Effects of Inorganic Salts on Curdlan Production and Structural Properties
Abstract
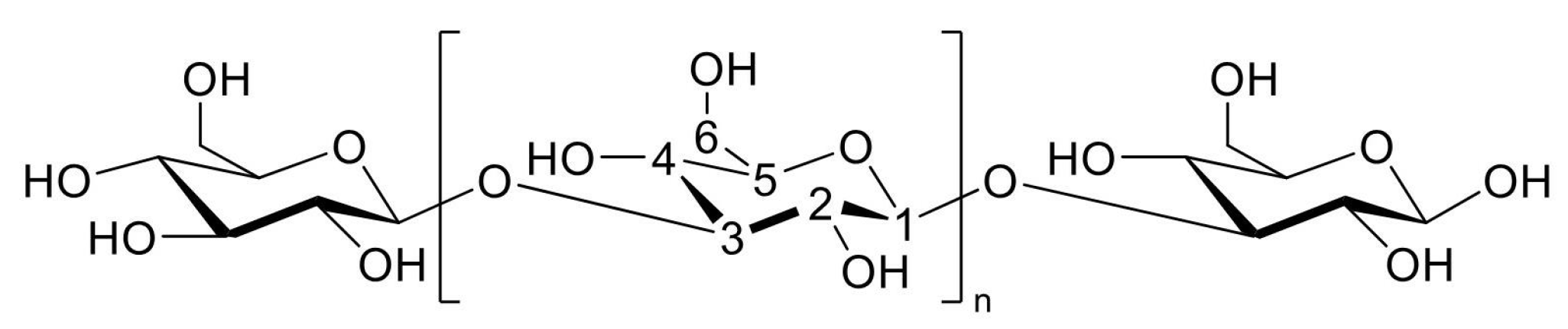
1. Introduction
2. Results and Discussion
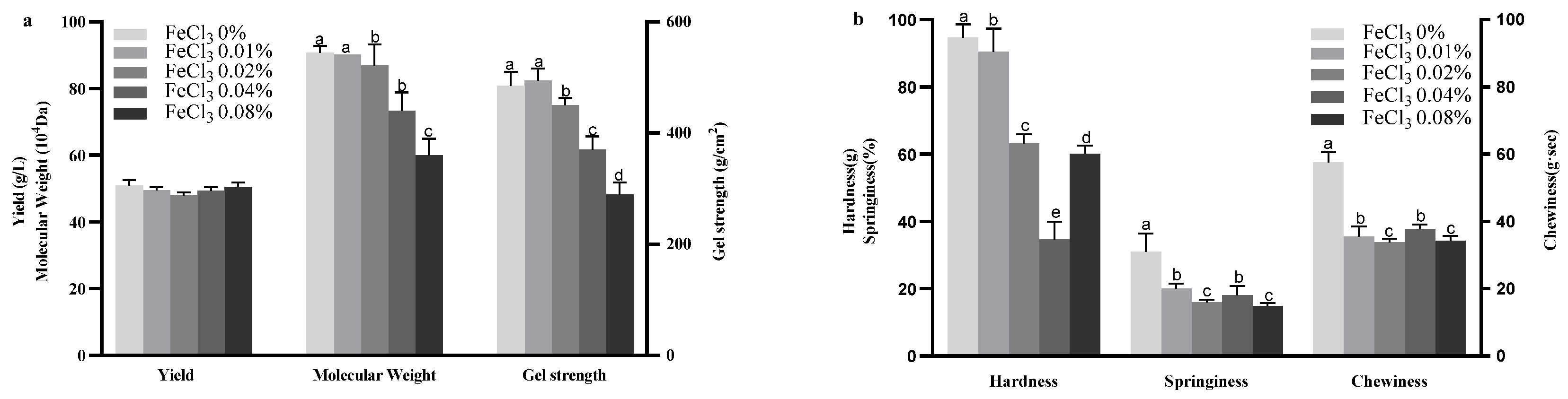
2.1. Effect of FeCl3 on Curdlan Production and Properties
2.2. Effect of FeSO4 on Curdlan Production and Properties
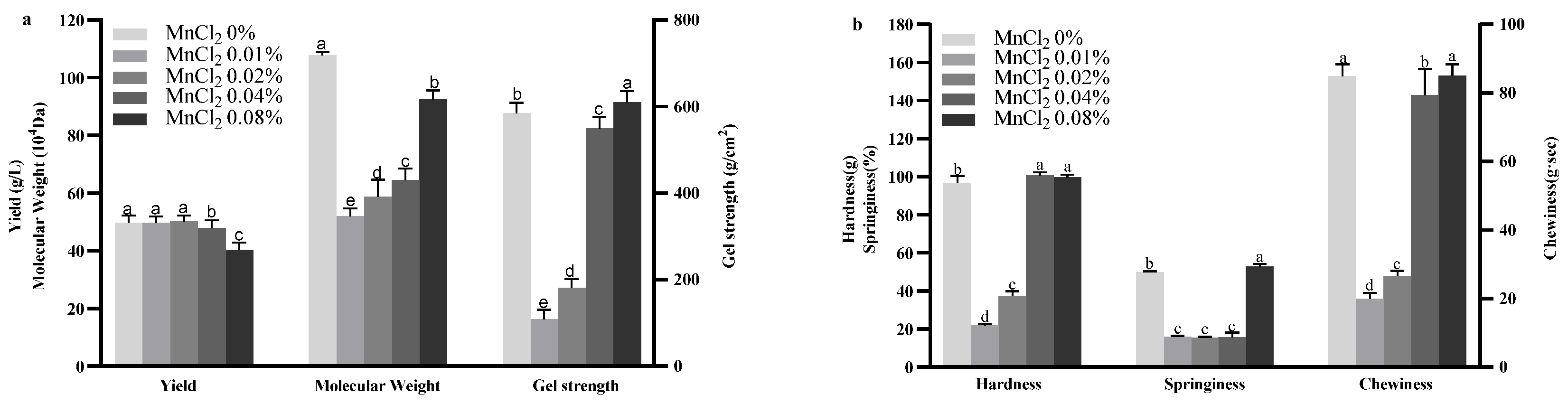
2.3. Effect of MnCl2 on Curdlan Production and Properties
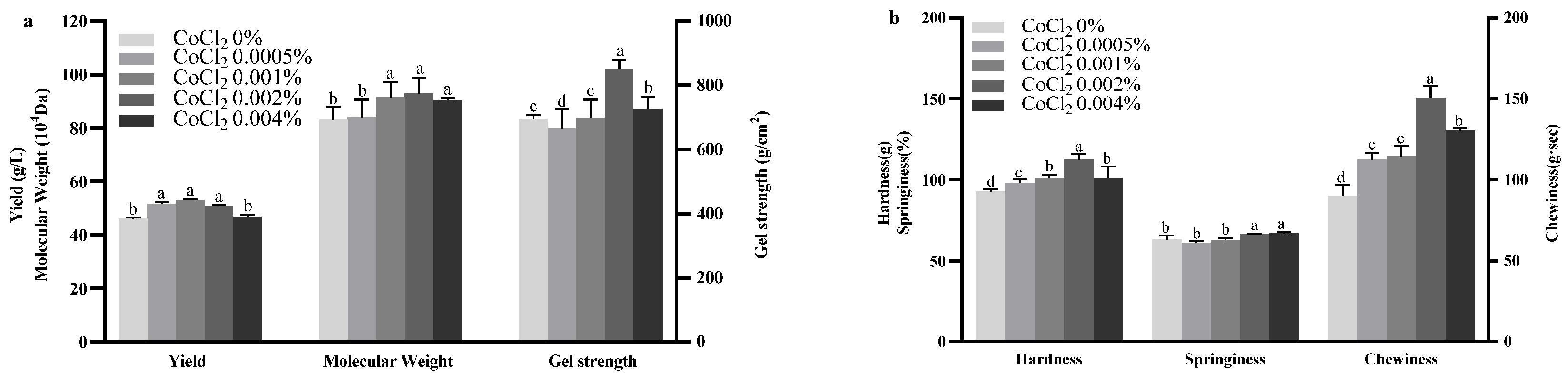
2.4. Effect of CoCl2 on Curdlan Production and Properties
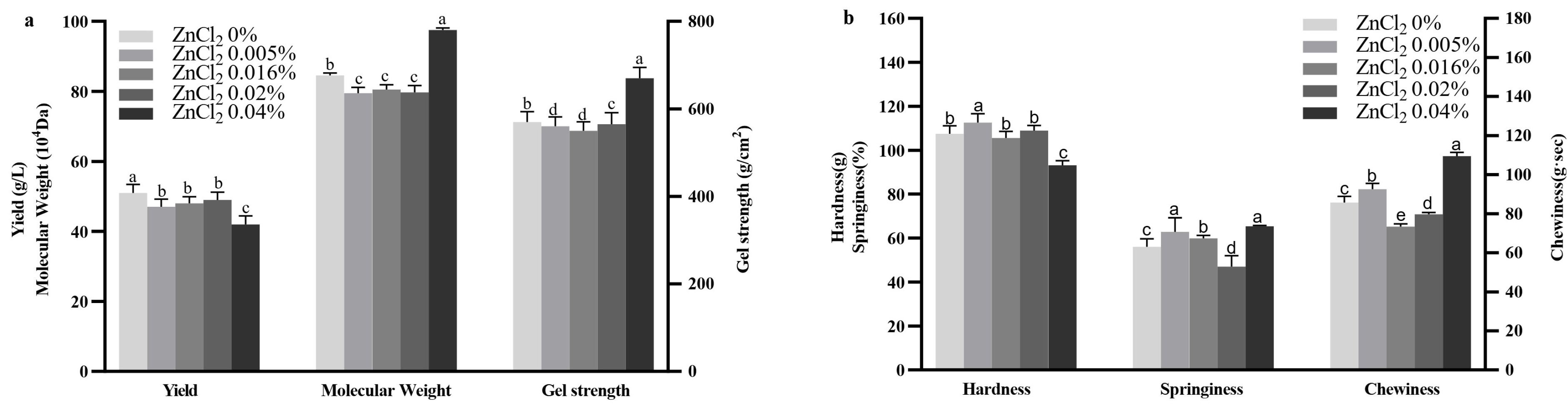
2.5. Effect of ZnCl2 on Curdlan Production and Properties
2.6. The Relationship Between Gel Strength and Molecular Weight
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Microorganisms
4.3. Mediums
4.4. Culture Conditions
4.5. Effect of Inorganic Salt Ions on the Gel Strength of Curdlan
4.6. Measurement of Curdlan Production
4.7. Curdlan Purification
4.8. Gel Strength and TPA Determination
4.9. Molecular Weight (MW) Determination
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acharya, K.; Mangolim, C.S.; Silva, T.T.d.; Fenelon, V.C.; Koga, L.N.; Ferreira, S.B.d.S.; Bruschi, M.L.; Matioli, G. Description of recovery method used for curdlan produced by Agrobacterium sp. IFO 13140 and its relation to the morphology and physicochemical and technological properties of the polysaccharide. PLoS ONE 2017, 12, e0171469. [Google Scholar] [CrossRef]
- Aquinas, N.; Bhat, M.R.; Selvaraj, S. A review presenting production, characterization, and applications of biopolymer curdlan in food and pharmaceutical sectors. Polym. Bull. 2021, 79, 6905–6927. [Google Scholar] [CrossRef]
- Andreassen, M.; Aquilina, G.; Bastos, M.L.; Boon, P.; Fallico, B.; FitzGerald, R.; Frutos Fernandez, M.J.; Grasl-Kraupp, B.; Gundert-Remy, U.; Gürtler, R.; et al. Safety evaluation of curdlan as a food additive. EFSA J. 2024, 22, 8985. [Google Scholar] [CrossRef]
- Verma, D.K.; Niamah, A.K.; Patel, A.R.; Thakur, M.; Singh Sandhu, K.; Chávez-González, M.L.; Shah, N.; Noe Aguilar, C. Chemistry and microbial sources of curdlan with potential application and safety regulations as prebiotic in food and health. Food Res. Int. 2020, 133, 109136. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Yu, Y.C.; Yu, S.H.; Chou, Y.C.; Lin, H.J.; Santoso, S.P.; Lin, S.P. Antimicrobial efficacy of carvacrol-loaded curdlan hydrogels for enhancing shelf-life in seafood packaging applications. Int. J. Food Microbiol. 2025, 428, 110976. [Google Scholar] [CrossRef]
