Application of a Dopa Derivative for the Formation of Gels in the Presence of Commercial Surfactants
Abstract
1. Introduction
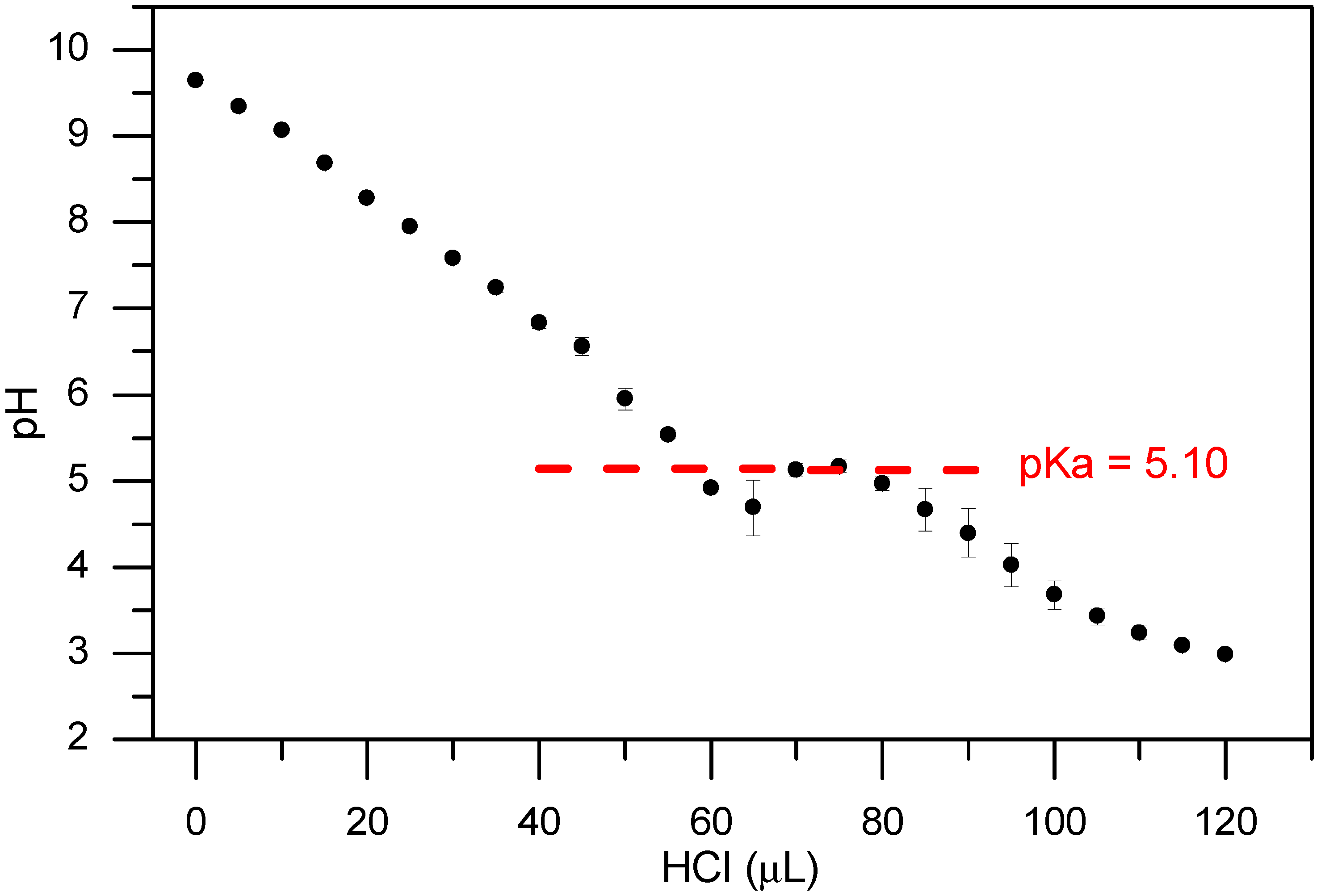
