Current Utilization of Gel-Based Scaffolds and Templates in Foot and Ankle Surgery—A Review
Abstract
1. Introduction
2. Types of Gel Scaffolds and Templates
2.1. Natural
2.1.1. Alginate
2.1.2. Chitosan
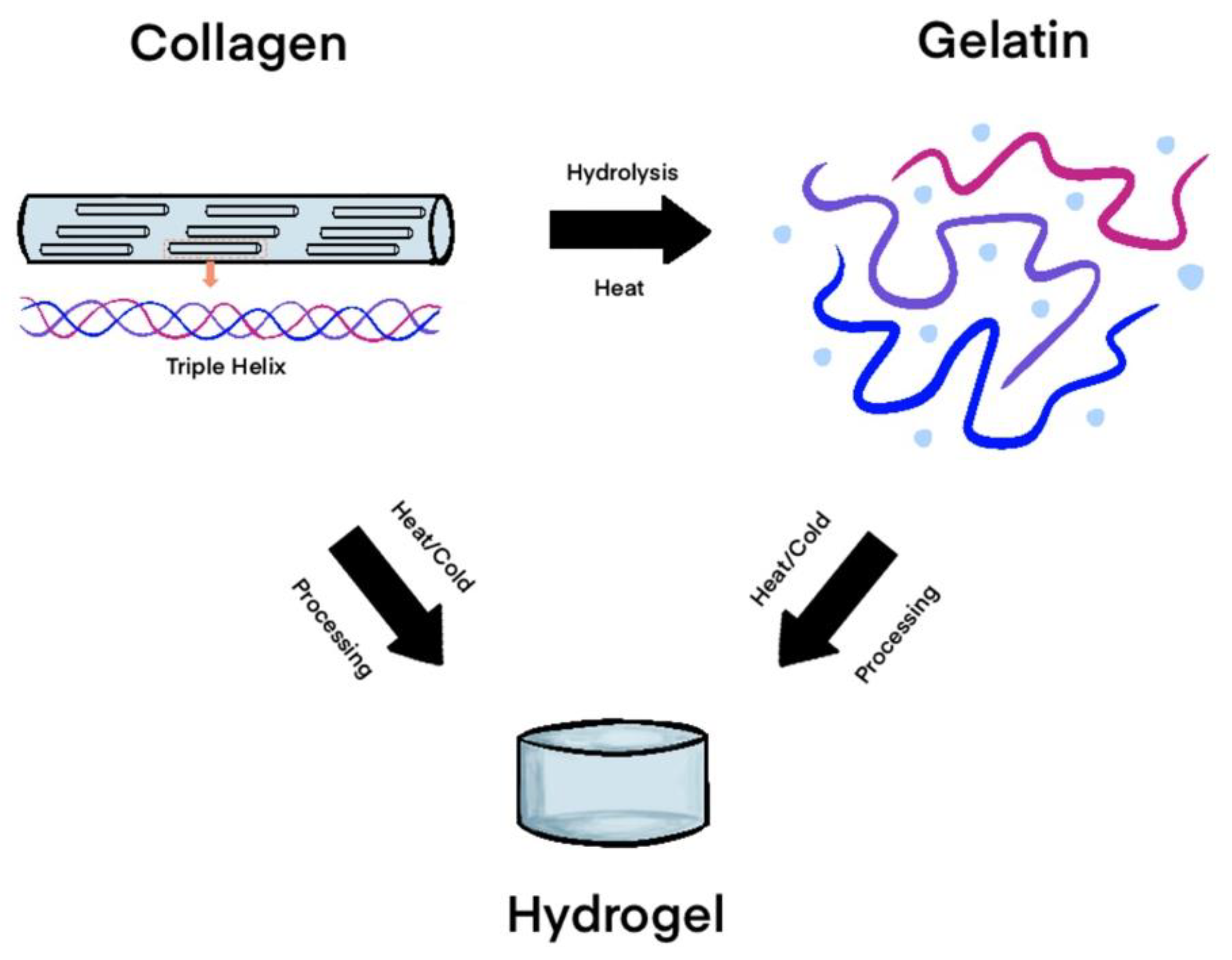
2.1.3. Collagen
2.1.4. Gelatin
2.1.5. Hyaluronic Acid
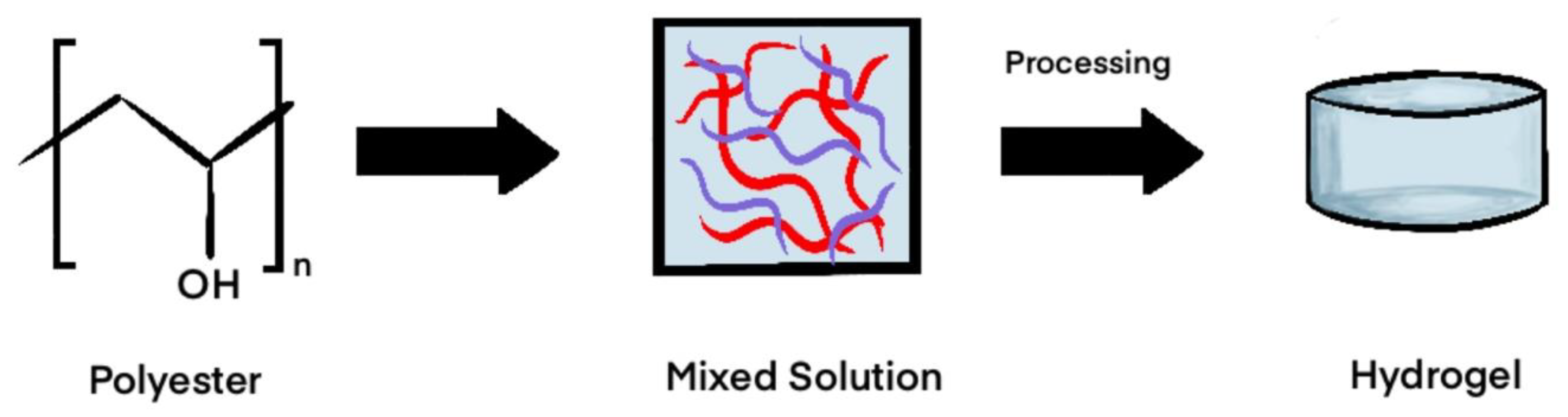
2.2. Synthetic
Polyester-Based Synthetics
2.3. Hybrids
2.3.1. Gelatin Methacrylate
2.3.2. Gelatin Nanohydroxyapatite
2.3.3. Hybrid Systems and Nanomaterials
3. Comparison of Gel Types
4. Molecular Mechanisms of Gel Interactions
5. Mechanobiology
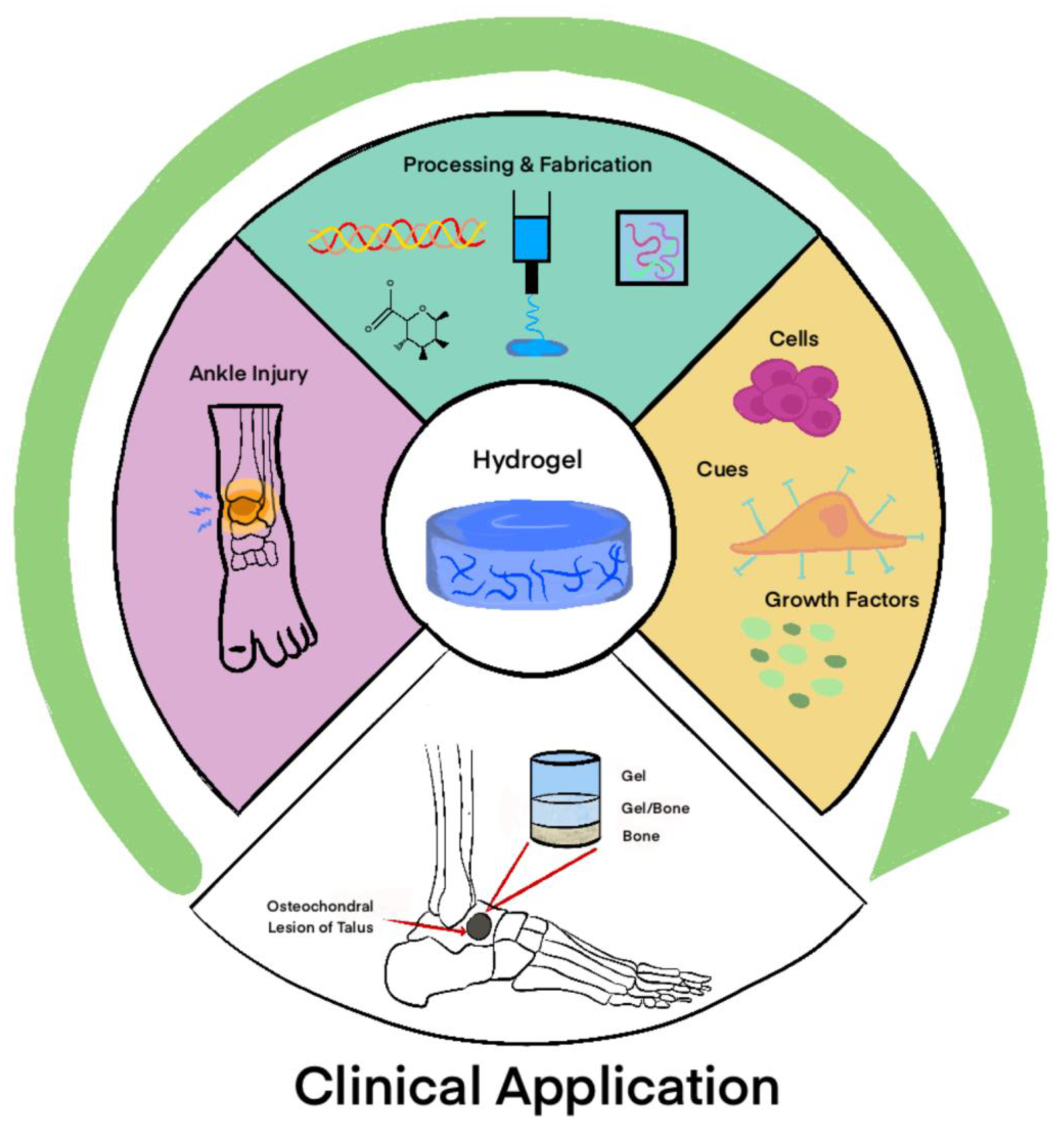
6. Applications of Gels in Foot and Ankle Surgery
6.1. Talar Osteochondral Defects
6.2. Osteoarthritis Applications
7. Challenges in Scaffold Application
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TERM | Tissue Engineering and Regenerative Medicine |
| ECM | Extracellular Matrix |
| HA | Hyaluronic Acid |
| PGA | polyglycolic acid |
| PLA | polylactic acid |
| PLGA | polylactic-co-glycolic acid |
| PVA | polyvinyl alcohol |
| PEO | polyethylene oxide |
| PEG | polyethylene glycol |
| PCL | Polycaprolactone |
| GelMA | gelatin methacrylate |
| GelnHA | gelatin nanohydroxyapatite |
| nHA | nano-sized hydroxyapatite |
| OCL | Osteochondral lesions |
| AOFAS | American Orthopaedic Foot & Ankle Society |
| VAS | Visual Analog Scale |
| PGA-HA | polyglycolic acid–hyaluronan |
| MTP | metatarsophalangeal |
| VEGF | vascular endothelial growth factor |
References
- Correia, S.I.; Pereira, H.; Silva-Correia, J.; Van Dijk, C.N.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Current concepts: Tissue engineering and regenerative medicine applications in the ankle joint. J. R. Soc. Interface 2014, 11, 20130784. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Li, Y.; Feng, L.; Wang, B.; Wang, M.; Wang, H.; Zhu, M.; Yang, Y.; Waldorff, E.I.; et al. Enhancing cartilage repair with optimized supramolecular hydrogel-based scaffold and pulsed electromagnetic field. Bioact. Mater. 2023, 22, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration. Biomolecules 2021, 11, 444. [Google Scholar] [CrossRef]
- Adak, A.; Castelletto, V.; Hamley, I.W.; Seitsonen, J.; Jana, A.; Ghosh, S.; Mukherjee, N.; Ghosh, S. Self-Assembly and Wound Healing Activity of Biomimetic Cycloalkane-Based Lipopeptides. ACS Appl. Mater. Interfaces 2024, 16, 58417–58426. [Google Scholar] [CrossRef]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Guo, B.; Lei, B.; Li, P.; Ma, P.X. Functionalized scaffolds to enhance tissue regeneration. Regen. Biomater. 2015, 2, 47–57. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Olabisi, R.M. Cell microencapsulation with synthetic polymers. J. Biomed. Mater. Res. A 2015, 103, 846–859. [Google Scholar] [CrossRef]
- Evans, S.F.; Chang, H.; Knothe Tate, M.L. Elucidating multiscale periosteal mechanobiology: A key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng. Part. B Rev. 2013, 19, 147–159. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Geng, L.; Yuan, Y.; Lu, J.; Wang, Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015, 5, 9409. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, B.M.; Butchko, E.A.; Wahby, M.N.; Breining, K.M.; Konz, A.E.; Grunlan, M.A. Shape Memory Polymer Bioglass Composite Scaffolds Designed to Heal Complex Bone Defects. ACS Biomater. Sci. Eng. 2024, 10, 6509–6519. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Lee, A.; Itel, F.; Maniura-Weber, K.; Petri-Fink, A.; Rothen-Rutishauser, B. The Effect of Substrate Properties on Cellular Behavior and Nanoparticle Uptake in Human Fibroblasts and Epithelial Cells. Nanomaterials 2024, 14, 342. [Google Scholar] [CrossRef]
