Easy-to-Apply Hydrogel Patch for Field Treatment and Monitoring of Equine Wounds
Abstract
1. Introduction
2. Results and Discussion
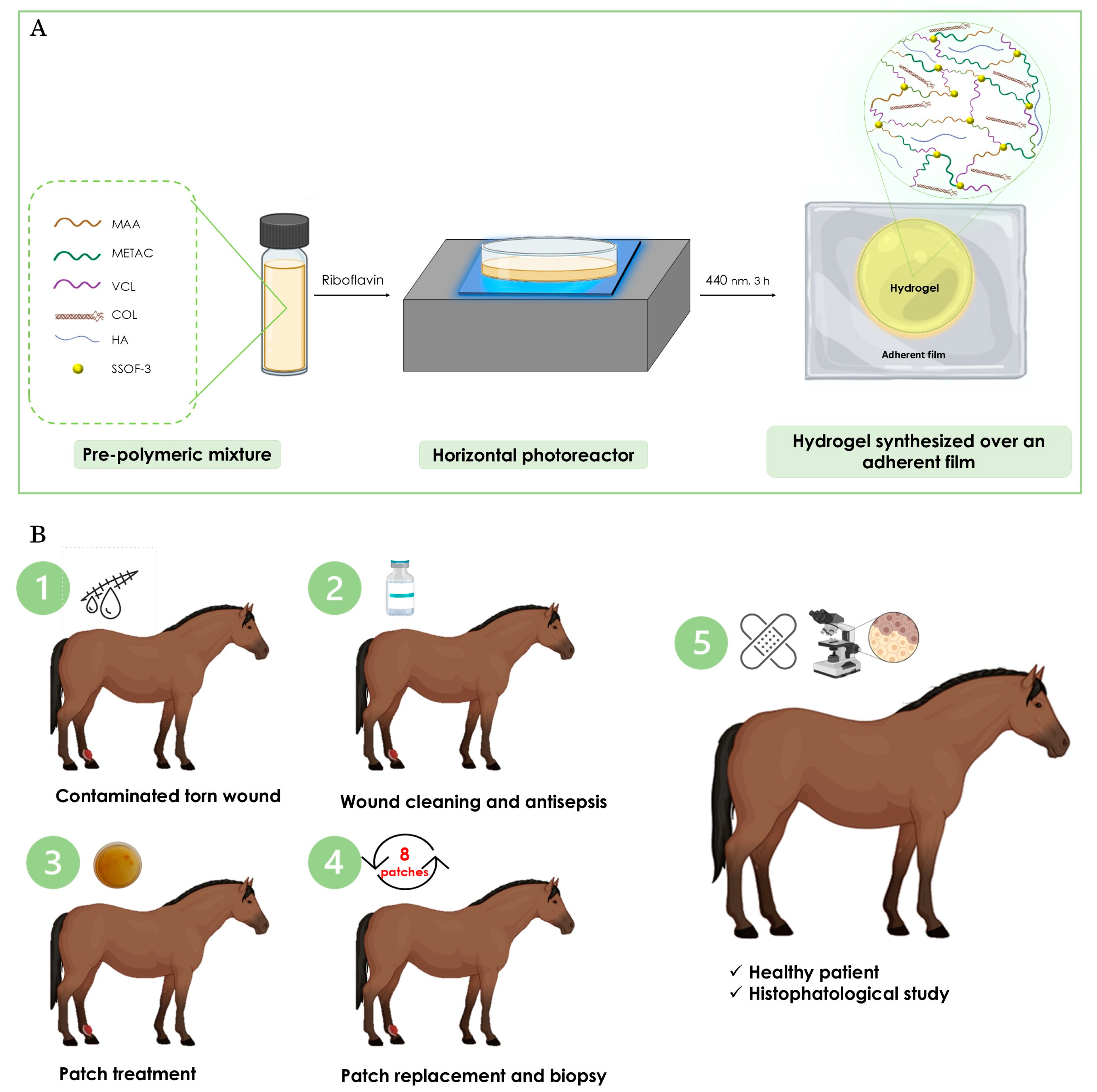
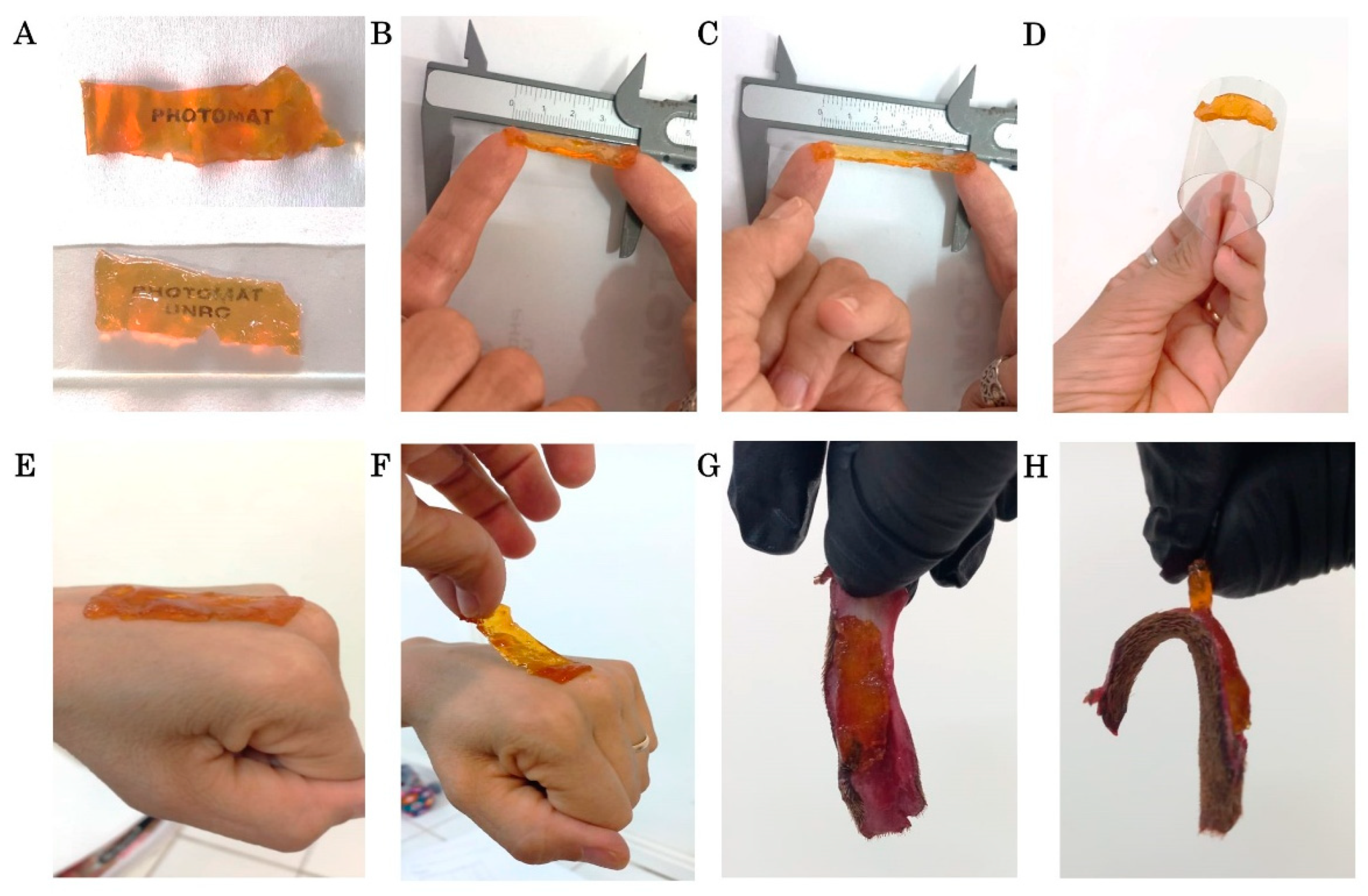
2.1. Synthesis of the Hydrogel Patch
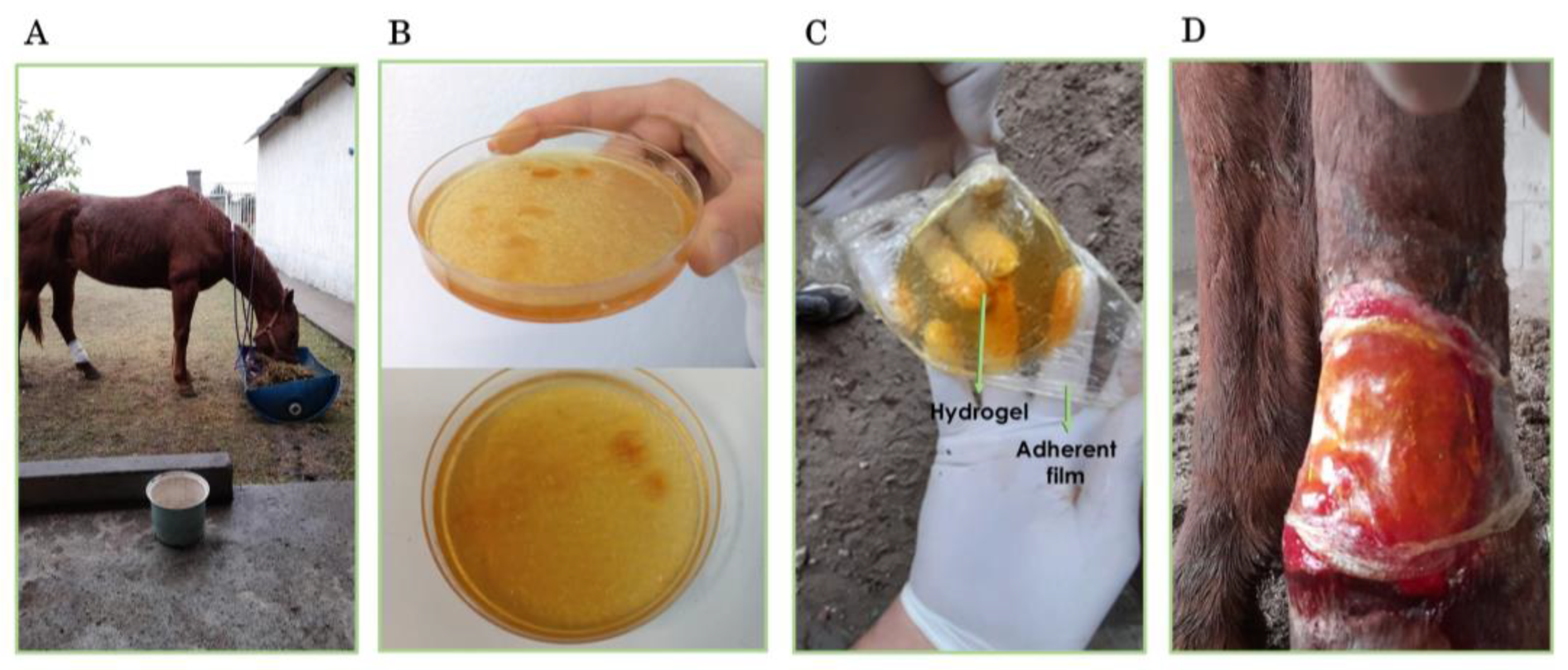
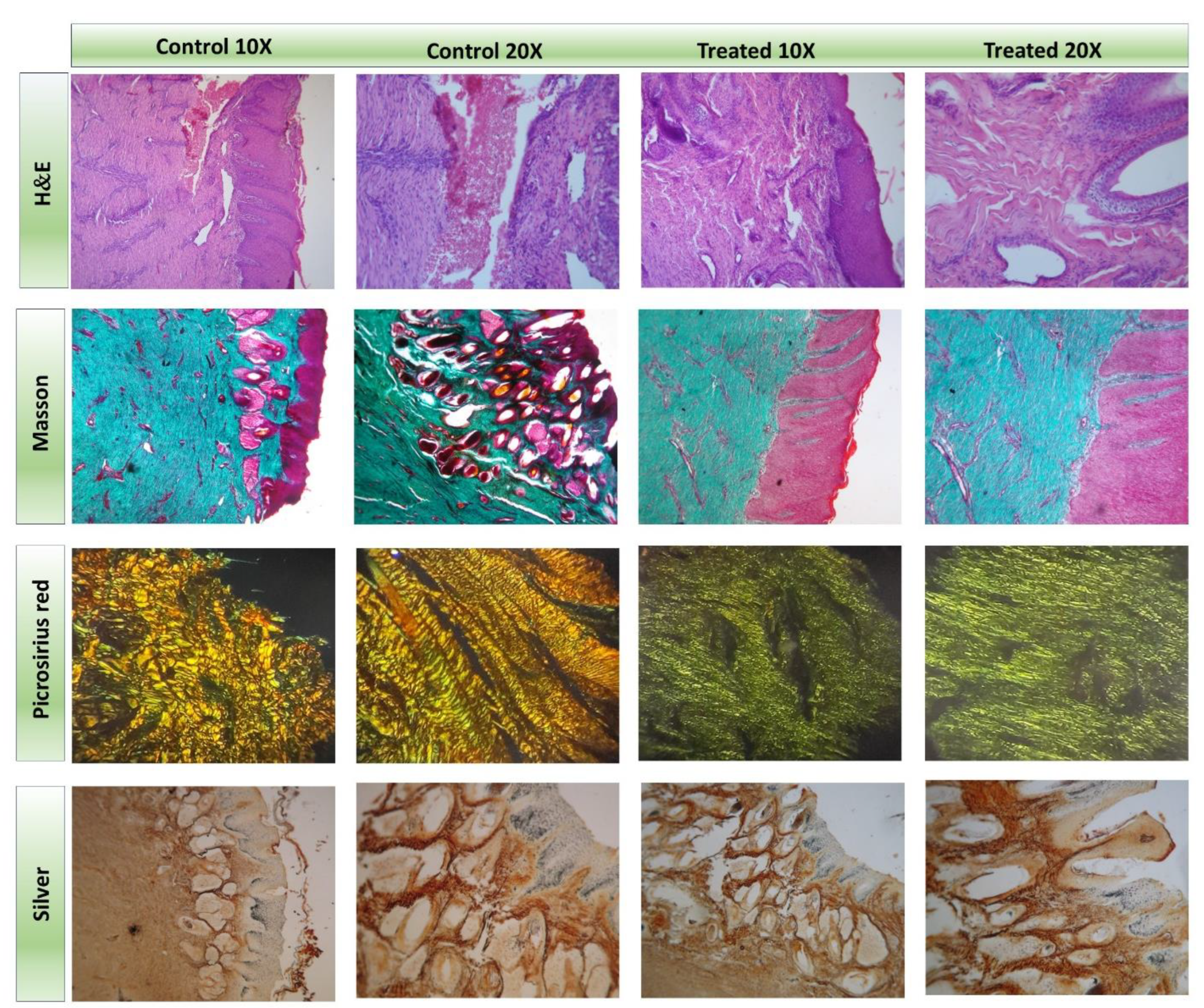
2.2. Application of the Hydrogel Patch in the Treatment of a Full-Thickness Wound in a Mare
2.3. Final Remarks
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogel Patch
4.2.1. Pre-Polymeric Mixture Preparation
4.2.2. Photopolymerization Process
4.2.3. Post-Processing and Characterization
4.3. Case Report
4.3.1. Clinical Findings
4.3.2. Diagnosis
4.3.3. Treatment
4.4. Histopathology and Histochemistry
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Boateng, S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Afzal, S.; Barkat, K.; Ashraf, M.U.; Khalid, I.; Mehmood, Y.; Shah, N.H.; Badshah, S.F.; Naeem, S.; Khan, S.A.; Kazi, M. Formulation and Characterization of Polymeric Cross-Linked Hydrogel Patches for Topical Delivery of Antibiotic for Healing Wound Infections. Polymers 2023, 15, 1652. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Budras, K.-D.; Sack, W.O.; Rock, S. Anatomy of the Horse: An Illustrated Text; Schlütersche: Hannover, Germany, 2003. [Google Scholar]
- Sparks, H.D.; Sigaeva, T.; Tarraf, S.; Mandla, S.; Pope, H.; Hee, O.; Di Martino, E.S.; Biernaskie, J.; Radisic, M.; Scott, W.M. Biomechanics of wound healing in an equine limb model: Effect of location and treatment with a peptide-modified collagen–chitosan hydrogel. ACS Biomater. Sci. Eng. 2020, 7, 265–278. [Google Scholar] [CrossRef]