2. Results and Discussion
3. Conclusions
4. Materials and Methods
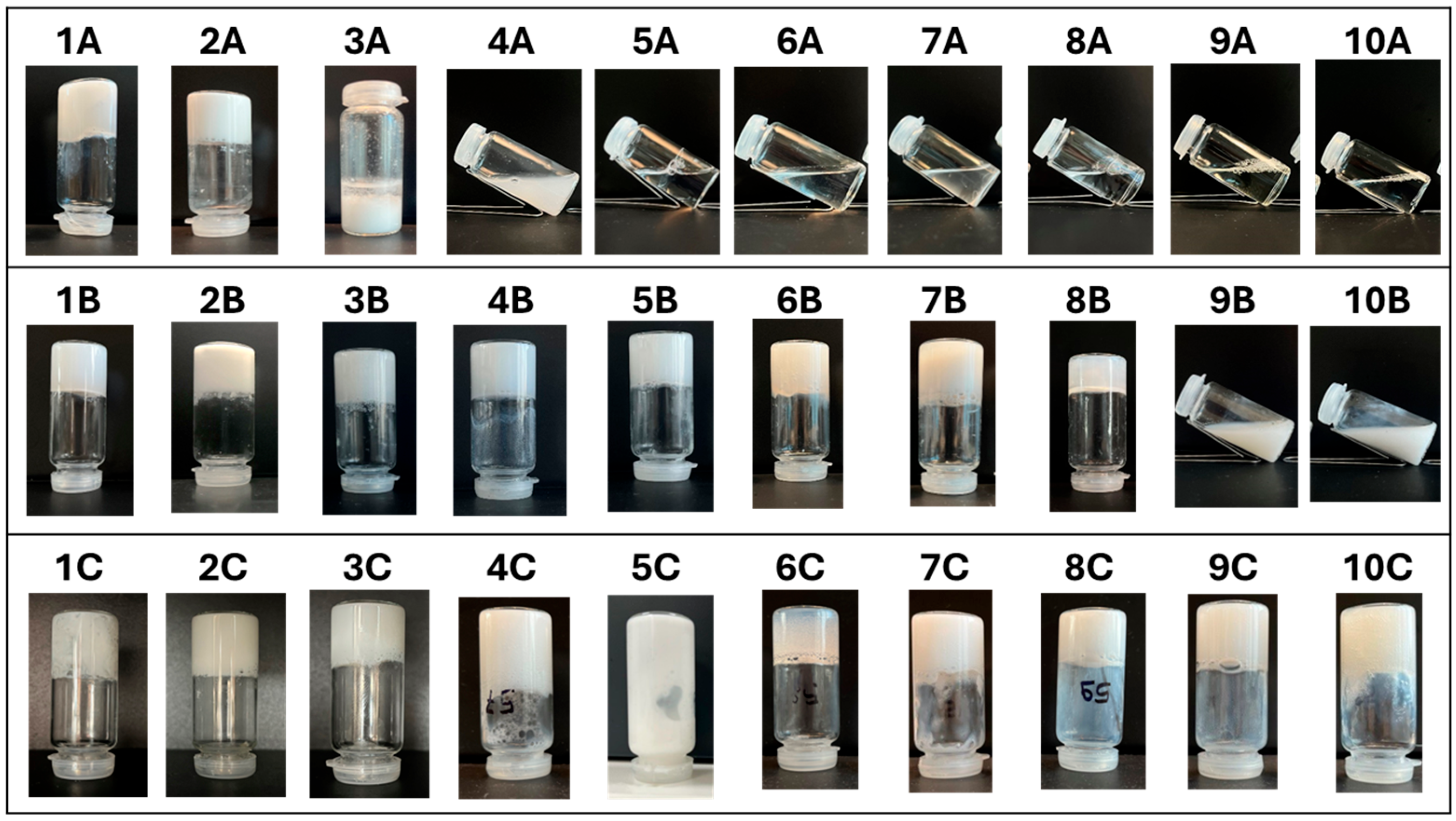
- Sample A contained 10% surfactant.