- Yi, E.J.; Kim, Y.I.; Song, J.H.; Ko, H.J.; Chang, S.Y. Intranasal immunization with curdlan induce Th17 responses and enhance protection against enterovirus 71. Vaccine 2023, 41, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Ganbold, T.; Bao, M.; Xiao, H.; Han, S.; Baigude, H. Tumor targeted siRNA delivery by adenosine receptor-specific curdlan nanoparticles. Int. J. Biol. Macromol. 2023, 253, 126845. [Google Scholar] [CrossRef]
- Bera, H.; Abbasi, Y.F.; Thakur, A. Curdlan/Clay Nanocomposite-Reinforced Alginate Beads as Drug Carriers. J. Polym. Environ. 2023, 32, 854–869. [Google Scholar] [CrossRef]
- West, T.P. Production of the Polysaccharide Curdlan by Agrobacterium species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation 2020, 6, 16. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhan, X.B.; Zheng, Z.Y.; Wu, J.R.; English, N.; Yu, X.B.; Lin, C.C. Improved curdlan fermentation process based on optimization of dissolved oxygen combined with pH control and metabolic characterization of Agrobacterium sp. ATCC 31749. Appl. Microbiol. Biotechnol. 2011, 93, 367–379. [Google Scholar] [CrossRef]
- García-Cruz, F.; Durán-Páramo, E.; Garín-Aguilar, M.A.; Del Toro, G.V.; Chairez, I. Parametric characterization of the initial pH effect on the polysaccharides production by Lentinula edodes in submerged culture. Food Bioprod. Process. 2020, 119, 170–178. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, L.; Ding, H.; Gao, M.; Zheng, Z.; Wu, J.; Zhan, X. Enhanced production of curdlan by coupled fermentation system of Agrobacterium sp. ATCC 31749 and Trichoderma harzianum GIM 3.442. Carbohydr. Polym. 2017, 157, 1687–1694. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, L.; Gao, M.; Wu, J.; Zhan, X. Effective production of biologically active water-soluble β-1,3-glucan by a coupled system of Agrobacterium sp. and Trichoderma harzianum. Prep. Biochem. Biotechnol. 2018, 48, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.B.; Lin, C.C.; Zhang, H.T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2011, 93, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Wang, W.; Zhang, Y.; Zhang, Y.; Han, H.; Liu, Y.; Zhou, S.; Dong, X. Optimizing Curdlan Synthesis: Engineering Agrobacterium tumefaciens ATCC31749 for Enhanced Production Using Dextrin as a Carbon Source. Fermentation 2024, 10, 240. [Google Scholar] [CrossRef]
