Simultaneous Hydrogen Production and Dye Decomposition in Alkaline Photocatalytic Process Using Calcined Xerogels of CuO-TiO2
Abstract
:1. Introduction
Recent Advances in Simultaneous H2 Evolution and Contaminant Degradation
2. Results and Discussion
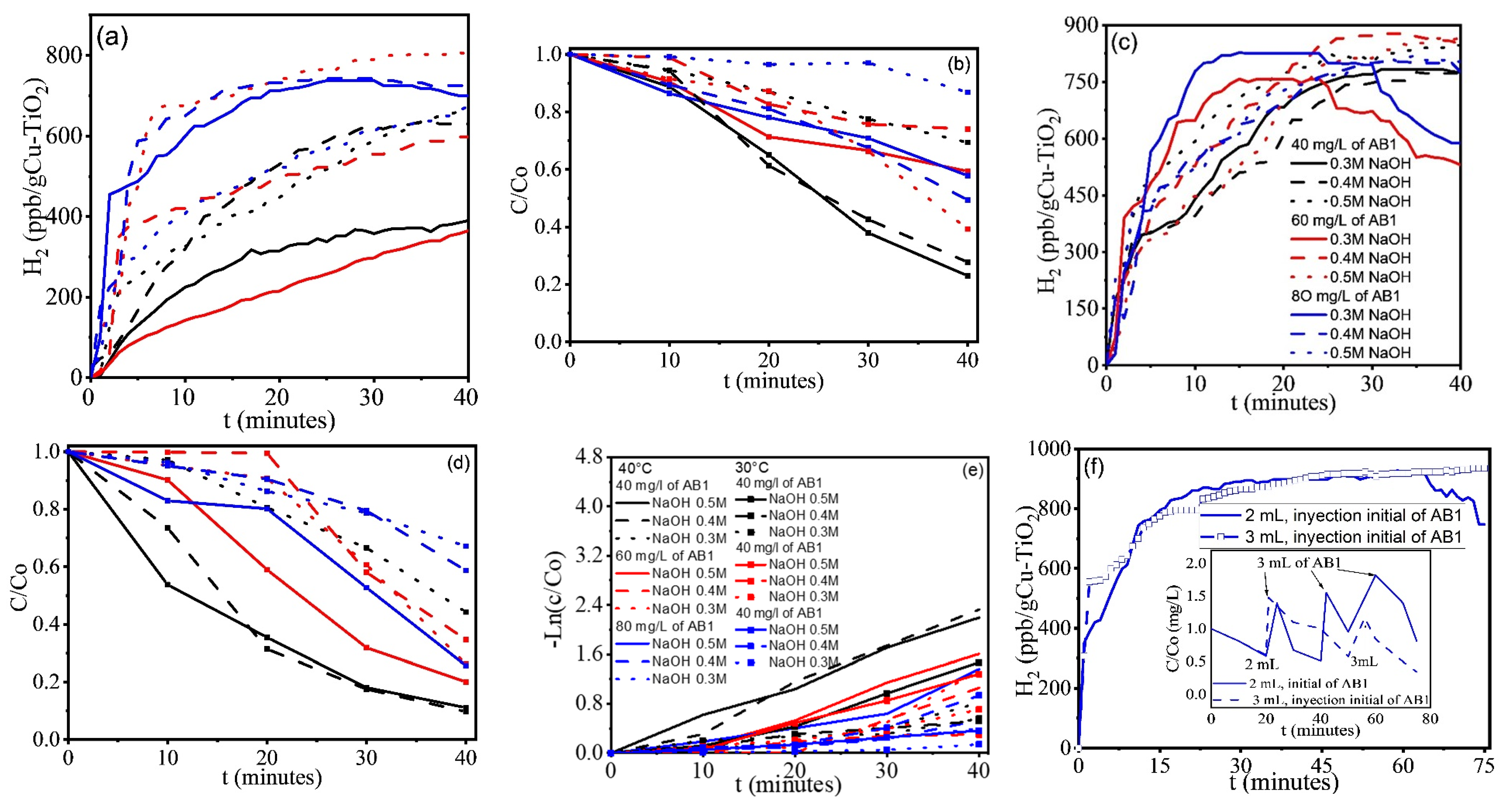
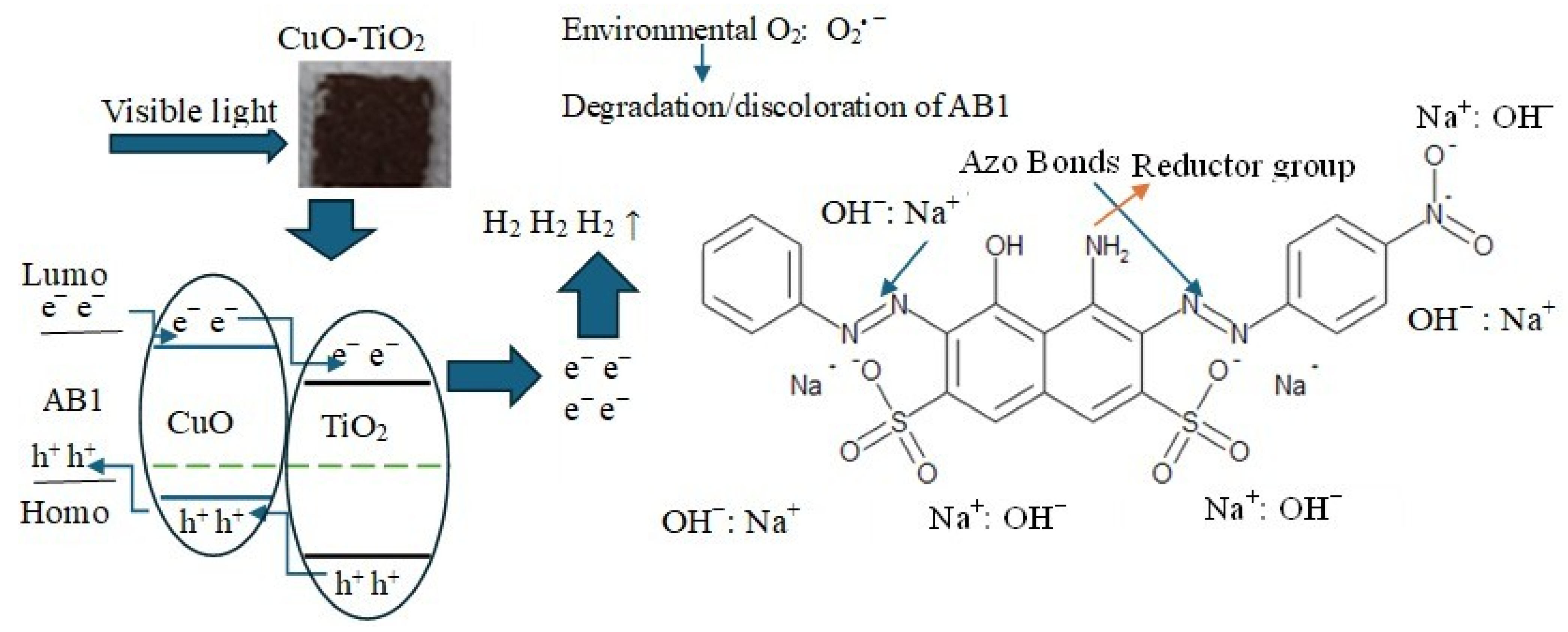
2.1. Hydrogen Evolution and Degradation of AB1

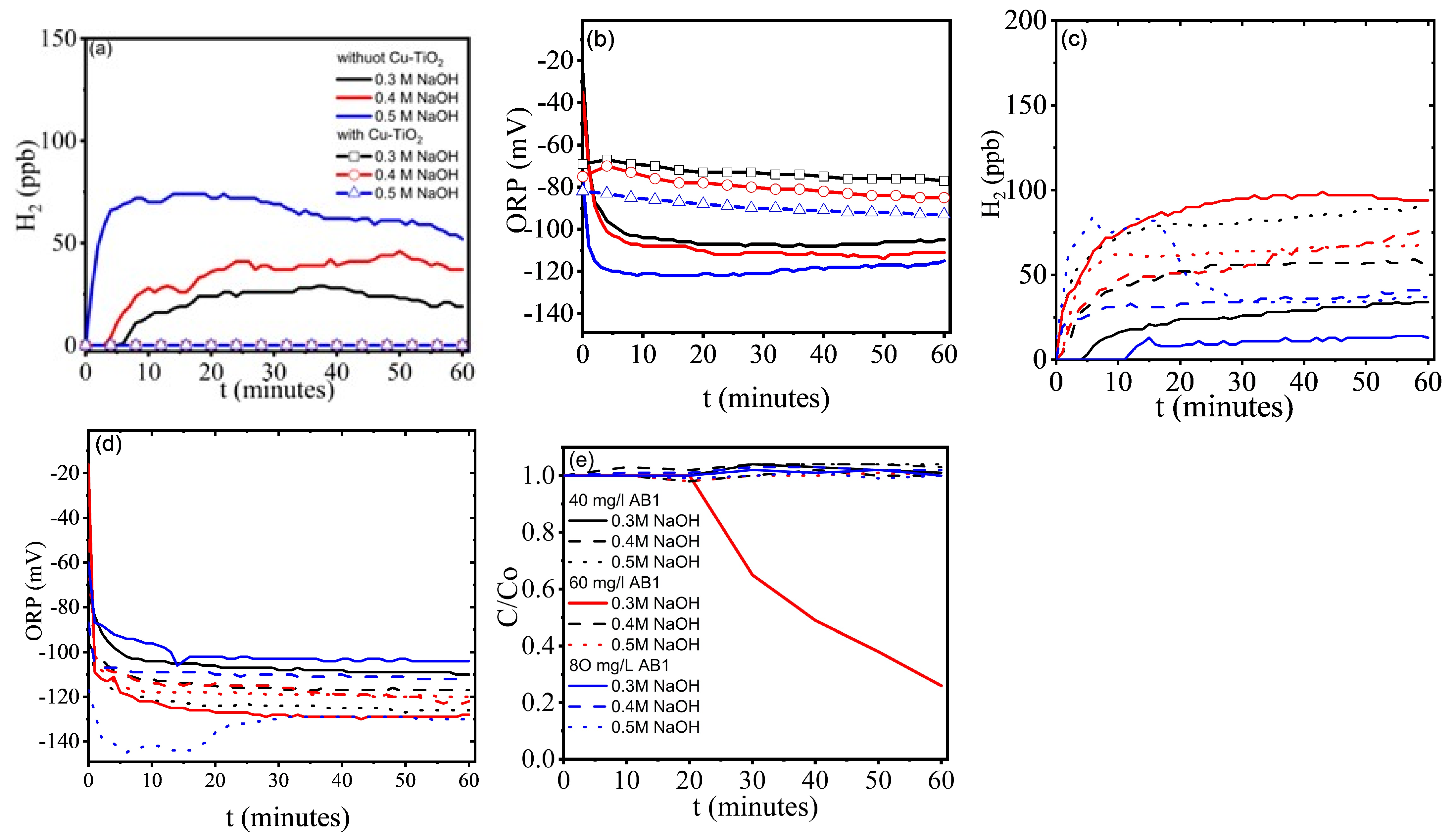
2.2. Discussion
- There are several routes for dye degradation, with the non-selective degradation by hydroxyl radicals being the most dominant in alkaline media.
- At temperatures greater than 40 °C, and even at 40 °C in some samples, the red coloration intensifies, and discoloration increases, possibly because higher temperatures reduce AB1 adsorption on the photocatalyst. Controlling the ambient temperature was a determining factor in increasing H2 production.
- The reaction pH shows minimal variation, likely because the processes taking place generate and consume OH−.
- The alkaline environment influences the blank tests: without dye and CuO-TiO2, a negative ORP develops over time.
- In the CuO-TiO2/NaOH blank tests, an increase in ORP and zero H2 production was observed.