- Sample B contained 10% surfactant + 1% Boc-L-DOPA(Bn)2-OH (equivalent to 0.105 mmol for 5 mL of the sample).
- Sample C contained 10% surfactant + 1% Boc-L-DOPA(Bn)2-OH and 4.5% Cocamidopropyl betaine.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Boc-L-Dopa(Bn)2-OH | (S)-N-(tert-butyloxycarbonyl)-3,4-bis(benzyloxy)-phenylalanine |
| LMWG | Low molecular weight gelator |
| CAPB | Cocamidopropyl betaine |
| AAS | N-substituted Amino Acid Surfactant |
References
- De Castro, M.; Roque, C.S.; Loureiro, A.; Guimarães, D.; Silva, C.; Ribeiro, A.; Cavaco-Paulo, A.; Noro, J. Exploring Bio-Based Alternatives to Cyclopentasiloxane: Paving the Way to Promising Silicone Substitutes. Colloids. Surf. A Physicochem. Eng. Asp. 2025, 707, 135915. [Google Scholar] [CrossRef]
- Chadha, G.D.; Singh, R. Circular Beauty: Sustainable Resource Recovery and Waste Management in the Cosmetic Industry. In Proceedings of the Technological Advancements in Waste Management: Challenges and Opportunities, Dhanbad, India, 4–5 December 2023; Kumar, V., Dubey, B.K., Yadav, K.D., Eds.; Springer Nature: Singapore, 2025; pp. 379–399. [Google Scholar]
- Reichmuth, N.; Huber, P.; Ott, R. High-Acyl Gellan Gum as a Functional Polyacrylate Substitute in Emulsions High-Acyl Gellan Gum as a Functional Polyacrylate Substitute in Emulsions. SOFW J. 2019, 145, 36–39. [Google Scholar]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Semenzato, A. Evaluating Natural Alternatives to Synthetic Acrylic Polymers: Rheological and Texture Analyses of Polymeric Water Dispersions. ACS Omega 2020, 5, 15280–15289. [Google Scholar] [CrossRef]
- Wu, Q.; Cheng, N.; Fang, D.; Wang, H.; Rahman, F.-U.; Hao, H.; Zhang, Y. Recent Advances on Application of Polysaccharides in Cosmetics. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100004. [Google Scholar] [CrossRef]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Busata, L.; Semenzato, A. Characterization of Polysaccharidic Associations for Cosmetic Use: Rheology and Texture Analysis. Cosmetics 2021, 8, 62. [Google Scholar] [CrossRef]
- Hirst, A.R.; Coates, I.A.; Boucheteau, T.R.; Miravet, J.F.; Escuder, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Low-Molecular-Weight Gelators: Elucidating the Principles of Gelation Based on Gelator Solubility and a Cooperative Self-Assembly Model. J. Am. Chem. Soc. 2008, 130, 9113–9121. [Google Scholar] [CrossRef]
