Preparation and Performance of PVDF-HFP/PAN-Based Gel Polymer Electrolytes
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
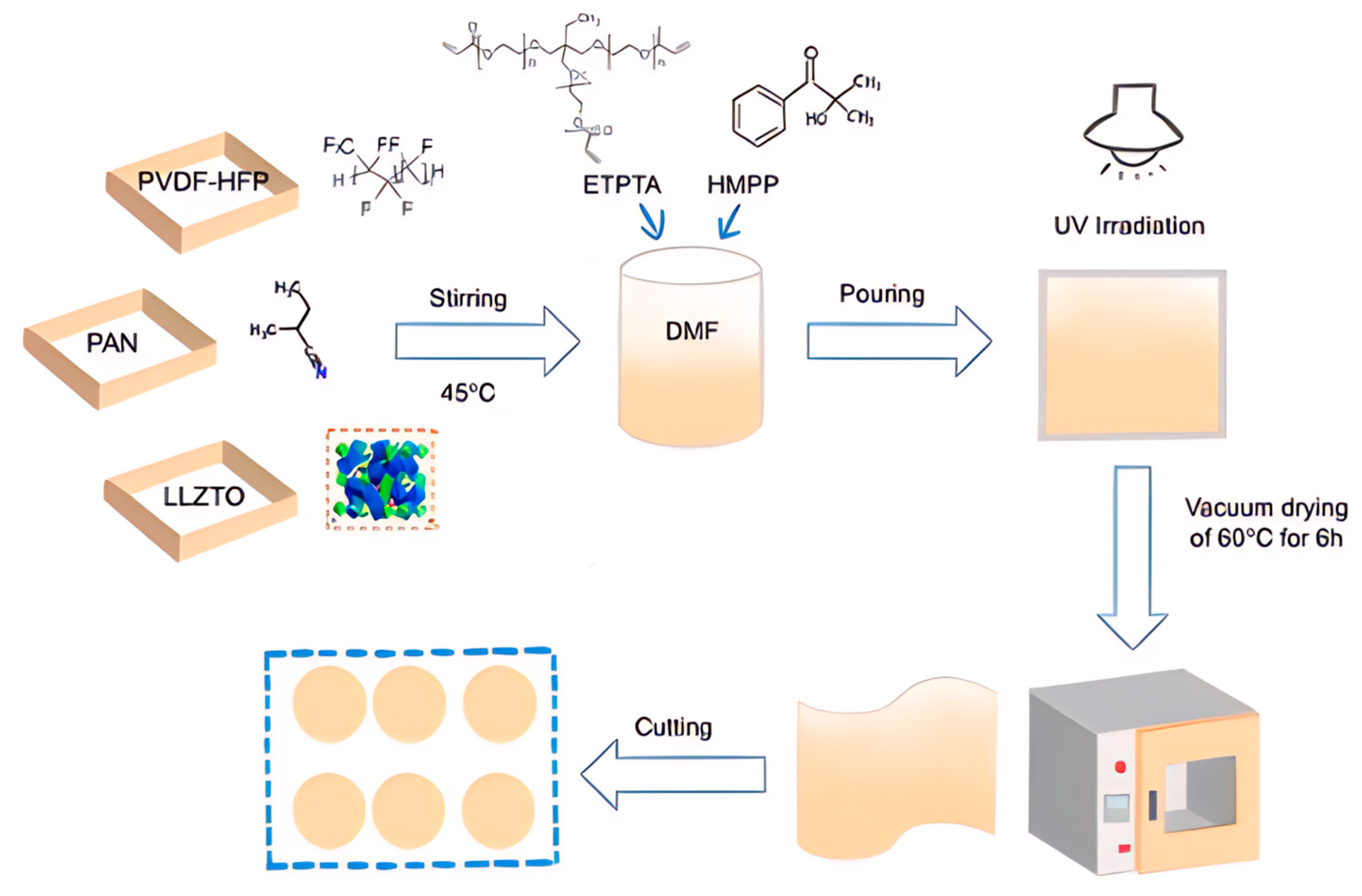
4.1. Preparation of Gel Polymer Electrolytes (GPEs)
4.2. Material Characterization
4.3. Electrochemical Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, H.; Zhang, N.; Lu, Y.; Zhang, Z.; Li, M.; Liu, J.; Zhang, N.; Song, W.; Zhao, Y.; Miao, Z. Strategies toward the development of high-energy-density lithium batteries. Microelectron. J. 2024, 88, 111666. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Liu, R.; Xue, J.; Xie, L.; Chen, H.; Deng, Z.; Yin, W. A Flexible Yet Robust 3D-Hybrid Gel Solid-State Electrolyte Based on Metal–Organic Frameworks for Rechargeable Lithium Metal Batteries. Gels 2024, 10, 812. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Xu, S.; Song, H. Stretchable ionic conductive gels for wearable human-activity detection. Chem. Eng. J. 2024, 489, 151231. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Lei, Y.; Jaumaux, P.; Tian, H.; Wang, G. Quasi-Solid Gel Electrolytes for Alkali Metal Battery Applications. Nano-Micro Lett. 2025, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mo, Z.; Liu, Z.; Hu, Y.; Du, C.; Liang, L.; Liu, Z.; Chen, G. Robust, Efficient, and Recoverable Thermocells with Zwitterion-Boosted Hydrogel Electrolytes for Energy-Autonomous and Wearable Sensing. Angew. Chem. Int. Ed. 2024, 63, e202405357. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Ding, D.; Tang, S.; Ma, Z.; Liu, C.; Yuan, Y. Solvent-Triggered, Ultra-Adhesive, Conductive, and Biocompatible Transition Gels for Wearable Devices. Small 2024, 20, 2310731. [Google Scholar] [CrossRef]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive Ionic Conductive Alginate/Gelatin Organohydrogels with Tunable Functions. Adv. Funct. Mater. 2024, 34, 2410663. [Google Scholar] [CrossRef]
- Kainat, S.; Anwer, J.; Hamid, A.; Gull, N.; Khan, S.M. Electrolytes in Lithium-Ion Batteries: Advancements in the Era of Twenties (2020’s). Mater. Chem. Phys. 2024, 313, 13. [Google Scholar] [CrossRef]