3. Conclusions
4. Materials and Methods
4.1. Sol-Gel Synthesis of CuO-TiO2 Calcined Xerogels
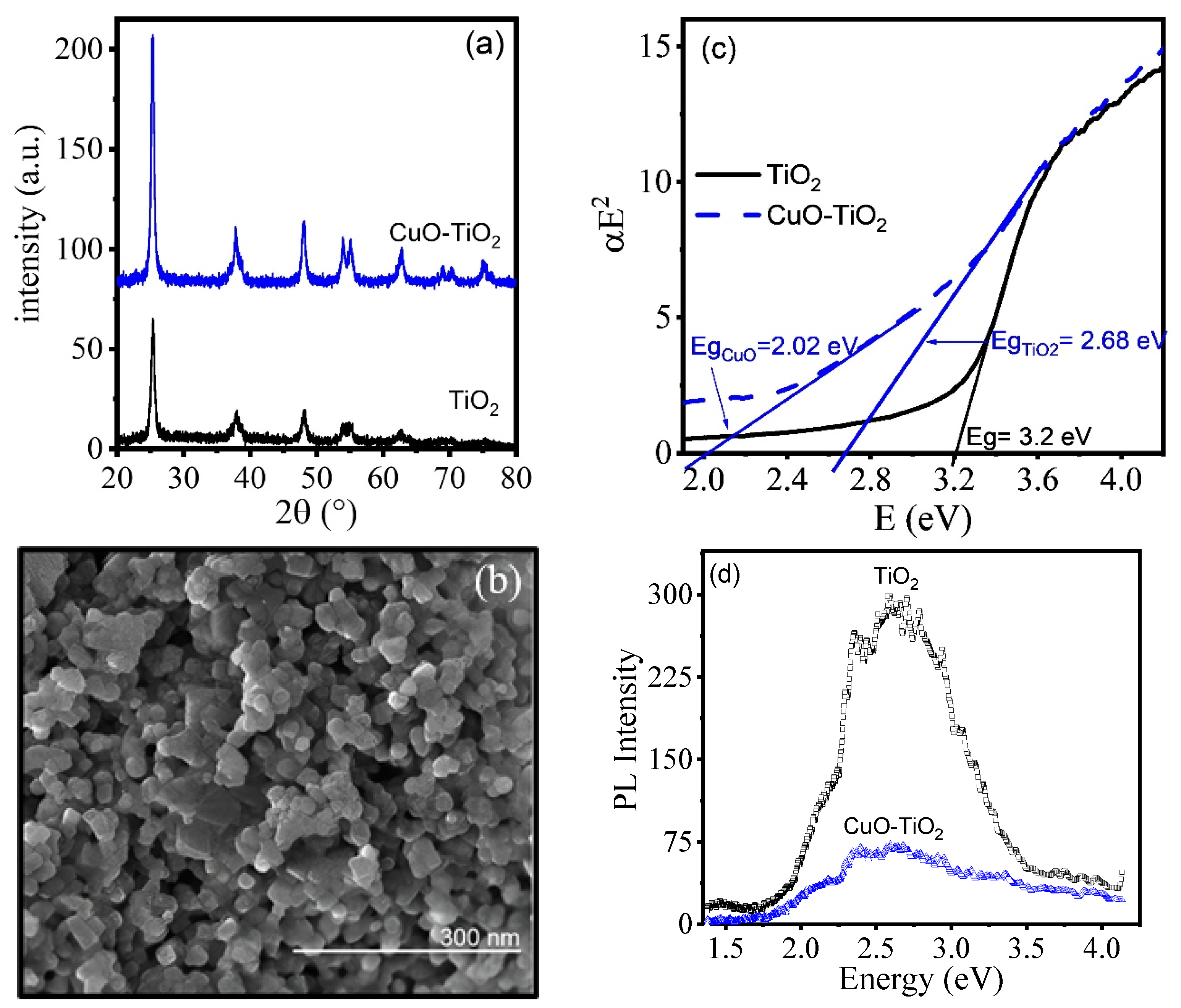
4.2. Characterization of CuO-TiO2 Calcinated Xerogels
4.3. H2 Evolution and AB1 Degradation Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Jang, J.; Yu, T. A facile aqueous-phase synthesis of Co-based nanostructures composed of cobalt hydroxide and cobalt oxide for enhanced photocatalytic activity of dye-sensitized H2 production. J. Alloys Compd. 2023, 942, 169078. [Google Scholar] [CrossRef]
- Olatomiwa, L.; Mekhilef, S.; Ismail, M.S.; Moghavvemi, M. Energy management strategies in hybrid renewable energy systems: A review. Renew. Sustain. Energy Rev. 2016, 62, 821–835. [Google Scholar] [CrossRef]
- Gomari, K.A.; Hafeez, H.Y.; Mohammed, J.; Dankawu, U.; Ndikilar, C.E.; Suleiman, A.B. A recent development and future prospect of g–C3N4–based photocatalyst for stable hydrogen (H2) generation via photocatalytic water-splitting. Int. J. Hydrogen Energy 2024, 85, 598–624. [Google Scholar] [CrossRef]
- Papiez, M.; Smiech, S.; Frodyma, K.; Borowiec, J. Decoupling is not enough-Evidence from fossil fuel use in over 130 countries. J. Clean. Prod. 2022, 379, 134856. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Al-Mazroai, L.S.; Dickinson, A.; Greaves, J.; James, D.; Millard, L.; Pedrono, F. Sustainable H2 gas production by photocatalysis. J. Photochem. Photobiol. A Chem. 2010, 216, 115–118. [Google Scholar] [CrossRef]
- Rand, D.A.J.; Dell, R.M. Hydrogen Energy, Challenges and Prospects; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Ramírez-Ortega, D.; Guerrero-Araque, D.; Acevedo-Peña, P.; Zanella, R. Producción fotocatalítica de hidrógeno empleando semiconductores modificados con nanopartículas metálicas. Mundo Nano 2023, 17, 1e–27e. [Google Scholar] [CrossRef]
- Ding, W.; Luo, J.; Gu, Q.; Liu, Z.-H. Ultrathin 2D ZnGa-borate-LDH nanosheets for boosting dye-sensitized photocatalytic coupled reaction of H2 production with pollutant degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130575. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, K.; Xu, M.; Fang, N.; Yu, W.; Chu, Y.; Shu, S. Photocatalysis for synergistic water remediation and H2 production: A review. Chem. Eng. J. 2023, 472, 145066. [Google Scholar] [CrossRef]