- Gao, H.; Xie, F.; Zhang, W.; Tian, J.; Zou, C.; Jia, C.; Jin, M.; Huang, J.; Chang, Z.; Yang, X.; et al. Characterization and improvement of curdlan produced by a high-yield mutant of Agrobacterium sp. ATCC 31749 based on whole-genome analysis. Carbohydr. Polym. 2020, 245, 116486. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Fu, R.; Yang, Y.; Cheng, R.; Li, J.; Wang, S.; Zhang, J. The chemical properties and hygroscopic activity of the exopolysaccharide lubcan from Paenibacillus sp. ZX1905. Int. J. Biol. Macromol. 2020, 164, 2641–2650. [Google Scholar] [CrossRef]
- Meng, Q.; Han, Y.; Zhu, H.; Yang, W.; Bello, A.; Deng, L.; Jiang, X.; Wu, X.; Sheng, S.; Xu, Y.; et al. Differences in distribution of functional microorganism at DNA and cDNA levels in cow manure composting. Ecotoxicol. Environ. Saf. 2020, 191, 110161. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Zhang, R.; Edgar, K.J. Properties, Chemistry, and Applications of the Bioactive Polysaccharide Curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Whistler, R. Industrial Gums: Polysaccharides and Their Derivatives; Elsevier: Amsterdam, The Netherlands, 1993; pp. 145–180. [Google Scholar]
- Ruffing, A.M.; Chen, R.R. Transcriptome profiling of a curdlan-producing Agrobacterium reveals conserved regulatory mechanisms of exopolysaccharide biosynthesis. Microb. Cell Factories 2012, 11, 17. [Google Scholar] [CrossRef]
- Survase, S.A.; Saudagar, P.S.; Bajaj, I.B.; Singhal, R.S. Scleroglucan: Fermentative production, downstream processing and applications. Food Technol. Biotechnol. 2007, 45, 107–118. [Google Scholar]
- Mıdık, F.; Tokatlı, M.; Bağder Elmacı, S.; Özçelik, F. Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles. Arch. Microbiol. 2020, 202, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Chen, H.; Xiao, W.; Li, C.; Peng, Z.; Li, Z.; Liu, J.; Lin, L.; Zeng, X. Inorganic salt starvation improves the polysaccharide production and CO2 fixation by Porphyridium purpureum. Bioprocess Biosyst. Eng. 2024, 47, 1017–1026. [Google Scholar] [CrossRef]