- Ji, W.; Luo, B.; Wang, Q.; Yu, G.; Zhang, Z.; Tian, Y.; Zhao, Z.; Zhao, R.; Wang, S.; Wang, X. Interface engineering enabling thin lithium metal electrodes down to 0.78 μm for garnet-type solid-state batteries. Nat. Commun. 2024, 15, 9920. [Google Scholar] [CrossRef]
- Hu, J.K.; Gao, Y.C.; Yang, S.J.; Wang, X.L.; Chen, X.; Liao, Y.L.; Li, S.; Liu, J.; Yuan, H.; Huang, J.Q. High Energy Density Solid-State Lithium Metal Batteries Enabled by In Situ Polymerized Integrated Ultrathin Solid Electrolyte/Cathode. Adv. Funct. Mater. 2024, 34, 2311633. [Google Scholar] [CrossRef]
- Gao, M.; Li, H.; Xu, L.; Xue, Q.; Wang, X.; Bai, Y.; Wu, C. Lithium metal batteries for high energy density: Fundamental electrochemistry and challenges. J. Energy Chem. 2021, 59, 666–687. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Meng, Y. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Castillo, J.; Robles-Fernandez, A.; Cid, R.; González-Marcos, J.A.; Armand, M.; Carriazo, D.; Zhang, H.; Santiago, A. Dehydrofluorination Process of Poly(vinylidene difluoride) PVdF-Based Gel Polymer Electrolytes and Its Effect on Lithium-Sulfur Batteries. Gels 2023, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Jiang, D.; Gao, J.; Cheng, L.; Li, G.; Luo, H.; Xu, Z.L.; Shin, D.M.; Wang, Y. Solid-State Electrolytes for Lithium Metal Batteries: State-of-the-Art and Perspectives. Adv. Funct. Mater. 2025, 35, 2411171. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent Advances in Biopolymer-Based Hydrogel Electrolytes for Flexible Supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef]
- Hao, Q.; Ma, X.; Gao, Y.; Chen, F.; Chen, X.; Qi, Y.; Li, N. Commercial carbonate based gel polymer electrolytes enable safe and stable high-voltage Li-metal batteries. Energy Storage Mater. 2024, 70, 103509. [Google Scholar] [CrossRef]
- Jia, R.; Wei, C.; Ma, B.; Li, L.; Yang, C.; Wang, B.; Tan, L.; Feng, J. Biopolymer-Based Gel Electrolytes for Advanced Zinc Ion Batteries: Progress and Perspectives. Adv. Funct. Mater. 2025, 35, 2417498. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zeng, J.; Chen, Y.; Huang, Y.; Liu, J.; Gan, J.; Li, T.; Zhang, H.; Zhong, L.; et al. Biopolymer-based gel electrolytes for electrochemical energy Storage: Advances and prospects. Prog. Mater. Sci. 2024, 144, 101264. [Google Scholar] [CrossRef]