- Lowry, J.C.; Bartley, G.B.; Garrity, J.A. The role of second-intention healing in periocular reconstruction. Ophthalmic Plast. Reconstr. Surg. 1997, 13, 174–188. [Google Scholar] [CrossRef]
- Bertone, A.L. Second-intention healing. Vet. Clin. N. Am. Equine Pract. 1989, 5, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Eriksson, E. Moist wound healing with commonly available dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.E.; Martínez, S.R.; Ibarra, L.E.; Gallastegui, A.; Martucci, J.F.; Palacios, R.E.; Chesta, C.A.; Gómez, M.L. Reusable antimicrobial antibiotic-free dressings obtained by photopolymerization. Biomater. Adv. 2023, 149, 213399. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.J.; Cries, L.; Jeffcott, L.B.; Hodgson, D.R.; Rose, R.J. Effects of 25% propylene glycol hydrogel (Solugel) on second intention wound healing in horses. Vet. Surg. 2002, 31, 309–313. [Google Scholar] [CrossRef]
- Nasra, S.; Patel, M.; Shukla, H.; Bhatt, M.; Kumar, A. Functional hydrogel-based wound dressings: A review on biocompatibility and therapeutic efficacy. Life Sci. 2023, 334, 122232. [Google Scholar] [CrossRef]
- Kolipaka, T.; Pandey, G.; Abraham, N.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Rajinikanth, P.; Tickoo, V.; Srivastava, S. Stimuli-responsive polysaccharide-based smart hydrogels for diabetic wound healing: Design aspects, preparation methods and regulatory perspectives. Carbohydr. Polym. 2024, 324, 121537. [Google Scholar] [CrossRef]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 279–291. [Google Scholar] [CrossRef]