- Van Esch, J.H. We Can Design Molecular Gelators, but Do We Understand Them? Langmuir 2009, 25, 8392–8394. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Jones, C.D.; Kershaw Cook, L.J.; Slater, A.G.; Yufit, D.S.; Steed, J.W. Scrolling in Supramolecular Gels: A Designer’s Guide. Chem. Mater. 2024, 36, 2799–2809. [Google Scholar] [CrossRef]
- Morris, K.L.; Chen, L.; Rodger, A.; Adams, D.J.; Serpell, L.C. Structural Determinants in a Library of Low Molecular Weight Gelators. Soft Matter 2015, 11, 1174–1181. [Google Scholar] [CrossRef]
- Tang, C.; Smith, A.M.; Collins, R.F.; Ulijn, R.V.; Saiani, A. Fmoc-Diphenylalanine Self-ASsembly Mechanism Induces Apparent PK a Shifts. Langmuir 2009, 25, 9447–9453. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A New Method for Maintaining Homogeneity during Liquid-Hydrogel Transitions Using Low Molecular Weight Hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Chen, L.; Raeburn, J.; Sutton, S.; Spiller, D.G.; Williams, J.; Sharp, J.S.; Griffiths, P.C.; Heenan, R.K.; King, S.M.; Paul, A.; et al. Tuneable Mechanical Properties in Low Molecular Weight Gels. Soft Matter 2011, 7, 9721–9727. [Google Scholar] [CrossRef]
- Okesola, B.O.; Vieira, V.M.P.; Cornwell, D.J.; Whitelaw, N.K.; Smith, D.K. 1,3:2,4-Dibenzylidene-d-Sorbitol (DBS) and Its Derivatives-Efficient, Versatile and Industrially-Relevant Low-Molecular-Weight Gelators with over 100 Years of History and a Bright Future. Soft Matter 2015, 11, 4768–4787. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, R.; Kraatz, H.-B. Sonication-Induced Coiled Fibrous Architectures of Boc-L-Phe-L-Lys(Z)-OMe. Chem.—Eur. J. 2013, 19, 1769–1777. [Google Scholar] [CrossRef]
- Cenciarelli, F.; Pieraccini, S.; Masiero, S.; Falini, G.; Giuri, D.; Tomasini, C. Experimental Correlation between Apparent PKa and Gelation Propensity in Amphiphilic Hydrogelators Derived from L-Dopa. Biomacromolecules 2024, 25, 5058–5067. [Google Scholar] [CrossRef]
- Singh, R.S. Supramolecular Gels from Bolaamphiphilic Molecules. J. Mol. Liq. 2024, 394, 123772. [Google Scholar] [CrossRef]
- Das, D.; Dasgupta, A.; Roy, S.; Mitra, R.N.; Debnath, S.; Das, P.K. Water Gelation of an Amino Acid-Based Amphiphile. Chem.—Eur. J. 2006, 12, 5068–5074. [Google Scholar] [CrossRef]
