Three-Dimensional Gel Dosimetry in a Simulated Postmastectomy with Expandable Prosthesis Radiotherapy
Abstract
1. Introduction
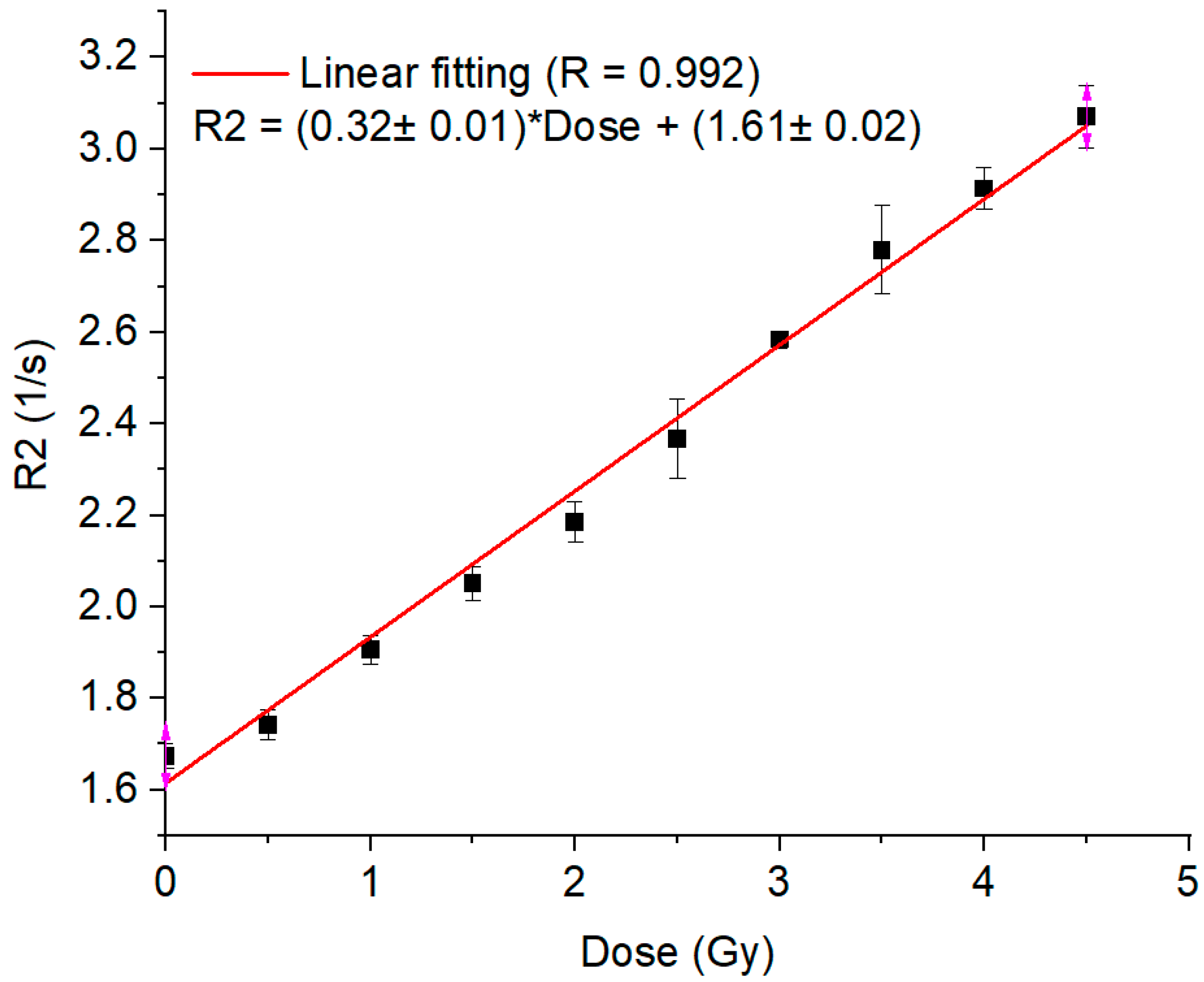