- Liang, M.; Dong, L.; Guo, Z.; Liu, L.; Fan, Z.; Wei, C.; Mi, S.; Sun, W. Collagen–Hyaluronic Acid Composite Hydrogels with Applications for Chronic Diabetic Wound Repair. ACS Biomater. Sci. Eng. 2023, 9, 5376–5388. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, Q.; Yang, K.; Chen, R.; Zhang, J.; Xiao, P.; Zhou, Y. Hyaluronic acid hydrogels with excellent self-healing capacity and photo-enhanced mechanical properties for wound healing. Int. J. Biol. Macromol. 2024, 267, 131235. [Google Scholar] [CrossRef]
- Lomas, A.; Ryan, C.; Sorushanova, A.; Shologu, N.; Sideri, A.; Tsioli, V.; Fthenakis, G.; Tzora, A.; Skoufos, I.; Quinlan, L.R. The past, present and future in scaffold-based tendon treatments. Adv. Drug Deliv. Rev. 2015, 84, 257–277. [Google Scholar] [CrossRef]
- Williams, Z.J.; Pezzanite, L.M.; Hendrickson, D.A. Review of skin grafting in equine wounds: Indications and techniques. Equine Vet. Educ. 2024, 36, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, L.A.; Milton, S.C.; Boswell, S.G.; Werre, S.R.; Brewster, C.C.; Jones, C.S.; Crisman, M.V. Evaluation of a hyaluronic acid-based biomaterial to enhance wound healing in the equine distal limb. J. Equine Vet. Sci. 2016, 44, 90–99. [Google Scholar] [CrossRef]
- Eggleston, R.B. Wound management: Wounds with special challenges. Vet. Clin. Equine Pract. 2018, 34, 511–538. [Google Scholar] [CrossRef]