- Vashist, A.; Ahmad, S. Hydrogels in tissue engineering: Scope and applications. Curr. Pharm. Biotechnol. 2015, 16, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Ghezzi, C.E.; McNamara, S.L.; Black, L.D., 3rd; Kaplan, D.L. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann. Biomed. Eng. 2015, 43, 657–680. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sanket, A.S.; Pradhan, S.; Sahoo, R.; Das, S.; Pany, S.; Douglas, T.E.L.; Dandela, R.; Liu, Q.; Rajadas, J.; et al. Designing of gradient scaffolds and their applications in tissue regeneration. Biomaterials 2023, 296, 122078. [Google Scholar] [CrossRef]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M.; Shastri, V.P. Optimization strategies on the structural modeling of gelatin/chitosan scaffolds to mimic human meniscus tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4777–4785. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Schaeffer, C.; Pfaff, B.N.; Cornell, N.J.; Salopek, L.S.; Shan, S.; Viyar, J.; Omesiete, W.; Griffin, D.R.; Cottler, P.S.; DeGeorge, B.R., Jr. Injectable Microannealed Porous Scaffold for Articular Cartilage Regeneration. Ann. Plast. Surg. 2020, 84, S446–S450. [Google Scholar] [CrossRef]
- Danilkowicz, R.; Murawski, C.; Pellegrini, M.; Walther, M.; Valderrabano, V.; Angthong, C.; Adams, S. Nonoperative and Operative Soft-Tissue and Cartilage Regeneration and Orthopaedic Biologics of the Foot and Ankle: An Orthoregeneration Network Foundation Review. Arthroscopy 2022, 38, 2350–2358. [Google Scholar] [CrossRef]
- Zhang, J.; Sen, A.; Cho, E.; Lee, J.S.; Webb, K. Poloxamine/fibrin hybrid hydrogels for non-viral gene delivery. J. Tissue Eng. Regen. Med. 2017, 11, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Ebhodaghe, S.O. Natural Polymeric Scaffolds for Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2021, 32, 2144–2194. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/ hydroxyapatite/collagen scaffolds. J. Transl. Med. 2015, 13, 152. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Bridges, T.N.; McCahon, J.A.; Parekh, S.G. Surgical Arthroscopy with Intra-articular Hyaluronic Acid/Alginate Adjunct in the Treatment of Ankle Osteoarthritis. Technol. Foot Ankle Surg. 2024, 23, 204–207. [Google Scholar] [CrossRef]
- Soltani, L.; Varmira, K.; Nazari, M. Comparison of the differentiation of ovine fetal bone-marrow mesenchymal stem cells towards osteocytes on chitosan/alginate/CuO-NPs and chitosan/alginate/FeO-NPs scaffolds. Sci. Rep. 2024, 14, 161. [Google Scholar] [CrossRef]
- Lin, T.H.; Wang, H.C.; Tseng, Y.L.; Yeh, M.L. A bioactive composite scaffold enhances osteochondral repair by using thermosensitive chitosan hydrogel and endothelial lineage cell-derived chondrogenic cell. Mater. Today Bio 2024, 28, 101174. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J. Biological Influence of Nonswelling Microgels on Cartilage Induction of Mouse Adipose-Derived Stem Cells. Biomed. Res. Int. 2019, 2019, 6508094. [Google Scholar] [CrossRef]
- Li, A.; Xue, Q.; Ye, Y.; Gong, P.; Deng, M.; Jiang, B. Study on TEMPO-Mediated Oxidation of N-Succinyl Chitosan and the Water Retention Property. Molecules 2020, 25, 4698. [Google Scholar] [CrossRef]
- Bao, G.; Jiang, T.; Ravanbakhsh, H.; Reyes, A.; Ma, Z.; Strong, M.; Wang, H.; Kinsella, J.M.; Li, J.; Mongeau, L. Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater. Horiz. 2020, 7, 2336–2347. [Google Scholar] [CrossRef]
- Akmeşe, R.; Ertan, M.B.; Kocaoğlu, H. Comparison of Chitosan-Based Liquid Scaffold and Hyaluronic Acid–Based Soft Scaffold for Treatment of Talus Osteochondral Lesions. Foot Ankle Int. 2020, 41, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Matevž, S.; Maja Navodnik, P.; Mojca, F.; Janja, J.; Nanča Čebron, L.; Igor, L.; Hélène Liette, L.; Vilma Urbančič, R. A randomized, placebo-controlled study of chitosan gel for the treatment of chronic diabetic foot ulcers (the CHITOWOUND study). BMJ Open Diabetes Res. Care 2024, 12, e004195. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, W.; Hu, L.; Yi, X.; Tang, F. Application of Hydrogels as Sustained-Release Drug Carriers in Bone Defect Repair. Polymers 2022, 14, 4906. [Google Scholar] [CrossRef]
- Alberts, B.; Heald, R.; Johnson, A.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell: Seventh International Student Edition with Registration Card; WW Norton & Company: New York, NY, USA, 2022. [Google Scholar]