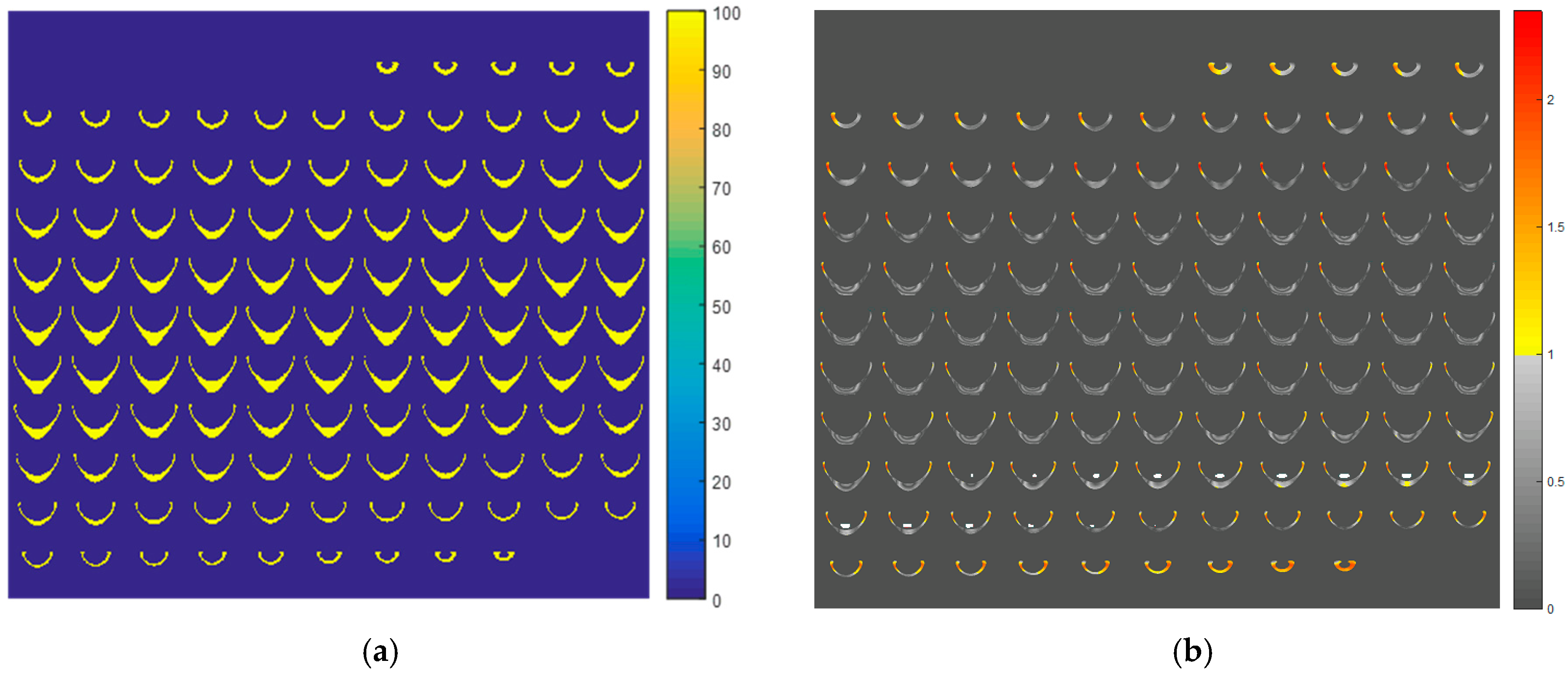