- Dong, J.J.; Xu, G.C.; Han, R.Z.; Ni, Y. Enhanced curdlan production with nitrogen feeding during polysaccharide synthesis by Rhizobium radiobacter. Carbohydr. Polym. 2016, 150, 385–391. [Google Scholar]
- Wu, J.; Han, X.; Ye, M.; Li, Y.; Wang, X.; Zhong, Q. Exopolysaccharides synthesized by lactic acid bacteria: Biosynthesis pathway, structure-function relationship, structural modification and applicability. Crit. Rev. Food Sci. Nutr. 2022, 63, 7043–7064. [Google Scholar] [CrossRef] [PubMed]
- Abedfar, A.; Hosseininezhad, M.; Rafe, A. Effect of microbial exopolysaccharide on wheat bran sourdough: Rheological, thermal and microstructural characteristics. Int. J. Biol. Macromol. 2020, 154, 371–379. [Google Scholar] [CrossRef]
- Yousefi, M.; Arianfar, A.; Hakimzadeh, V.; Rafe, A. Enhancing the Texture and Sensory Properties of Pickled Cucumbers with Different Brine Solutions. Foods 2025, 14, 336. [Google Scholar] [CrossRef]
- Al-jarrah, A.M.; Abdul Rahman, A.; Shahrim, I.; Razak, N.N.A.N.A.; Ababneh, B.; Tousi, E.T. Effect of inorganic salts and glucose additives on dose–response, melting point and mass density of genipin gel dosimeters. Phys. Medica 2016, 32, 36–41. [Google Scholar] [CrossRef]
- Hu, B.; Cen, S.; Sun, W.; Zhan, S.; Jia, R.; Ou, C.; Huang, T. Effects of different phosphorylation times and pH on fish gelatin: Functional properties, structural and mechanism analysis. Food Hydrocoll. 2024, 152, 109876. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhu, Q.; Wu, S.J. Preparation and gel properties of low molecular weight curdlan by hydrolysis of curdlan with commercial α-amylase. Carbohydr. Polym. 2014, 113, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Yi, Z.; Ma, L.; Tan, Y.; Cao, X.; Liu, D.; Li, X. Influences of carboxymethyl chitosan upon stabilization and gelation of O/W Pickering emulsions in the presence of inorganic salts. Carbohydr. Polym. 2024, 331, 121902. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, L.; Wang, Y.; Liu, S.; Cao, J.; Li, H.; Liu, X. Salt ions improve soybean protein isolate/curdlan complex fat substitutes: Effect of molecular interactions on freeze-thaw stability. Int. J. Biol. Macromol. 2024, 272, 132774. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; He, N.; Huang, X.; Song, X.; Chen, J.; Lin, J.; Jin, Y. FeCl3-catalyzed oxidative amidation of benzylic C–H bonds enabled by a photogenerated chlorine-radical. Chem. Commun. 2023, 59, 10299–10302. [Google Scholar] [CrossRef]