- Naka, Y.; Kitano, S.; Irie, S.; Matsusaki, M. Wholly vascularized millimeter-sized engineered tissues by cell-sized microscaffolds. Mater. Today Bio 2020, 6, 100054. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-Printed Polycaprolactone/β-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Younger, A.; Wing, K.; Penner, M.; Cresswell, M. A study to evaluate the safety of platelet-derived growth factor for treatment of osteochondral defects of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1250–1258. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Kruppke, B.; Ray, S.; Alt, V.; Rohnke, M.; Kern, C.; Kampschulte, M.; Heinemann, C.; Budak, M.; Adam, J.; Döhner, N. Gelatin-modified calcium/strontium hydrogen phosphates stimulate bone regeneration in osteoblast/osteoclast co-culture and in osteoporotic rat femur defects—In vitro to in vivo translation. Molecules 2020, 25, 5103. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Schönfelder, J.; Tugtekin, S.M.; Wetzel, C.; Hacker, M.C.; Schulz-Siegmund, M. Stabilization and Sterilization of Pericardial Scaffolds by Ultraviolet and Low-Energy Electron Irradiation. Tissue Eng. Part. C Methods 2018, 24, 717–729. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Agrali, O.B.; Yildirim, S.; Ozener, H.O.; Köse, K.N.; Ozbeyli, D.; Soluk-Tekkesin, M.; Kuru, L. Evaluation of the Effectiveness of Esterified Hyaluronic Acid Fibers on Bone Regeneration in Rat Calvarial Defects. Biomed. Res. Int. 2018, 2018, 3874131. [Google Scholar] [CrossRef]
- Choi, C.-E.; Paul, A. Mineral nanoparticles and nanocomposite hydrogels with osteoinductive properties for bone regeneration. Explor. BioMat X 2025, 2, 101332. [Google Scholar] [CrossRef]
- Alturki, A.M. Rationally design of electrospun polysaccharides polymeric nanofiber webs by various tools for biomedical applications: A review. Int. J. Biol. Macromol. 2021, 184, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, J.; Takahashi, M.; Hatakeyama, T.; Hatakeyama, H. Gelation of hyaluronic acid through annealing. Polym. Int. 2000, 49, 1604–1608. [Google Scholar] [CrossRef]
- Madau, M.; Le Cerf, D.; Dulong, V.; Picton, L. Hyaluronic Acid Functionalization with Jeffamine® M2005: A Comparison of the Thermo-Responsiveness Properties of the Hydrogel Obtained through Two Different Synthesis Routes. Gels 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Alarake, N.Z.; Frohberg, P.; Groth, T.; Pietzsch, M. Mechanical properties and biocompatibility of in situ enzymatically cross-linked gelatin hydrogels. Int. J. Artif. Organs 2017, 40, 159–168. [Google Scholar] [CrossRef]
- Kemençe, N.; Bölgen, N. Gelatin- and hydroxyapatite-based cryogels for bone tissue engineering: Synthesis, characterization, in vitro and in vivo biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef]
- Sahapaibounkit, P.; Prasertsung, I.; Mongkolnavin, R.; Wong, C.S.; Damrongsakkul, S. A two-step method using air plasma and carbodiimide crosslinking to enhance the biocompatibility of polycaprolactone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar]
- Mohan, N.; Mohanan, P.V.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef]
- Kim, B.J.; Arai, Y.; Choi, B.; Park, S.; Ahn, J.; Han, I.-B.; Lee, S.-H. Restoration of articular osteochondral defects in rat by a bi-layered hyaluronic acid hydrogel plug with TUDCA-PLGA microsphere. J. Ind. Eng. Chem. 2018, 61, 295–303. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Pérez González, R.; Sáez-Martínez, V.; Ruiz Pérez, J.; Vilas-Vilela, J.L. Synthesis and Characterization of Covalently Crosslinked pH-Responsive Hyaluronic Acid Nanogels: Effect of Synthesis Parameters. Polymers 2019, 11, 742. [Google Scholar] [CrossRef]
- Gao, L.; Beninatto, R.; Oláh, T.; Goebel, L.; Tao, K.; Roels, R.; Schrenker, S.; Glomm, J.; Venkatesan, J.K.; Schmitt, G.; et al. A Photopolymerizable Biocompatible Hyaluronic Acid Hydrogel Promotes Early Articular Cartilage Repair in a Minipig Model In Vivo. Adv. Healthc. Mater. 2023, 12, e2300931. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Prinz, J.; Eschweiler, J.; Schenker, H.; Weber, C.; Maffulli, N.; Lecouturier, S.; Hildebrand, F.; Greven, J. Fibrin glue does not promote migration and proliferation of bone marrow derived mesenchymal stem cells in collagenic membranes: An in vitro study. Sci. Rep. 2022, 12, 20660. [Google Scholar] [CrossRef]