- Wongyongnoi, P.; Serivalsatit, K.; Hunsom, M.; Pruksathorn, K. Simultaneous green synthesis of H2 and decolorization of distillery effluent by photocatalysis via gold-decorated TiO2 photocatalysts. Int. J. Hydrogen Energy 2024, 80, 646–658. [Google Scholar] [CrossRef]
- Yang, L.; Hong, Y.; Liu, E.; Zhang, X.; Wang, L.; Lin, X.; Shi, J. Significant enhancement of photocatalytic H2 production simultaneous with dye degradation over Ni2P modified In2O3 nanocomposites. Sep. Purif. Technol. 2021, 263, 118366. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Shabanloo, A.; Fazlzadeh, M.; Poureshgh, Y. Investigation of operational parameters influencing in treatment of dye from water by electro-Fenton process. Desalin. Water Treat. 2016, 57, 24387–24394. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Piao, C.; Li, S.; Tang, J.; Fang, D.; Zhang, Z.; Wang, J. Construction of fixed Z-scheme Ag|AgBr/Ag/TiO2 photocatalyst composite film for malachite green degradation with simultaneous hydrogen production. J. Power Sources 2020, 469, 228430. [Google Scholar] [CrossRef]
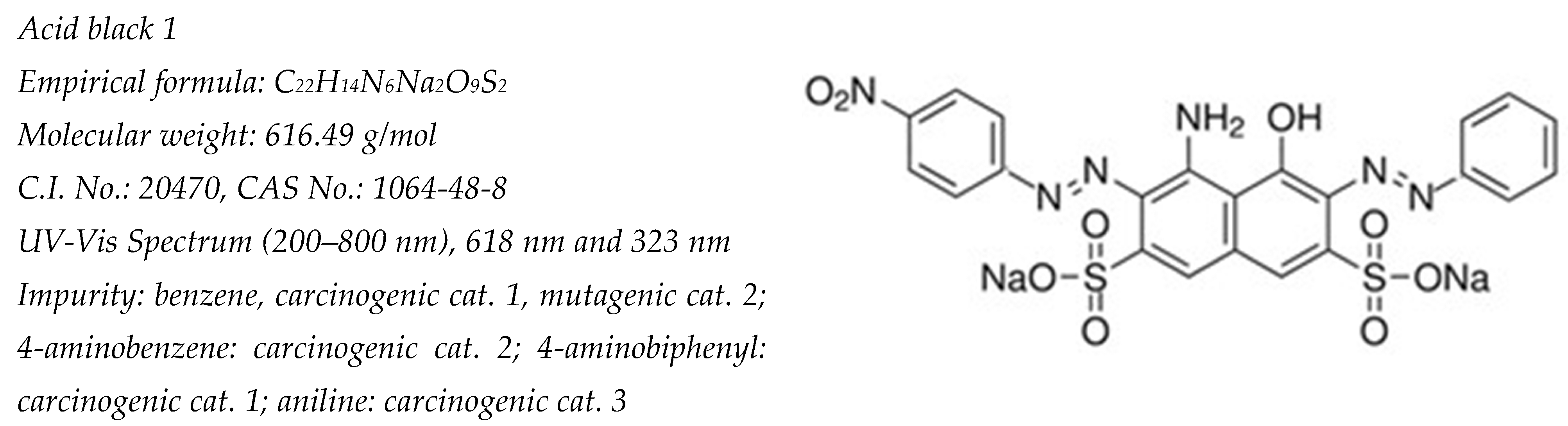
- European Commission. Directorate-General for Health and Consumers, Opinion on Acid Black 1 COLIPA n° B15, European Commission. 2010. Available online: https://data.europa.eu/doi/10.2772/22711 (accessed on 1 September 2024).
- Yan, J.; Wu, R.; Jin, G.; Jia, L.; Feng, G.; Tong, X. The hybrid Pt nanoclusters/Ru nanowires catalysts accelerating alkaline hydrogen evolution reaction. Adv. Powder Mater. 2024, 3, 100214. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Z.; Jin, T.; Jiang, T.; Ding, L.; Shen, S.; Zhang, J.; Zhong, W. An in-situ phase transformation induced mesoporous heterointerface for the alkaline hydrogen evolution reaction. RSC Adv. 2024, 14, 14886–14893. [Google Scholar] [CrossRef] [PubMed]
- Benedet, M.; Rizzi, G.A.; Gasparotto, A.; Zeng, L.; Pagot, G.; Olsson, E.; Di Noto, V.; Maccato, C.; Barreca, D. Efficient photoactivated hydrogen evolution promoted by CuxO–gCN–TiO2–Au (x = 1, 2) nanoarchitectures. RSC Adv. 2024, 14, 7221–7228. [Google Scholar] [CrossRef]