- Mondal, B.; Gupta, V.K.; Hansda, B.; Bhoumik, A.; Mondal, T.; Majumder, H.K.; Edwards-Gayle, C.J.C.; Hamley, I.W.; Jaisankar, P.; Banerjee, A. Amino Acid Containing Amphiphilic Hydrogelators with Antibacterial and Antiparasitic Activities. Soft Matter 2022, 18, 7201–7216. [Google Scholar] [CrossRef]
- Wang, T.; Ménard-Moyon, C.; Bianco, A. Self-Assembly of Amphiphilic Amino Acid Derivatives for Biomedical Applications. Chem. Soc. Rev. 2022, 51, 3535–3560. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Rosa, M.; Diaferia, C.; Fernandes, C. A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels. Gels 2024, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Restu, W.K.; Nishida, Y.; Kataoka, T.; Morimoto, M.; Ishida, K.; Mizuhata, M.; Maruyama, T. Palmitoylated Amino Acids as Low-Molecular-Weight Gelators for Ionic Liquids. Colloid. Polym. Sci. 2017, 295, 1109–1116. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, X. A Low Molecular Weight Gel Formed by Cationic Surfactant 1-Dodecylpyridinium Bromide in Acetone/Water: Its Characterisation and Implication for Enzyme Immobilisation. Supramol. Chem. 2015, 27, 21–27. [Google Scholar] [CrossRef]
- Xie, H.; Asad Ayoubi, M.; Lu, W.; Wang, J.; Huang, J.; Wang, W. A Unique Thermo-Induced Gel-to-Gel Transition in a PH-Sensitive Small-Molecule Hydrogel. Sci. Rep. 2017, 7, 8456. [Google Scholar] [CrossRef]
- Raghavan, S.R. Distinct Character of Surfactant Gels: A Smooth Progression from Micelles to Fibrillar Networks. Langmuir 2009, 25, 8382–8385. [Google Scholar] [CrossRef] [PubMed]
- Oda, R.; Huc, I.; Candau, S.J. Gemini Surfactants as New, Low Molecular Weight Gelators of Organic Solvents and Water. Angew. Chem. Int. Ed. 1998, 37, 2689–2691. [Google Scholar] [CrossRef]
- Akin-Ige, F.; Amin, S. Stimuli-Responsive Bio-Based Surfactant-Polymer Gels. Colloids. Surf. A Physicochem. Eng. Asp. 2024, 703, 135149. [Google Scholar] [CrossRef]
- Leung, P.S.; Goddard, E.D. Gels from Dilute Polymer/Surfactant Solutions. Langmuir 1991, 7, 608–609. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Effects of Surfactants on Gel Behavior. Am. J. Drug Deliv. 2003, 1, 77–101. [Google Scholar] [CrossRef]