- Vonk, L.A.; de Windt, T.S.; Slaper-Cortenbach, I.C.; Saris, D.B. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: A concise review. Stem Cell Res. Ther. 2015, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, K.; Mohammadpour, M.; Alavinasab Ardebili, S.A.; Eshaghi Malekshah, R.; Samadian, H. Fabrication and examination of polyorganophosphazene/polycaprolactone-based scaffold with degradation, in vitro and in vivo behaviors suitable for tissue engineering applications. Sci. Rep. 2022, 12, 18407. [Google Scholar] [CrossRef]
- Xavier, M.; Farez, N.; Salvatierra, P.L.; Jardini, A.L.; Kharmandayan, P.; Feldman, S. Biological performance of a bioabsorbable Poly (L-Lactic Acid) produced in polymerization unit: In vivo studies. F1000Research 2021, 10, 1275. [Google Scholar] [CrossRef]
- Giuliani, A.; Moroncini, F.; Mazzoni, S.; Belicchi, M.L.; Villa, C.; Erratico, S.; Colombo, E.; Calcaterra, F.; Brambilla, L.; Torrente, Y.; et al. Polyglycolic acid-polylactic acid scaffold response to different progenitor cell in vitro cultures: A demonstrative and comparative X-ray synchrotron radiation phase-contrast microtomography study. Tissue Eng. Part. C Methods 2014, 20, 308–316. [Google Scholar] [CrossRef]
- Pihlajamäki, H.K.; Salminen, S.T.; Tynninen, O.; Böstman, O.M.; Laitinen, O. Tissue restoration after implantation of polyglycolide, polydioxanone, polylevolactide, and metallic pins in cortical bone: An experimental study in rabbits. Calcif. Tissue Int. 2010, 87, 90–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G.M. Tailoring the Interface of Biomaterials to Design Effective Scaffolds. J. Funct. Biomater. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Sojo, F.; Arvelo, F.; Sabino, M.A. Functional electrospun poly (lactic acid) scaffolds for biomedical applications: Experimental conditions, degradation and biocompatibility study. Mol. Cell. Biomech. 2013, 10, 85. [Google Scholar]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Dalzoppo, D.; Lora, S.; Sartore, L.; Folin, M.; Parnigotto, P.P.; Grandi, C. Tailored PVA/ECM scaffolds for cartilage regeneration. Biomed. Res. Int. 2014, 2014, 762189. [Google Scholar] [CrossRef]
- Park, J.; Lakes, R.S. Biomaterials: An Introduction; Springer Science & Business Media: Cham, Switzerland, 2007. [Google Scholar]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet, R. Preventing bacterial adhesion on scaffolds for bone tissue engineering. Int. J. Bioprint. 2025, 2, 20–34. [Google Scholar] [CrossRef]
- Bracho-Sanchez, E.; Xia, C.Q.; Clare-Salzler, M.J.; Keselowsky, B.G. Micro and Nano Material Carriers for Immunomodulation. Am. J. Transplant. 2016, 16, 3362–3370. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bang, S.; Kim, S.; Jo, S.Y.; Kim, B.-C.; Hwang, Y.; Noh, I. Synthesis and in vitro characterizations of porous carboxymethyl cellulose-poly (ethylene oxide) hydrogel film. Biomater. Res. 2015, 19, 12. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol–coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef]
- Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Modrák, M.; Trebuňová, M.; Balogová, A.F.; Hudák, R.; Živčák, J. Biodegradable Materials for Tissue Engineering: Development, Classification and Current Applications. J. Funct. Biomater. 2023, 14, 159. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Wang, X.C.; Peng, X.F.; Turng, L.S. Shish-kebab-structured poly(ε-caprolactone) nanofibers hierarchically decorated with chitosan-poly(ε-caprolactone) copolymers for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6955–6965. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jia, Y.; Yao, C.; Lu, Y. PCL/PEG core/sheath fibers with controlled drug release rate fabricated on the basis of a novel combined technique. Int. J. Pharm. 2014, 469, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Ng, H.Y.; Lin, Y.H.; Lin, T.J.; Kao, C.T.; Shie, M.Y. The Synergistic Effect of Cyclic Tensile Force and Periodontal Ligament Cell-Laden Calcium Silicate/Gelatin Methacrylate Auxetic Hydrogel Scaffolds for Bone Regeneration. Cells 2022, 11, 2069. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.B.; Bauman, L.A.; Zhao, B. 3D Printing Organogels with Bioderived Cyrene for High-Resolution Customized Hydrogel Structures. Langmuir 2025, 41, 646–653. [Google Scholar] [CrossRef]