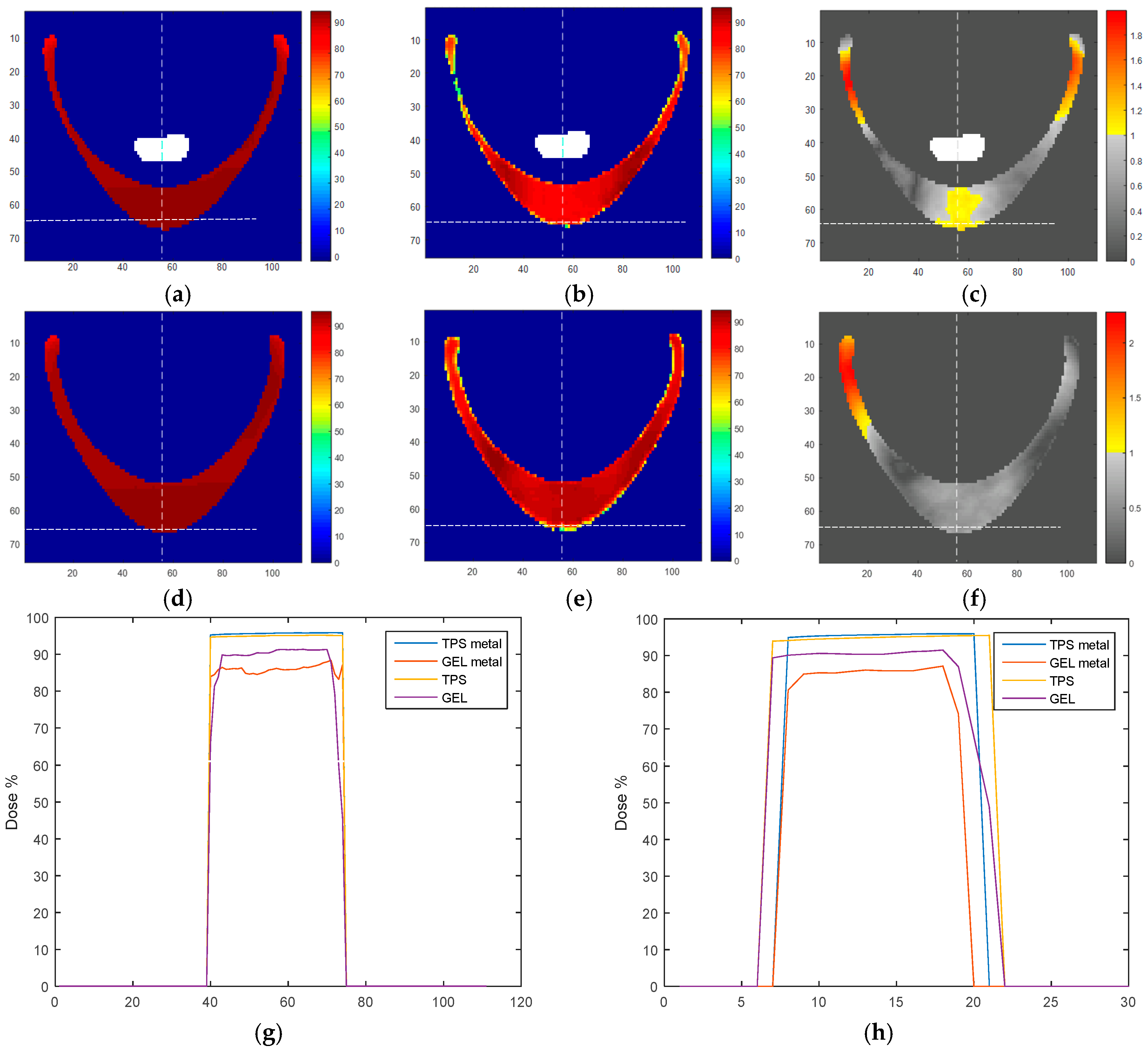
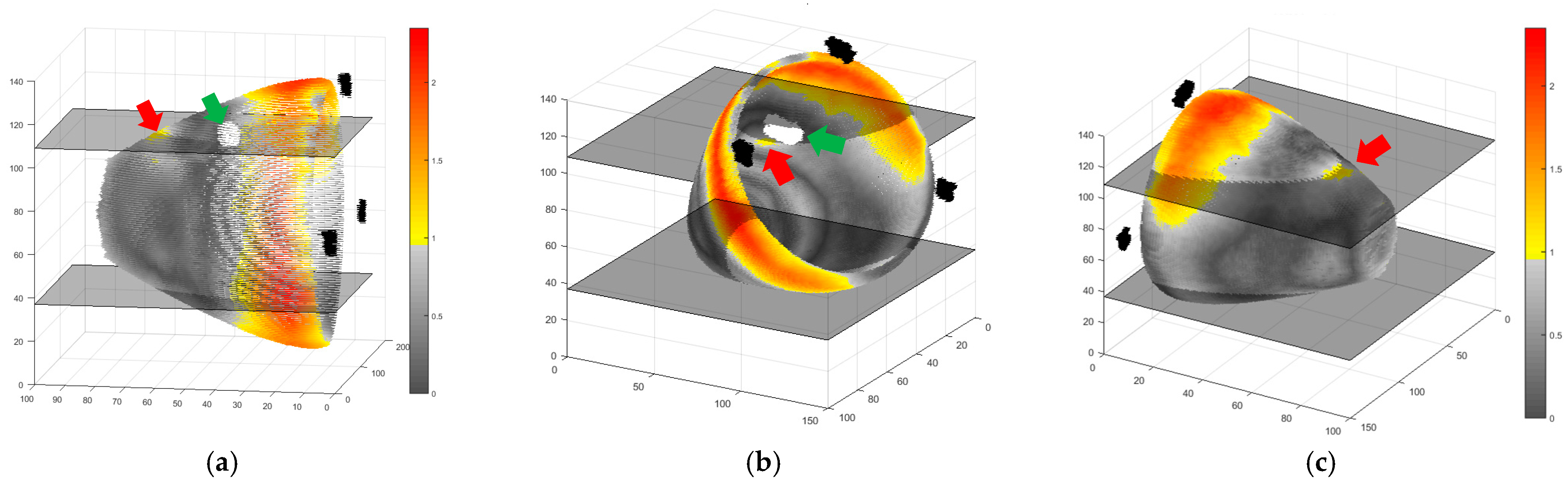