- Brizard, A.M.; Stuart, M.C.A.; van Esch, J.H. Self-Assembled Interpenetrating Networks by orthogonal Self Assembly of Surfactants And. Faraday Discuss 2009, 143, 345–357. [Google Scholar] [CrossRef]
- Heeres, A.; Van Der Pol, C.; Stuart, M.; Friggeri, A.; Feringa, B.L.; Van Esch, J. Orthogonal Self-Assembly of Low Molecular Weight Hydrogelators and Surfactants. J. Am. Chem. Soc. 2003, 125, 14252–14253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Liu, X.Y.; Strom, C.S.; Xiong, J.Y. Engineering of Small Molecule Organogels by Design of the Nanometer Structure of Fiber Networks. Adv. Mater. 2006, 18, 2574–2578. [Google Scholar] [CrossRef]
- Aramaki, K.; Koitani, S.; Takimoto, E.; Kondo, M.; Stubenrauch, C. Hydrogelation with a Water-Insoluble Organogelator-Surfactant Mediated Gelation (SMG). Soft Matter 2019, 15, 8896–8904. [Google Scholar] [CrossRef] [PubMed]
- Falcone, N.; Kraatz, H.B. Supramolecular Assembly of Peptide and Metallopeptide Gelators and Their Stimuli-Responsive Properties in Biomedical Applications. Chem. Eur. J. 2018, 24, 14316–14328. [Google Scholar] [CrossRef] [PubMed]
- Firipis, K.; Nisbet, D.R.; Franks, S.J.; Kapsa, R.M.I.; Pirogova, E.; Williams, R.J.; Quigley, A. Enhancing Peptide Biomaterials for Biofabrication. Polymers 2021, 13, 2590. [Google Scholar] [CrossRef]
- Datta, D.; Nagaraj, R.; Chaudhary, N. Water-Alcohol Bigels from Fatty Acylated Dipeptides. J. Phys. Chem. B 2020, 124, 577–588. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Shariati Pour, S.R.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. [Google Scholar] [CrossRef]
- Toronyi, A.Á.; Giuri, D.; Martiniakova, S.; Tomasini, C. Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics 2023, 10, 38. [Google Scholar] [CrossRef]
- Cenciarelli, F.; Falini, G.; Giuri, D.; Tomasini, C. Controlled Lactonization of O-Coumaric Esters Mediated by Supramolecular Gels. Gels 2023, 9, 350. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-assembling of fibers inside an injectable calcium phosphate bone cement: A feasibility study. Mater. Today Chem. 2022, 24, 100991–101004. [Google Scholar] [CrossRef]
- Hibbs, J. Anionic Surfactants. In Chemistry and Technology of Surfactants; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 91–132. ISBN 9780470988596. [Google Scholar]