- Cinar, A.G.; Munir, I.; Yesiloz, G. Investigating Physical Properties of Hybrid Hyaluronic Acid and Collagen Compositions of GelMA Microgels toward Tissue Engineering and Organ-on-Chip Applications. ACS Appl. Polym. Mater. 2023, 5, 8121–8132. [Google Scholar] [CrossRef]
- Reyna-Urrutia, V.A.; Estevez, M.; González-González, A.M.; Rosales-Ibáñez, R. 3D scaffolds of caprolactone/chitosan/polyvinyl alcohol/hydroxyapatite stabilized by physical bonds seeded with swine dental pulp stem cell for bone tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 81. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Li, F.; Wang, A.; Wang, C. Analysis of friction between articular cartilage and polyvinyl alcohol hydrogel artificial cartilage. J. Mater. Sci. Mater. Med. 2016, 27, 87. [Google Scholar] [CrossRef]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef] [PubMed]
- McMurtrey, R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 2014, 11, 066009. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, Z.; Nasrollahzadeh, M.; Daemi, H.; Baghaban Eslaminejad, M.; Shabani, A.A.; Dadashpour, M.; Mirmohammadkhani, M.; Nasrabadi, D. Micro- and nanotechnology in biomedical engineering for cartilage tissue regeneration in osteoarthritis. Beilstein J. Nanotechnol. 2022, 13, 363–389. [Google Scholar] [CrossRef]
- Paul, A.; Manoharan, V.; Krafft, D.; Assmann, A.; Uquillas, J.A.; Shin, S.R.; Hasan, A.; Hussain, M.A.; Memic, A.; Gaharwar, A.K.; et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 2016, 4, 3544–3554. [Google Scholar] [CrossRef]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Pacelli, S.; Maloney, R.; Chakravarti, A.R.; Whitlow, J.; Basu, S.; Modaresi, S.; Gehrke, S.; Paul, A. Controlling Adult Stem Cell Behavior Using Nanodiamond-Reinforced Hydrogel: Implication in Bone Regeneration Therapy. Sci. Rep. 2017, 7, 6577. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, P.; Lach, S. Hydrogels as Scaffolds in Bone-Related Tissue Engineering and Regeneration. Macromol. Biosci. 2023, 23, 2300152. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Designing Hydrogels for Immunomodulation in Cancer Therapy and Regenerative Medicine. Adv. Mater. 2024, 36, e2308894. [Google Scholar] [CrossRef]
- Vernerey, F.J.; Lalitha Sridhar, S.; Muralidharan, A.; Bryant, S.J. Mechanics of 3D Cell-Hydrogel Interactions: Experiments, Models, and Mechanisms. Chem. Rev. 2021, 121, 11085–11148. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Zhang, Q.Y.; Feng, Z.Y.; Huang, K.; Zou, C.Y.; Fan, M.H.; Zhang, Y.Q.; Zhang, J.Y.; Li-Ling, J.; Tan, B.; et al. Hydrogel-based immunoregulation of macrophages for tissue repair and regeneration. Int. J. Biol. Macromol. 2024, 268, 131643. [Google Scholar] [CrossRef]
- Butenko, S.; Nagalla, R.R.; Guerrero-Juarez, C.F.; Palomba, F.; David, L.M.; Nguyen, R.Q.; Gay, D.; Almet, A.A.; Digman, M.A.; Nie, Q.; et al. Hydrogel crosslinking modulates macrophages, fibroblasts, and their communication, during wound healing. Nat. Commun. 2024, 15, 6820. [Google Scholar] [CrossRef]
- Samanta, S.; Rangasami, V.K.; Sarlus, H.; Samal, J.R.K.; Evans, A.D.; Parihar, V.S.; Varghese, O.P.; Harris, R.A.; Oommen, O.P. Interpenetrating gallol functionalized tissue adhesive hyaluronic acid hydrogel polarizes macrophages to an immunosuppressive phenotype. Acta Biomater. 2022, 142, 36–48. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, L.; Wang, Z.; Dang, W.; Chen, H.; Li, T.; Liu, Y.; Tan, W. Molecular-Cellular Two-Pronged Reprogramming of Inflammatory Soft-Tissue Interface with an Immunosuppressive Pure DNA Hydrogel. Nano Lett. 2025, 25, 5087–5096. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Qin, M.; Cheng, W.; Wang, W.; Cao, Y. Understanding and Regulating Cell-Matrix Interactions Using Hydrogels of Designable Mechanical Properties. J. Biomed. Nanotechnol. 2021, 17, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Guo, B.; Wu, D.; Yang, F.; Ding, Y. Advances of natural hydrogel-based vascularization strategies for soft tissue repair. Front. Mater. 2024, 11, 1446035. [Google Scholar] [CrossRef]
