P.R.; Tutsch, K.D.; Rago, R.; Arzoomanian, R.; Alberti, D.; Pomplun, M.; Church, D.; Marnocha, R.; Cheng, A.L.; Kehrli, N. Renal clearance, tissue distribution, and CA-125 responses in a phase I trial of suramin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1998, 4, 1429–1436. [Google Scholar]
- Chen, X.; Yuk, H.; Wu, J.; Nabzdyk, C.S.; Zhao, X. Instant tough bioadhesive with triggerable benign detachment. Proc. Natl. Acad. Sci. USA 2020, 117, 15497–15503. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T. In vitro enhanced skin permeation and retention of imiquimod loaded in β-cyclodextrin nanosponge hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Development and applications of PLGA hydrogels for sustained delivery of therapeutic agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Klecker Jr, R.W.; Collins, J.M. Quantification of suramin by reverse-phase ion-pairing high-performance liquid chromatography. J. Liq. Chromatogr. 1985, 8, 1685–1696. [Google Scholar] [CrossRef]
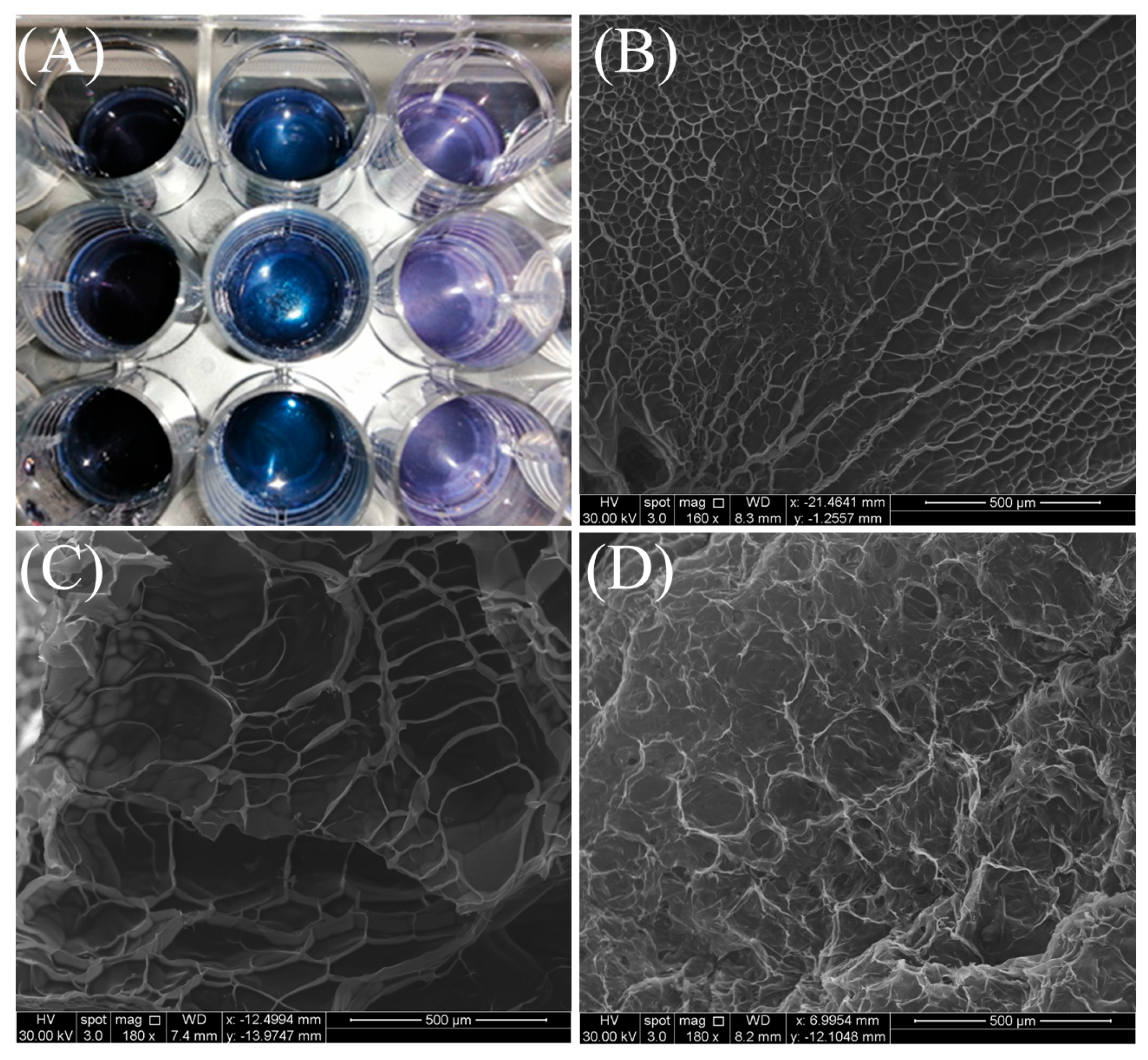


| Genipin % w/w | Swelling Ratio (%) |
|---|---|
| 1 | 205 ± 5.1 |
| 3 | 63 ± 11.6 |
| 5 | 41 ± 7.2 |
| Sample % Genipin | Higuchi k | Higuchi R2 | k0 | R2 | First-Order k1 | R2 | Peppas k | n | R2 |
|---|---|---|---|---|---|---|---|---|---|
| 1% | 43.75 | 0.93 | 15.57 | 0.89 | 0.36 | 0.98 | 38.88 | 0.58 | 0.94 |
| 3% | 33.59 | 0.95 | 10.88 | 0.89 | 0.44 | 0.98 | 32.47 | 0.52 | 0.95 |
| 5% | 22.58 | 0.97 | 6.58 | 0.92 | 0.52 | 0.97 | 23.72 | 0.46 | 0.98 |
| Sample (% Genipin) | Flux (µg/cm2/h) | Drug Retention in Membrane (µg/cm2) |
|---|---|---|
| 1% | 12.7 ± 4.3 a | 48.8 ± 6.8 a |
| 3% | 8.0 ± 2.6 b | 31.61 ± 0.1 b |
| 5% | 6.5 ± 1.3 b,c | 5.75 ± 0.4 c |
| Suramin Sol | 6.0 ± 2.6 c | 15.5 ± 3.9 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encinas-Basurto, D.; Ruiz, V.H.; Schnellmann, R.G.; Mansour, H.M. Evaluation of Carboxymethyl Chitosan–Genipin Hydrogels as Reservoir Systems for Suramin Delivery in Epithelial Tissues. Gels 2025, 11, 312. https://doi.org/10.3390/gels11050312
Encinas-Basurto D, Ruiz VH, Schnellmann RG, Mansour HM. Evaluation of Carboxymethyl Chitosan–Genipin Hydrogels as Reservoir Systems for Suramin Delivery in Epithelial Tissues. Gels. 2025; 11(5):312. https://doi.org/10.3390/gels11050312
Chicago/Turabian StyleEncinas-Basurto, David, Victor H. Ruiz, Rick G. Schnellmann, and Heidi M. Mansour. 2025. "Evaluation of Carboxymethyl Chitosan–Genipin Hydrogels as Reservoir Systems for Suramin Delivery in Epithelial Tissues" Gels 11, no. 5: 312. https://doi.org/10.3390/gels11050312
APA StyleEncinas-Basurto, D., Ruiz, V. H., Schnellmann, R. G., & Mansour, H. M. (2025). Evaluation of Carboxymethyl Chitosan–Genipin Hydrogels as Reservoir Systems for Suramin Delivery in Epithelial Tissues. Gels, 11(5), 312. https://doi.org/10.3390/gels11050312