- Ribeiro, G.; Carvalho, L.; Borges, J.; Prazeres, J. The best protocol to treat equine skin wounds by second intention healing: A scoping review of the literature. Animals 2024, 14, 1500. [Google Scholar] [CrossRef]
- Harman, R.M.; Theoret, C.L.; Van de Walle, G.R. The horse as a model for the study of cutaneous wound healing. Adv. Wound Care 2021, 10, 381–399. [Google Scholar] [CrossRef]
- Caston, S.S. Wound care in horses. Vet. Clin. Equine Pract. 2012, 28, 83–100. [Google Scholar] [CrossRef]
- Mespoulhes-Riviere, C.; Martens, A.; Bogaert, L.; Wilderjans, H. Factors affecting outcome of extensor tendon lacerations in the distal limb of horses. Vet. Comp. Orthop. Traumatol. 2008, 21, 358–364. [Google Scholar]
- Theoret, C.; Bolwell, C.; Riley, C. A cross-sectional survey on wounds in horses in New Zealand. New Zealand Vet. J. 2016, 64, 90–94. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, N.; Du, S.; Gao, Y.; Suo, H.; Yang, J.; Tao, J.; Zhu, J.; Zhang, L. Transparent photothermal hydrogels for wound visualization and accelerated healing. Fundam. Res. 2022, 2, 268–275. [Google Scholar] [CrossRef]
- Zambroni, M.E.; Martínez, S.R.; Cagnetta, G.E.; Ibarra, L.E.; Posadaz, A.; Martucci, J.F.; Romanini, S.; Aramayo, E.A.; Cabral, A.L.; Bertone, P. Multifunctional skin dressings synthesized via one-pot photopolymerization: Advancing wound healing and infection prevention strategies. Polym. Adv. Technol. 2024, 35, e6490. [Google Scholar] [CrossRef]