- Hall-manning, T.J.; Holland, G.H.; Rennie, G.; Revell, P.; Hines, J.; Barratt, M.D.; Basketter, D.A. Skin Irritation Potential of Mixed Surfactant Systems. Food Chem. Toxicol. 1998, 36, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Clendennen, S.K.; Boaz, N.W. Betaine Amphoteric Surfactants-Synthesis, Properties, and Applications. In Biobased Surfactants: Synthesis, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–469. ISBN 9780128127056. [Google Scholar]
- Smith, W.P. Epidermal and Dermal Effects of Topical Lactic Acid. J. Am. Acad. Dermatol. 1996, 35, 388–391. [Google Scholar] [CrossRef]
- Alsaheb, R.A.A.; Aladdin, A.; Othman, Z.; Malek, R.A.; Leng, O.M.; Aziz, R.; Enshasy, H.A. El Lactic Acid Applications in Pharmaceutical and Cosmeceutical Industries. J. Chem. Pharm. Res. 2015, 7, 729–735. [Google Scholar]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of Supramolecular Gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological Properties of Peptide-Based Hydrogels for Biomedical and Other Applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Winter, H.H.; Mours, M. Rheology of Polymers Near Liquid-Solid Transitions. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1997; pp. 165–234. ISBN 3-540-62713-8. [Google Scholar]
- Liu, Y.; Winter, H.H.; Perry, S.L. Linear Viscoelasticity of Complex Coacervates. Adv. Colloid. Interface Sci. 2017, 239, 46–60. [Google Scholar] [CrossRef]
- Tanna, V.A.; Zhou, Y.; Winter, H.H. Effect of Platelet Size in a Soft Nanocomposite: Physical Gelation and Yielding. J. Rheol. 2018, 62, 791–800. [Google Scholar] [CrossRef]
- Gaucher, A.; Dutot, L.; Barbeau, O.; Hamchaoui, W.; Wakselman, M.; Mazaleyrat, J.P. Synthesis of Terminally Protected (S)-Β3-H-DOPA by Arndt-Eistert Homologation: An Approach to Crowned β-Peptides. Tetrahedron Asymmetry 2005, 16, 857–864. [Google Scholar] [CrossRef]
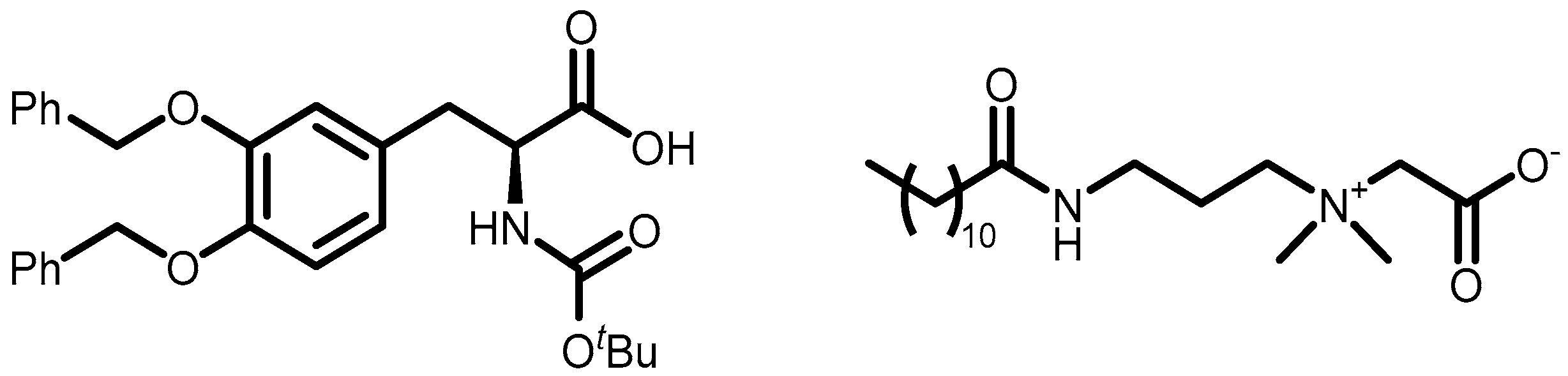


| Trade Name | Class | Number | Chemical Structure |
|---|---|---|---|
| EVERSOFTTM ACS | Alaninate | 1 | 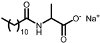 |
| GALSOFT SCG | Glycinate | 2 | 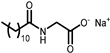 |
| PROTELAN AGL 95 | Akylglutamate | 3 | 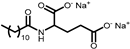 |
| PROTELAN LS 9011/SL | Sarcosinate | 4 | 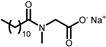 |
| PUREACT WS CONC | Taurate | 5 | 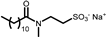 |