- St-Onge, V.; Cui, M.; Rochon, S.; Daigle, J.-C.; Claverie, J.P. Reducing crystallinity in solid polymer electrolytes for lithium-metal batteries via statistical copolymerization. Commun. Mater. 2021, 2, 83. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Faizan, M.; Ali, B.; Bhuyan, M.M.; Bari, G.A.K.M.R.; Nam, K.W. Review on the Polymeric and Chelate Gel Precursor for Li-Ion Battery Cathode Material Synthesis. Gels 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Islam, M.; Raut, B.; Yun, S.; Kim, H.Y.; Nam, K.-W. A comprehensive review of functional gel polymer electrolytes and applications in lithium-ion battery. Gels 2024, 10, 563. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Yang, H.; Zhao, Y.; Shan, Y. A review of composite polymer electrolytes for solid-state lithium-sulfur batteries: Synthesis methods, optimal design, and critical challenges. Chem. Eng. J. 2024, 484, 149433. [Google Scholar] [CrossRef]
- Ma, X.; Liu, M.; Wu, Q.; Guan, X.; Wang, F.; Liu, H.; Xu, J. Composite Electrolytes Prepared by Improving the Interfacial Compatibility of Organic–Inorganic Electrolytes for Dendrite-Free, Long-Life All-Solid Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 53828–53839. [Google Scholar] [CrossRef]
- Halder, B.; Mohamed, M.G.; Kuo, S.-W.; Elumalai, P. Review on composite polymer electrolyte using PVDF-HFP for solid-state lithium-ion battery. Mater. Today Chem. 2024, 36, 101926. [Google Scholar] [CrossRef]
- Jie, J.; Liu, Y.; Cong, L.; Zhang, B.; Lu, W.; Zhang, X.; Liu, J.; Xie, H.; Sun, L. High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. Joural Energy Chem. 2020, 29, 9. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Yang, C.; Han, P.; Zhang, L.; Gao, L.; Wu, Z.; Liu, B.; Liu, R. PVdF-HFP-Based Gel Polymer Electrolyte with Semi-Interpenetrating Networks For Dendrite-Free Lithium Metal Battery. Acta Metall. Sin. 2021, 34, 417–424. [Google Scholar] [CrossRef]