- Gholipour, M.R.; Dinh, C.T.; Béland, F.; Do, T.O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Zhang, Z.; Liao, S.; Deng, Y.; Wang, X.; Ye, Q.; Wang, K. Hydrothermal preparation of Sn3O4/TiO2 nanotube arrays as effective photocatalysts for boosting photocatalytic dye degradation and hydrogen production. Ceram. Int. 2023, 49, 5977–5985. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; Zhao, Y.; Deng, Y.; Yang, W.; Ye, Y.; Wang, K. Construction of Z-scheme Bi2O3/CeO2 heterojunction for enhanced photocatalytic capacity of TiO2 NTs. Spectrochim. Acta Part A 2024, 304, 123405. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Zhao, S.; Li, C.; Wang, R.; Cao, D.; Liu, G. Construction of Bi-assisted modified CdS/TiO2 nanotube arrays with ternary S-scheme heterojunction for photocatalytic wastewater treatment and hydrogen production. Fuel 2022, 322, 124163. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Ohtani, B.; Bahena Uribe, D.; Colbeau-Justin, C.; Rau, S.; Rodríguez-López, J.L.; Remita, H. Heterojunction of CuO nanoclusters with TiO2 for photo-oxidation of organic compounds and for hydrogen production. J. Chem. Phys. 2020, 153, 034705. [Google Scholar] [CrossRef] [PubMed]
- Dolgonos, A.; Mason, T.-O.; Poeppelmeier, K.-R. Direct optical band gap measurement in polycrystalline semiconductors: A critical look at the Tauc method. J. Solid State Chem. 2016, 240, 43–48. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Rybak-Wilusz, E.; Cholewa, M.; Wisz, G.; Yavorskyi, R. Review of the development of copper oxides with titanium dioxide thin-film solar cells. AIP Adv. 2020, 10, 010701. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, S. Sacrificial agents for photocatalytic hydrogen production: Effects, cost, and development. Chem Catal. 2022, 2, 1502–1505. [Google Scholar] [CrossRef]
- López-Ayala, S.; Rincón, M.E. Catalytic and photocatalytic performance of mesoporous CuxO–TiO2. J. Photochem. Photobiol. A Chem. 2011, 222, 249–257. [Google Scholar] [CrossRef]

| Experiment | [NaOH] M | [AB1] mg/L | [NaOH] M | [AB1] mg/L | [NaOH] M | [AB1] mg/L |
|---|---|---|---|---|---|---|
| Only NaOH | 0.3 | --- | 0.4 | --- | 0.5 | --- |
| CuO-TiO2/NaOH | 0.3 | --- | 0.4 | --- | 0.5 | --- |