- Zambroni, M.E.; Gallastegui, A.; Martucci, J.F.; Ibarra, L.E.; Martínez, S.R.; Cagnetta, G.E.; Palacios, R.E.; Chesta, C.A.; Gomez, M.L. Parches Dermatológicos Autoesterilizantes Conteniendo Amonios Cuaternarios, Silsesquioxanos y Polímeros Conjugados y un Procedimiento Para la Obtención de Dichos Parches. Argentinian Patent- Tracking Number 20220101385, 5 May 2022. [Google Scholar]
- Parnell, L.K.; Volk, S.W. The evolution of animal models in wound healing research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 238. [Google Scholar]
- Gallastegui, A.; Zambroni, M.E.; Chesta, C.A.; Palacios, R.E.; Gomez, M.L. New bifunctional cross-linkers/co-initiators for vinyl photopolymerization: Silsesquioxanes-B2 vitamin as eco-friendly hybrid photoinitiator systems. Polymer 2021, 221, 123605. [Google Scholar] [CrossRef]
- Anantama, N.A.; Du Cheyne, C.; Martens, A.; Roth, S.P.; Burk, J.; De Spiegelaere, W.; Michler, J.K. The granulation (t) issue: A narrative and scoping review of basic and clinical research of the equine distal limb exuberant wound healing disorder. Vet. J. 2022, 280, 105790. [Google Scholar] [CrossRef]
- Iacopetti, I.; Perazzi, A.; Ferrari, V.; Busetto, R. Application of platelet-rich gel to enhance wound healing in the horse: A case report. J. Equine Vet. Sci. 2012, 32, 123–128. [Google Scholar] [CrossRef]
- Dart, A.; Perkins, N.; Dart, C.; Jeffcott, L.; Canfield, P. Effect of bandaging on second intention healing of wounds of the distal limb in horses. Aust. Vet. J. 2009, 87, 215–218. [Google Scholar] [CrossRef]