- Yadav, P.; Hosen, M.S.; Dammala, P.K.; Ivanchenko, P.; Van Mierlo, J.; Berecibar, M. Development of composite solid polymer electrolyte for solid-state lithium battery: Incorporating LLZTO in PVDF-HFP/LiTFSI. Solid State Ion. 2023, 399, 116308. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.; Kim, J.Y.; Kim, J.; Choi, M.J.; Han, S.; Kim, S.; Kim, W.; Jang, H.W.; Park, J. Unveiling crystal orientation-dependent interface property in composite cathodes for solid-state batteries by in situ microscopic probe. Nat. Commun. 2024, 15, 7947. [Google Scholar] [CrossRef]
- Lei, J.; Lu, H.; Chen, J.; Yang, J.; Nuli, Y.; Wang, J. Crosslinked polyacrylonitrile precursor for S@pPAN composite cathode materials for rechargeable lithium batteries. J. Energy Chem. 2022, 65, 186–193. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Z.; Cui, C.; Wang, H.; Cao, W.; Hou, Z.; Zhu, D.; Yang, Y.; Zhang, T. An Ultrathin, Flexible Solid Electrolyte with High Ionic Conductivity Enhanced by a Mutual Promotion Mechanism. ACS Appl. Mater. Interfaces 2022, 14, 45373–45381. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, T.; Lv, Y.; Fang, J.; Dong, S.; Yao, S. “Polymer in ceramic” type LLZTO/PEO/PVDF composite electrolyte with high lithium migration number for solid-state lithium batteries. Ionics 2024, 30, 787–798. [Google Scholar] [CrossRef]
- Shan, C.; Wang, R.; Zhang, X.; Li, L.; Sun, W.; Gao, M.; Pan, H.; Liu, Y. Remarkably improved ionic conductivity and lithium metal compatibility of LLZTO-Li4 (BH4) 3I composite solid-state electrolyte. J. Alloys Compd. 2024, 980, 173554. [Google Scholar] [CrossRef]
- Busche, M.R.; Weiss, M.; Leichtweiss, T.; Fiedler, C.; Drossel, T.; Geiss, M.; Kronenberger, A.; Weber, D.A.; Janek, J. The Formation of the Solid/Liquid Electrolyte Interphase (SLEI) on NASICON-Type Glass Ceramics and LiPON. Adv. Mater. Interfaces 2020, 7, 2000380. [Google Scholar] [CrossRef]
- Seol, J.-c.; Balasubramaniam, R.; Aravindan, V.; Thangavel, R.; Lee, Y.-S. Ameliorating the electrode/electrolyte interface compatibility in Li-ion solid-state batteries with plasticizer. J. Alloys Compd. 2022, 927, 167077. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Zhang, J.G. Hierarchical porous silicon structures with extraordinary mechanical strength as high-performance lithium-ion battery anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef]