- Hazur, J.; Endrizzi, N.; Schubert, D.W.; Boccaccini, A.R.; Fabry, B. Stress relaxation amplitude of hydrogels determines migration, proliferation, and morphology of cells in 3-D culture. Biomater. Sci. 2021, 10, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Kim, T.H.; Alsberg, E. Reversible dynamic mechanics of hydrogels for regulation of cellular behavior. Acta Biomater. 2021, 136, 88–98. [Google Scholar] [CrossRef]
- Rosenthal, R.M.; Featherall, J.; Putko, R.M.; McGlone, P.J.; Feeley, S.M.; Panarello, N.M.; Lilley, B.M.; Rabin, S.; Lewis, D.C.; Parkes, C.W.; et al. Time-sensitive injuries for the sports medicine surgeon—“Sports Medicine Trauma”, Part 1: Upper Extremity. Orthop. Rev. 2024, 16, 119. [Google Scholar] [CrossRef]
- Bajuri, M.Y.; Sabri, S.; Mazli, N.; Sarifulnizam, F.A.; Mohd Apandi, H. Osteochondral Injury of the Talus Treated with Cell-Free Hyaluronic Acid-Based Scaffold (Hyalofast®)—A Reliable Solution. Cureus 2021, 13, e17928. [Google Scholar] [CrossRef]
- Dahmen, J.; Lambers, K.T.A.; Reilingh, M.L.; van Bergen, C.J.A.; Stufkens, S.A.S.; Kerkhoffs, G. No superior treatment for primary osteochondral defects of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2142–2157. [Google Scholar] [CrossRef]
- Yontar, N.S.; Aslan, L.; Can, A.; Ogut, T. One step treatment of talus osteochondral lesions with microfracture and cell free hyaluronic acid based scaffold combination. Acta Orthop. Traumatol. Turc. 2019, 53, 372–375. [Google Scholar] [CrossRef]
- Tahta, M.; Akkaya, M.; Gursoy, S.; Isik, C.; Bozkurt, M. Arthroscopic treatment of osteochondral lesions of the talus: Nanofracture versus hyaluronic acid-based cell-free scaffold with concentration of autologous bone marrow aspirate. J. Orthop. Surg. 2017, 25, 2309499017717870. [Google Scholar] [CrossRef]
- Kanatlı, U.; Eren, A.; Eren, T.K.; Vural, A.; Geylan, D.E.; Öner, A.Y. Single-Step Arthroscopic Repair with Cell-Free Polymer-Based Scaffold in Osteochondral Lesions of the Talus: Clinical and Radiological Results. Arthroscopy 2017, 33, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- El Masry, S.; Boden, A.L.; DiGiovanni, G.M.; Cororaton, A.D.; Ellis, S.J. A Comparison of PROMIS Scores of Metatarsophalangeal Joint Arthrodesis and Polyvinyl Alcohol Hydrogel Implant Hemiarthroplasty for Hallux Rigidus. JB JS Open Access 2024, 9, e23. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, A.T.; Chopra, A.; Madi, N.M.; Tabarestani, T.Q.; Fletcher, A.N.; Parekh, S.G. Polyvinyl Alcohol Hydrogel Hemiarthroplasty of First Metatarsophalangeal Joint Hallux Rigidus. Cureus 2024, 16, e58583. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.J.; Dhillon, R.; Wingo, T.; Lin, C.C.; Samsonov, A.P.; Azam, M.T.; Kennedy, J.G. Polyvinyl alcohol hydrogel implant for the treatment of hallux rigidus is associated with a high complication rate and moderate failure rate at short-term follow-up: A systematic review. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 1765–1778. [Google Scholar] [CrossRef]
- Mermerkaya, M.U.; Adli, H. A comparison between metatarsal head-resurfacing hemiarthroplasty and total metatarsophalangeal joint arthroplasty as surgical treatments for hallux rigidus: A retrospective study with short- to midterm follow-up. Clin. Interv. Aging 2016, 11, 1805–1813. [Google Scholar] [CrossRef]
- Nurmukhametov, M.R.; Makarov, M.A.; Makarov, S.A.; Bialik, E.I.; Biaik, V.E.; Nesterenko, V.A. The Use of Autologous Matrix-Induced Chondrogenesis as a Surgical Treatment for Patients with the First Metatarsophalangeal Joint Osteoarthritis: Immediate and Medium-Term Results. Cartilage 2021, 13, 1354s–1365s. [Google Scholar] [CrossRef]