| AB1/NaOH | 0.3 | 40 | 0.4 | 40 | 0.5 | 40 |
| AB1/NaOH | 0.3 | 60 | 0.4 | 60 | 0.5 | 60 |
| AB1/NaOH | 0.3 | 80 | 0.4 | 80 | 0.5 | 80 |
| CuO-TiO2/AB1/NaOH | 0.3 | 40 | 0.4 | 40 | 0.5 | 40 |
| CuO-TiO2/AB1/NaOH | 0.3 | 60 | 0.4 | 60 | 0.5 | 60 |
| CuO-TiO2/AB1/NaOH | 0.3 | 80 | 0.4 | 80 | 0.5 | 80 |
| [AB1]/[NaOH] mg/L/M | T (°C) | k (min−1) | R2 |
|---|---|---|---|
| 40 | 30 | 0.0459 | 99 |
| 40 | 30 | 0.014 | 0.985 |
| 60 | 30 | 0.0403 | 1 |
| 80 | 30 | 0.009 | 0.99 |
| 40 | 40 | 0.055 | 0.99 |
| 40 | 40 | 0.066 | 0.99 |
| 60 | 40 | 0.0512 | 0.99 |
| 1st Injection | 2nd Injection | 3rd Injection | Hydrogen Production | |||
|---|---|---|---|---|---|---|
| t, min | AB1, mL | t, min | AB1, mL | t, min | AB1, mL | ppb/gcat/h |
| 24 | 2 | 42 | 3 | 51 | 3 | 1927 |
| 21 | 3 | 56 | 3 | --- | --- | 5050 |
| Nanomaterial | EtOH/Ti Molar Ratio | H+/Ti Molar Ratio | Cu/Ti Molar Ratio | H2O/Ti Molar Ratio | Calcined’s Characteristics |
|---|---|---|---|---|---|
| Cu-TiO2 | 45 | 0.23 | 0.08 | 4.8 | 1.5 °C/min to 450 °C for 1 h |
| TiO2 | 45 | 0.23 | --- | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ayala, S.; Menchaca Campos, E.C.; Méndez-Rojas, M.A.; Rincón, M.E. Simultaneous Hydrogen Production and Dye Decomposition in Alkaline Photocatalytic Process Using Calcined Xerogels of CuO-TiO2. Gels 2025, 11, 319. https://doi.org/10.3390/gels11050319
López-Ayala S, Menchaca Campos EC, Méndez-Rojas MA, Rincón ME. Simultaneous Hydrogen Production and Dye Decomposition in Alkaline Photocatalytic Process Using Calcined Xerogels of CuO-TiO2. Gels. 2025; 11(5):319. https://doi.org/10.3390/gels11050319
Chicago/Turabian StyleLópez-Ayala, Susana, Elsa C. Menchaca Campos, Miguel A. Méndez-Rojas, and Marina E. Rincón. 2025. "Simultaneous Hydrogen Production and Dye Decomposition in Alkaline Photocatalytic Process Using Calcined Xerogels of CuO-TiO2" Gels 11, no. 5: 319. https://doi.org/10.3390/gels11050319
APA StyleLópez-Ayala, S., Menchaca Campos, E. C., Méndez-Rojas, M. A., & Rincón, M. E. (2025). Simultaneous Hydrogen Production and Dye Decomposition in Alkaline Photocatalytic Process Using Calcined Xerogels of CuO-TiO2. Gels, 11(5), 319. https://doi.org/10.3390/gels11050319