- Hun, Q.; Lan, L.; Lu, X.; Hu, Q.; Liang, X.; Guo, Y.; Wang, Y. Bilayer Heterostructure Electrolytes Were Prepared by a UV-Curing Process for High Temperature Lithium-Ion Batteries. Polymers 2024, 16, 2972. [Google Scholar] [CrossRef]
- Guan, D.; Huang, Y.; He, M.; Hu, G.; Du, K. Multilayer PEO/LLZTO composite electrolyte enables high-performance solid-state Li-ion batteries. Ionics 2021, 27, 4127–4134. [Google Scholar] [CrossRef]
- Zhuang, H.; Ma, W.; Xie, J.; Liu, X.; Li, B.; Jiang, Y.; Huang, S.; Chen, Z.; Zhao, B. Solvent-free synthesis of PEO/garnet composite electrolyte for high-safety all-solid-state lithium batteries. J. Alloys Compd. 2021, 860, 157915. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Pei, N.; Chen, Z.; Li, R.; Fu, L.; Zhang, P.; Zhao, J. The critical role of fillers in composite polymer electrolytes for lithium battery. Nano-Micro Lett. 2023, 15, 74. [Google Scholar] [CrossRef]
- Yoon, S.A.; Oh, N.R.; Yoo, A.R.; Lee, H.G.; Lee, H.C. Preparation and Characterization of Ta-substituted Li7La3Zr2-xO12 Garnet Solid Electrolyte by Sol-Gel Processing. J. Korean Ceram. Soc. 2017, 54, 278–284. [Google Scholar] [CrossRef]
- Tong, B.; Song, Z.; Wu, H.; Wang, X.; Feng, W.; Zhou, Z.; Zhang, H. Ion transport and structural design of lithium-ion conductive solid polymer electrolytes:a perspective. Mater. Future 2022, 1, 042103. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, X.; Xiang, Y.; Liu, K. Strategies to enhance Li^(+) transference number in liquid electrolytes for better lithium batteries. Nano Res. 2023, 16, 8055–8071. [Google Scholar] [CrossRef]
- Lee, Y.G.; Hong, S.; Pan, B.; Wu, X.; Dickey, E.C.; Whitacre, J.F. Improving Bulk and Interfacial Lithium Transport in Garnet-Type Solid Electrolytes through Microstructure Optimization for High-Performance All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2024, 16, 60340–60347. [Google Scholar] [CrossRef]
- Xu, K.; Xu, C.; Jiang, Y.; Cai, J.; Ni, J.; Lai, C. Sandwich structured PVDF-HFP-based composite solid electrolytes for solid-state lithium metal batteries. Ionics 2022, 28, 3243–3253. [Google Scholar] [CrossRef]