- Shimozono, Y.; Hurley, E.T.; Kennedy, J.G. Early Failures of Polyvinyl Alcohol Hydrogel Implant for the Treatment of Hallux Rigidus. Foot Ankle Int. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Verdonk, P.; Drobnic, M.; Dulic, O.; Gavrilovic, G.; Patrascu, J.M.; Zaslav, K.; Kwiatkowski, G.; Altschuler, N.; et al. Aragonite-Based Scaffold for the Treatment of Joint Surface Lesions in Mild to Moderate Osteoarthritic Knees: Results of a 2-Year Multicenter Prospective Study. Am. J. Sports Med. 2021, 49, 588–598. [Google Scholar] [CrossRef]
- Condello, V.; Filardo, G.; Madonna, V.; Andriolo, L.; Screpis, D.; Bonomo, M.; Zappia, M.; Dei Giudici, L.; Zorzi, C. Use of a Biomimetic Scaffold for the Treatment of Osteochondral Lesions in Early Osteoarthritis. Biomed. Res. Int. 2018, 2018, 7937089. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Ndreu Halili, A.; Engin Vrana, N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Pravata, V.M.; Omelková, M.; Stavridis, M.P.; Desbiens, C.M.; Stephen, H.M.; Lefeber, D.J.; Gecz, J.; Gundogdu, M.; Õunap, K.; Joss, S.; et al. An intellectual disability syndrome with single-nucleotide variants in O-GlcNAc transferase. Eur. J. Hum. Genet. 2020, 28, 706–714. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Shanmugavadivu, A.; Lekhavadhani, S.; Babu, S.; Suresh, N.; Selvamurugan, N. Magnesium-incorporated biocomposite scaffolds: A novel frontier in bone tissue engineering. J. Magnes. Alloys 2024, 12, 2231–2248. [Google Scholar] [CrossRef]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.; Guldberg, R.; Stayton, P.S. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Rothe, R.; Hauser, S.; Neuber, C.; Laube, M.; Schulze, S.; Rammelt, S.; Pietzsch, J. Adjuvant Drug-Assisted Bone Healing: Advances and Challenges in Drug Delivery Approaches. Pharmaceutics 2020, 12, 428. [Google Scholar] [CrossRef]
- Gaihre, B.; Jayasuriya, A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 330–339. [Google Scholar] [CrossRef]
- Abdollahi, F.; Saghatchi, M.; Paryab, A.; Malek Khachatourian, A.; Stephens, E.D.; Toprak, M.S.; Badv, M. Angiogenesis in bone tissue engineering via ceramic scaffolds: A review of concepts and recent advancements. Biomater. Adv. 2024, 159, 213828. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Qian, E.; Kang, Y. Effects of Macro-/Micro-Channels on Vascularization and Immune Response of Tissue Engineering Scaffolds. Cells 2021, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Vajda, J.; Milojević, M.; Maver, U.; Vihar, B. Microvascular Tissue Engineering-A Review. Biomedicines 2021, 9, 589. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, C.; He, L.; Zhou, J.; Chen, T.; Ouyang, L.; Guo, X.; Qu, Y.; Luo, Z.; Duan, D. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact. Mater. 2022, 16, 271–284. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Tu, Z.; Dai, W.; Xue, Y.; Tang, C.; Gao, W.; Mao, C.; Lei, B.; Lin, C. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics 2020, 10, 4929–4943. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y.; Sheng, S.; Yang, H.; Li, Z.; Han, Q.; Zhang, Q.; Su, J. DNA-based hydrogels for bone regeneration: A promising tool for bone organoids. Mater. Today Bio 2025, 31, 101502. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Baidya, A.; Annabi, N. Molecular design of an ultra-strong tissue adhesive hydrogel with tunable multifunctionality. Bioact. Mater. 2023, 29, 214–229. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Griffin, S.; Rathi, H.; Gupta, A.; Sharma, B.; van Bavel, D. Cost-effectiveness analysis of arthroscopic injection of a bioadhesive hydrogel implant in conjunction with microfracture for the treatment of focal chondral defects of the knee—An Australian perspective. J. Med. Econ. 2022, 25, 712–721. [Google Scholar] [CrossRef]
- Dumville, J.C.; Stubbs, N.; Keogh, S.J.; Walker, R.M.; Liu, Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 2015, Cd011226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef]