- Martínez, S.R.; Caverzan, M.; Ibarra, L.E.; Aiassa, V.; Bohl, L.; Porporatto, C.; Gómez, M.L.; Chesta, C.A.; Palacios, R.E. Light-activated conjugated polymer nanoparticles to defeat pathogens associated with bovine mastitis. J. Photochem. Photobiol. B Biol. 2024, 257, 112971. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W. Modified elastic tissue-Masson trichrome stain. Stain Technol. 1984, 59, 213–216. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambroni, M.E.; Bertone, P.A.; Cabral, A.L.; Boatti, A.S.; Romanini, S.V.; Martínez, S.R.; Gómez, M.L. Easy-to-Apply Hydrogel Patch for Field Treatment and Monitoring of Equine Wounds. Gels 2025, 11, 328. https://doi.org/10.3390/gels11050328
Zambroni ME, Bertone PA, Cabral AL, Boatti AS, Romanini SV, Martínez SR, Gómez ML. Easy-to-Apply Hydrogel Patch for Field Treatment and Monitoring of Equine Wounds. Gels. 2025; 11(5):328. https://doi.org/10.3390/gels11050328
Chicago/Turabian StyleZambroni, María Emilia, Patricia A. Bertone, Ana Lucía Cabral, Andrés S. Boatti, Silvia V. Romanini, Sol R. Martínez, and María Lorena Gómez. 2025. "Easy-to-Apply Hydrogel Patch for Field Treatment and Monitoring of Equine Wounds" Gels 11, no. 5: 328. https://doi.org/10.3390/gels11050328
APA StyleZambroni, M. E., Bertone, P. A., Cabral, A. L., Boatti, A. S., Romanini, S. V., Martínez, S. R., & Gómez, M. L. (2025). Easy-to-Apply Hydrogel Patch for Field Treatment and Monitoring of Equine Wounds. Gels, 11(5), 328. https://doi.org/10.3390/gels11050328