| STEPAN MILD® PCL | Sulphonate | 6 |  |
| ISELUX® | Alkylmethyl isothionate | 7 | 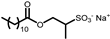 |
| ZETESOL MG FS | Alkylsulphate | 8 |  |
| SETACIN 103 SPEZIAL | Solphosuccinate | 9 |  |
| NANSA® LSS 38 AV | Olefinesulphonate | 10 |  |
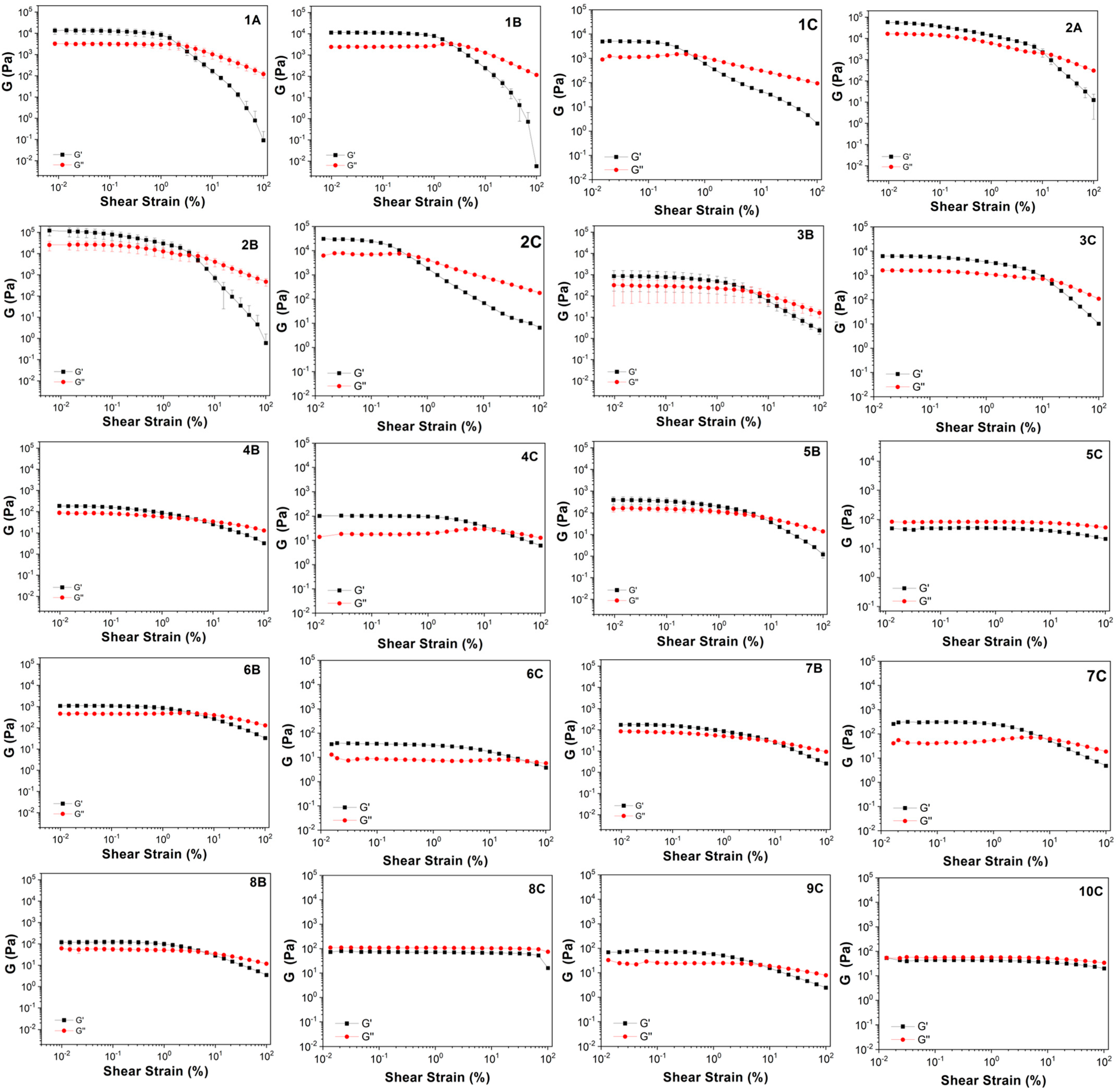
| Sample | G′ (Pa) | G″ (Pa) | tanδ | G* | η* (Pa) |
|---|---|---|---|---|---|
| 1A | 13,426.3 | 3207.2 | 0.248 | 13,811.7 | 2198.2 |
| 1B | 11,300.7 | 2420.4 | 0.215 | 11,557.0 | 1839.4 |
| 1C | 5037.4 | 1105.7 | 0.219 | 5157.3 | 820.8 |
| 2A | 49,688.7 | 15,778.3 | 0.317 | 52,134.0 | 8297.4 |
| 2B | 103,926.3 | 26,462.0 | 0.267 | 107,256.3 | 17,070.6 |
| 2C | 29,747.0 | 7826.8 | 0.263 | 30,760.0 | 4895.6 |
| 3B | 838.6 | 296.6 | 0.365 | 889.9 | 141.6 |
| 3C | 6179.6 | 1597.4 | 0.258 | 6382.7 | 1015.8 |
| 4B | 180.0 | 86.0 | 0.478 | 199.5 | 31.8 |
| 4C | 105.4 | 18.5 | 0.175 | 107.0 | 17.0 |
| 5B | 376.3 | 153.8 | 0.421 | 406.7 | 64.7 |
| 5C | 44.0 | 81.7 | 1.856 | 92.8 | 14.8 |
| 6B | 1094.6 | 457.2 | 0.424 | 1187.3 | 189.0 |
| 6C | 38.1 | 7.4 | 0.195 | 38.8 | 6.2 |
| 7B | 175.8 | 79.9 | 0.458 | 193.2 | 30.7 |
| 7C | 312.9 | 42.7 | 0.136 | 315.8 | 50.3 |
| 8B | 118.8 | 57.2 | 0.494 | 132.1 | 21.0 |
| 8C | 77.6 | 110.3 | 1.421 | 134.9 | 21.5 |
| 9C | 74.5 | 23.2 | 0.312 | 78.1 | 12.4 |
| 10C | 39.3 | 57.7 | 1.468 | 69.9 | 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinelli, S.; Cenciarelli, F.; Giuri, D.; Tomasini, C. Application of a Dopa Derivative for the Formation of Gels in the Presence of Commercial Surfactants. Gels 2025, 11, 320. https://doi.org/10.3390/gels11050320
Chinelli S, Cenciarelli F, Giuri D, Tomasini C. Application of a Dopa Derivative for the Formation of Gels in the Presence of Commercial Surfactants. Gels. 2025; 11(5):320. https://doi.org/10.3390/gels11050320
Chicago/Turabian StyleChinelli, Sofia, Fabia Cenciarelli, Demetra Giuri, and Claudia Tomasini. 2025. "Application of a Dopa Derivative for the Formation of Gels in the Presence of Commercial Surfactants" Gels 11, no. 5: 320. https://doi.org/10.3390/gels11050320
APA StyleChinelli, S., Cenciarelli, F., Giuri, D., & Tomasini, C. (2025). Application of a Dopa Derivative for the Formation of Gels in the Presence of Commercial Surfactants. Gels, 11(5), 320. https://doi.org/10.3390/gels11050320