- Li, C.S.; Xu, Y.; Li, J.; Qin, S.H.; Huang, S.W.; Chen, X.M.; Luo, Y.; Gao, C.T.; Xiao, J.H. Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration. Biomed. Eng. Online 2025, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xin, W.; Ji, J.; Xu, J.; Zheng, L.; Qu, X.; Yue, B. 3D-Printed Hydrogels in Orthopedics: Developments, Limitations, and Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 845342. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Guan, Y.; Li, S. Innovative hydrogel solutions for articular cartilage regeneration: A comprehensive review. Int. J. Surg. 2024, 110, 7984–8001. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Baradaran, B.; de la Guardia, M.; Oroojalian, F.; Mokhtarzadeh, A. Cutting-edge progress and challenges in stimuli responsive hydrogel microenvironment for success in tissue engineering today. J. Control. Release 2020, 328, 514–531. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147.0. [Google Scholar] [CrossRef]
- Altschuler, N.; Zaslav, K.R.; Di Matteo, B.; Sherman, S.L.; Gomoll, A.H.; Hacker, S.A.; Verdonk, P.; Dulic, O.; Patrascu, J.M.; Levy, A.S.; et al. Aragonite-Based Scaffold Versus Microfracture and Debridement for the Treatment of Knee Chondral and Osteochondral Lesions: Results of a Multicenter Randomized Controlled Trial. Am. J. Sports Med. 2023, 51, 957–967. [Google Scholar] [CrossRef]
- de Caro, F.; Vuylsteke, K.; Van Genechten, W.; Verdonk, P. Acellular Aragonite-Based Scaffold for the Treatment of Joint Surface Lesions of the Knee: A Minimum 5-Year Follow-Up Study. Cartilage 2024, 15, 399–406. [Google Scholar] [CrossRef]
- Conte, P.; Anzillotti, G.; Crawford, D.C.; Dasa, V.; Flanigan, D.C.; Nordt, W.E.; Scopp, J.M.; Meislin, R.J.; Strauss, E.J.; Strickland, S.M.; et al. Differential analysis of the impact of lesions’ location on clinical and radiological outcomes after the implantation of a novel aragonite-based scaffold to treat knee cartilage defects. Int. Orthop. 2024, 48, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide-Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Three-dimensional printing of extracellular matrix (ECM)-mimicking scaffolds: A critical review of the current ECM materials. J. Biomed. Mater. Res. A 2020, 108, 2324–2350. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D bioprinting in tissue engineering and regenerative medicine: Current progress and challenges. Int. J. Bioprint. 2023, 9, 759. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Dong, Q.; Fei, X.; Zhang, H.; Zhu, X.; Ruan, J. Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3879. [Google Scholar] [CrossRef]

| Type | Advantages | Disadvantages | Examples | |
|---|---|---|---|---|
| Natural | Strong Biocompatibility | Processing Difficulties | Alginate, Chitosan, Collagen, Gelatin, Hyaluronic Acid | [8,29,31,42,62] |
| Synthetic | Standardized Processing, Limited Variability | Biocompatibility Issues | PGA, PLA, PLGA, PVA, PEO, PEG, PCL | [7,73,74,84,85,86] |
| Hybrid | Strong Mechanical and Chemical Properties | Minimal | GelMA, GelnHA | [50,93,95,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralph, J.E.; Lauck, B.J.; Colson, C.B.; Ebangwese, S.; O’Neill, C.N.; Anastasio, A.T.; Adams, S.B. Current Utilization of Gel-Based Scaffolds and Templates in Foot and Ankle Surgery—A Review. Gels 2025, 11, 316. https://doi.org/10.3390/gels11050316
Ralph JE, Lauck BJ, Colson CB, Ebangwese S, O’Neill CN, Anastasio AT, Adams SB. Current Utilization of Gel-Based Scaffolds and Templates in Foot and Ankle Surgery—A Review. Gels. 2025; 11(5):316. https://doi.org/10.3390/gels11050316
Chicago/Turabian StyleRalph, Julia E., Bradley J. Lauck, Charles B. Colson, Santita Ebangwese, Conor N. O’Neill, Albert T. Anastasio, and Samuel B. Adams. 2025. "Current Utilization of Gel-Based Scaffolds and Templates in Foot and Ankle Surgery—A Review" Gels 11, no. 5: 316. https://doi.org/10.3390/gels11050316
APA StyleRalph, J. E., Lauck, B. J., Colson, C. B., Ebangwese, S., O’Neill, C. N., Anastasio, A. T., & Adams, S. B. (2025). Current Utilization of Gel-Based Scaffolds and Templates in Foot and Ankle Surgery—A Review. Gels, 11(5), 316. https://doi.org/10.3390/gels11050316