- Duan, T.; Cheng, H.; Liu, Y.; Sun, Q.; Nie, W.; Lu, X.; Dong, P.; Song, M.-K. A multifunctional Janus layer for LLZTO/PEO composite electrolyte with enhanced interfacial stability in solid-state lithium metal batteries. Energy Storage Mater. 2024, 65, 103091. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, R.; Nie, Q.; Zhao, P.; Li, Y.; Hong, Y.; Chen, S.; Li, C.; Sun, B.; Fan, H. Synergetic regulation of SEI mechanics and crystallographic orientation for stable lithium metal pouch cells. Nat. Commun. 2024, 15, 4454. [Google Scholar] [CrossRef]
- Hood, Z.D.; Chen, X.; Sacci, R.L.; Liu, X.; Veith, G.M.; Mo, Y.; Niu, J.; Dudney, N.J.; Chi, M. Elucidating Interfacial Stability between Lithium Metal Anode and Li Phosphorus Oxynitride via In Situ Electron Microscopy. Nano Lett. 2021, 21, 151–157. [Google Scholar] [CrossRef] [PubMed]



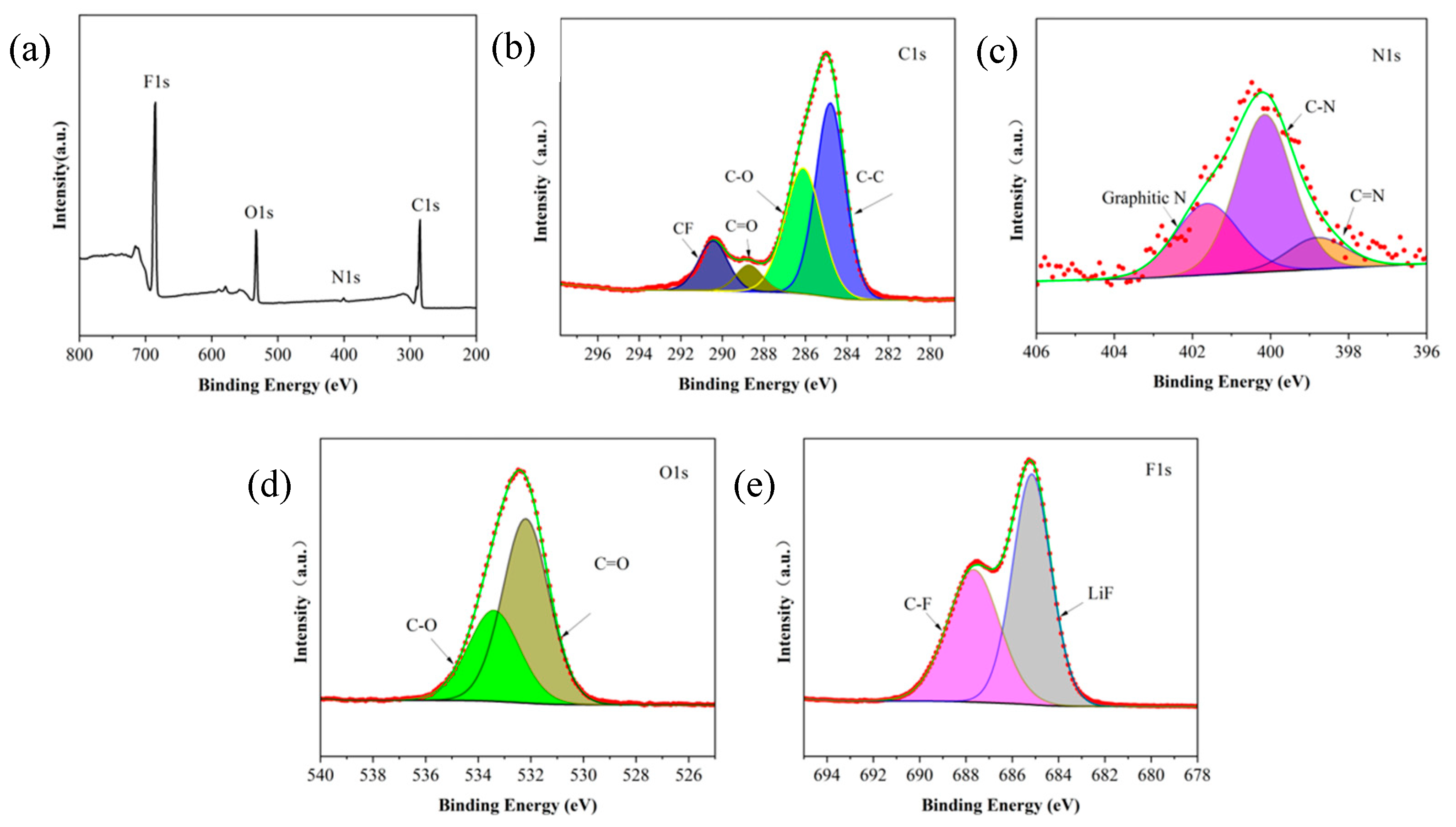


| GPEs | Mechanical Stability (MPa) | Ion Conductivity (S/cm) | Electrochemical Window (V) | Discharge Capacities (mAh/g) | Capacity Retention (0.2 C, 200 Cycles) |
|---|---|---|---|---|---|
| PE | 2.16 | 9.1 × 10−5 | 4.3 | 120.1 | short-circuited after 143 cycles |
| PPE | 3.1 | 6.1 × 10−5 | 4.4 | 135.2 | 53.4% |
| PPEL | 4.35 | 2.8 × 10−4 | 4.8 | 164.3 | 89.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Lan, L.; Hun, Q.; Lu, X.; Wei, J.; Liang, X.; Shen, P.; Long, Y.; Guo, Y. Preparation and Performance of PVDF-HFP/PAN-Based Gel Polymer Electrolytes. Gels 2025, 11, 317. https://doi.org/10.3390/gels11050317
Yao X, Lan L, Hun Q, Lu X, Wei J, Liang X, Shen P, Long Y, Guo Y. Preparation and Performance of PVDF-HFP/PAN-Based Gel Polymer Electrolytes. Gels. 2025; 11(5):317. https://doi.org/10.3390/gels11050317
Chicago/Turabian StyleYao, Xiubing, Lingxiao Lan, Qiankun Hun, Xuanan Lu, Jianghua Wei, Xinghua Liang, Pengcheng Shen, Ying Long, and Yifeng Guo. 2025. "Preparation and Performance of PVDF-HFP/PAN-Based Gel Polymer Electrolytes" Gels 11, no. 5: 317. https://doi.org/10.3390/gels11050317
APA StyleYao, X., Lan, L., Hun, Q., Lu, X., Wei, J., Liang, X., Shen, P., Long, Y., & Guo, Y. (2025). Preparation and Performance of PVDF-HFP/PAN-Based Gel Polymer Electrolytes. Gels, 11(5), 317. https://doi.org/10.3390/gels11050317