- Maire du Poset, A.; Lerbret, A.; Zitolo, A.; Cousin, F.; Assifaoui, A. Design of polygalacturonate hydrogels using iron(II) as cross-linkers: A promising route to protect bioavailable iron against oxidation. Carbohydr. Polym. 2018, 188, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, N.; Maina, N.H.; Vikgren, P.; Sontag-Strohm, T. Gelation of cereal β-glucan at low concentrations. Food Hydrocoll. 2017, 73, 60–66. [Google Scholar] [CrossRef]
- Cao, M.; Bai, Y.; Su, J.; Wang, Y.; Feng, J.; Zhang, Q. Denitrification performance of the nitrate-dependent manganese redox strain Dechloromonas sp. YZ8 under copper ion (Cu(II)) stress: Promotion mechanism and immobilization efficacy. J. Hazard. Mater. 2024, 479, 135748. [Google Scholar] [CrossRef]
- Gotoh, T.; Matsushima, K.; Kikuchi, K.I. Adsorption of Cu and Mn on covalently cross-linked alginate gel beads. Chemosphere 2004, 55, 57–64. [Google Scholar] [CrossRef]
- Cheng, P.; Chang, T.; Wang, C.; Yao, C.; Zhou, C.; Liu, T.; Wang, G.; Yan, X.; Ruan, R. High cobalt exposure facilitates bioactive exopolysaccharides production with a novel molecular structure in Botryococcus braunii. Chem. Eng. J. 2022, 442, 136294. [Google Scholar] [CrossRef]
- Eysturskarð, J. Mechanical Properties of Gelatin Gels; Effect of Molecular Weight and Molecular Weight Distribution; Norwegian University of Science and Technology: Trondheim, Norway, 2010. [Google Scholar]
- Cai, Z.; Zhang, H. Recent progress on curdlan provided by functionalization strategies. Food Hydrocoll. 2017, 68, 128–135. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, J.; Wang, Z.; Hang, H.; Zhao, W.; Zhuang, Y.; Chu, J. Kinetic analysis of curdlan production by Alcaligenes faecalis with maltose, sucrose, glucose and fructose as carbon sources. Bioresour. Technol. 2018, 259, 319–324. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, H.; Huang, Y.; Wu, S.; Tian, J.; Niu, Y.; Zou, C.; Jia, C.; Jin, M.; Huang, J.; et al. Glutamine synthetase gene glnA plays a vital role in curdlan biosynthesis of Agrobacterium sp. CGMCC 11546. Int. J. Biol. Macromol. 2020, 165, 222–230. [Google Scholar] [CrossRef]
- Kim, M.-K.; Ryu, K.-E.; Choi, W.-A.; Rhee, Y.-H.; Lee, I.-Y. Enhanced production of (1 → 3)-β-d-glucan by a mutant strain of Agrobacterium species. Biochem. Eng. J. 2003, 16, 163–168. [Google Scholar] [CrossRef]
- Mohsin, A.; Sun, J.; Khan, I.M.; Hang, H.; Tariq, M.; Tian, X.; Ahmed, W.; Niazi, S.; Zhuang, Y.; Chu, J.; et al. Sustainable biosynthesis of curdlan from orange waste by using Alcaligenes faecalis: A systematically modeled approach. Carbohydr. Polym. 2019, 205, 626–635. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Q.; Ofosu, F.K.; Yu, X. Isolation and characterization of curdlan produced by Agrobacterium HX1126 using α-lactose as substrate. Int. J. Biol. Macromol. 2015, 81, 498–503. [Google Scholar] [CrossRef]
- Tao, H.; Wang, B.; Wen, H.; Cui, B.; Zhang, Z.; Kong, X.; Wang, Y. Improvement of the textural characteristics of curdlan gel by the formation of hydrogen bonds with erythritol. Food Hydrocoll. 2021, 117, 106648. [Google Scholar] [CrossRef]
- Szwengiel, A.; Wiesner, M. Effect of metal ions on levan synthesis efficiency and its parameters by levansucrase from Bacillus subtilis. Int. J. Biol. Macromol. 2019, 128, 237–243. [Google Scholar] [CrossRef]
- Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Deng, J.Z.; Rustandi, R.R.; Swartz, A.; Shieh, Y.; Baker, J.B.; Vlasak, J.; Wang, S.; Loughney, J.W. SEC coupled with in-line multiple detectors for the characterization of an oncolytic Coxsackievirus. Mol. Ther.-Oncolytics 2022, 24, 139–147. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Yao, B.; Yue, S.; Chang, Z.; Yang, X.; Gao, H. Effects of Inorganic Salts on Curdlan Production and Structural Properties. Gels 2025, 11, 313. https://doi.org/10.3390/gels11050313
Zhu X, Yao B, Yue S, Chang Z, Yang X, Gao H. Effects of Inorganic Salts on Curdlan Production and Structural Properties. Gels. 2025; 11(5):313. https://doi.org/10.3390/gels11050313
Chicago/Turabian StyleZhu, Xinyi, Bowei Yao, Siyang Yue, Zhongyi Chang, Xuexia Yang, and Hongliang Gao. 2025. "Effects of Inorganic Salts on Curdlan Production and Structural Properties" Gels 11, no. 5: 313. https://doi.org/10.3390/gels11050313
APA StyleZhu, X., Yao, B., Yue, S., Chang, Z., Yang, X., & Gao, H. (2025). Effects of Inorganic Salts on Curdlan Production and Structural Properties. Gels, 11(5), 313. https://doi.org/10.3390/gels11050313