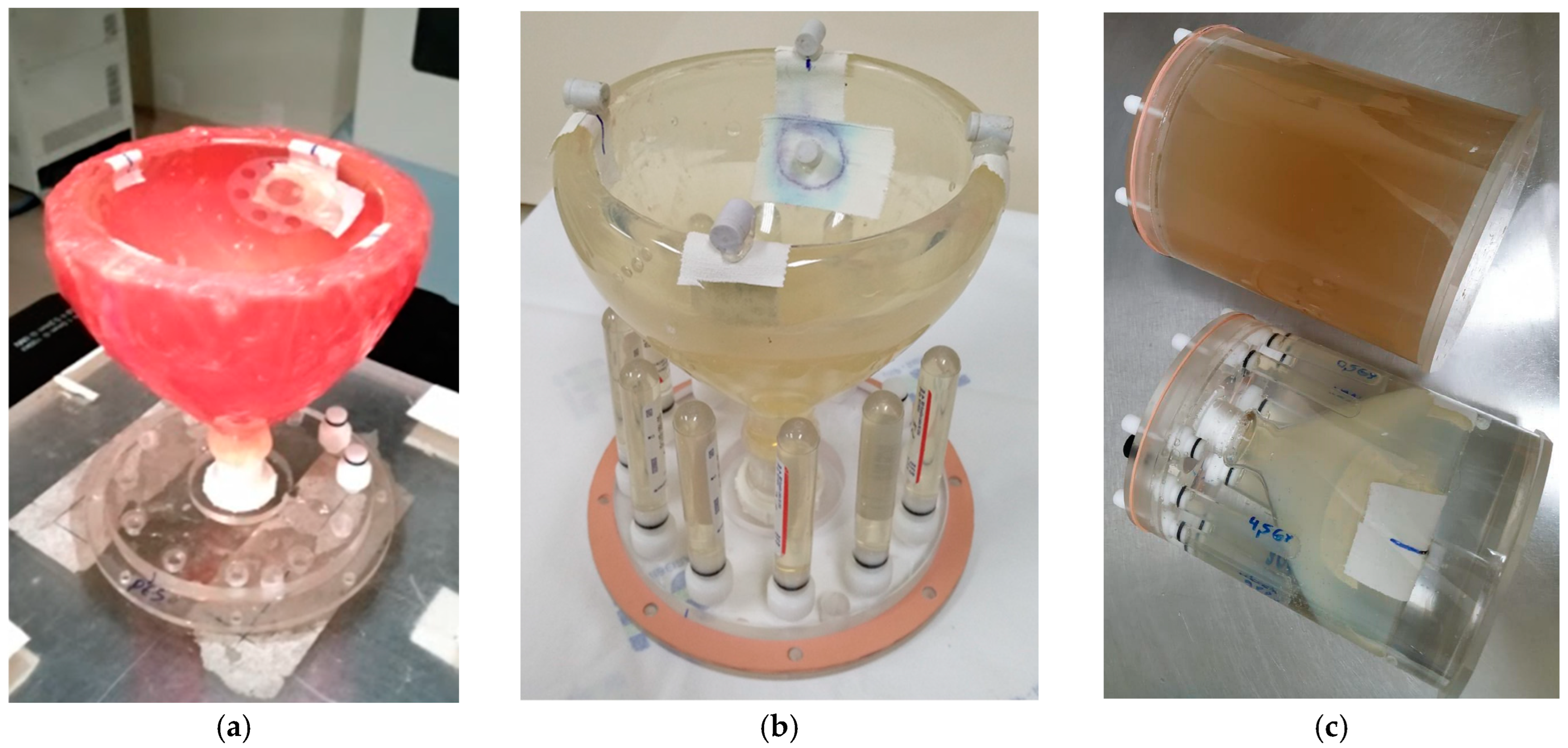
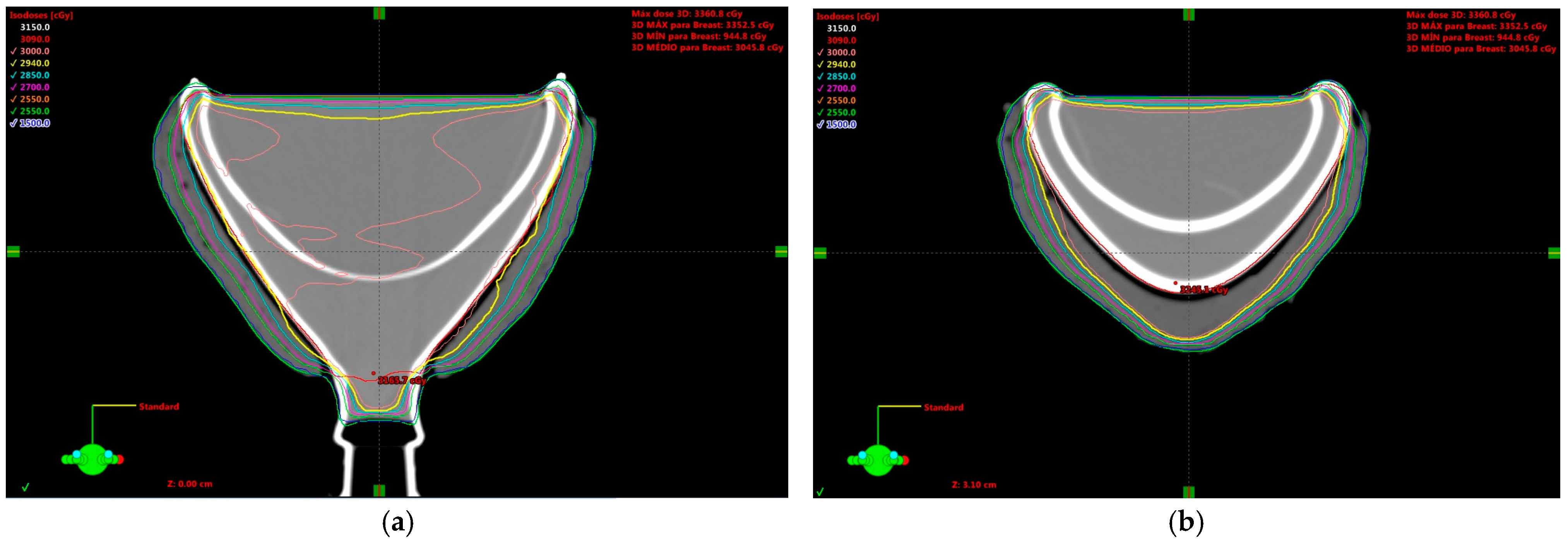
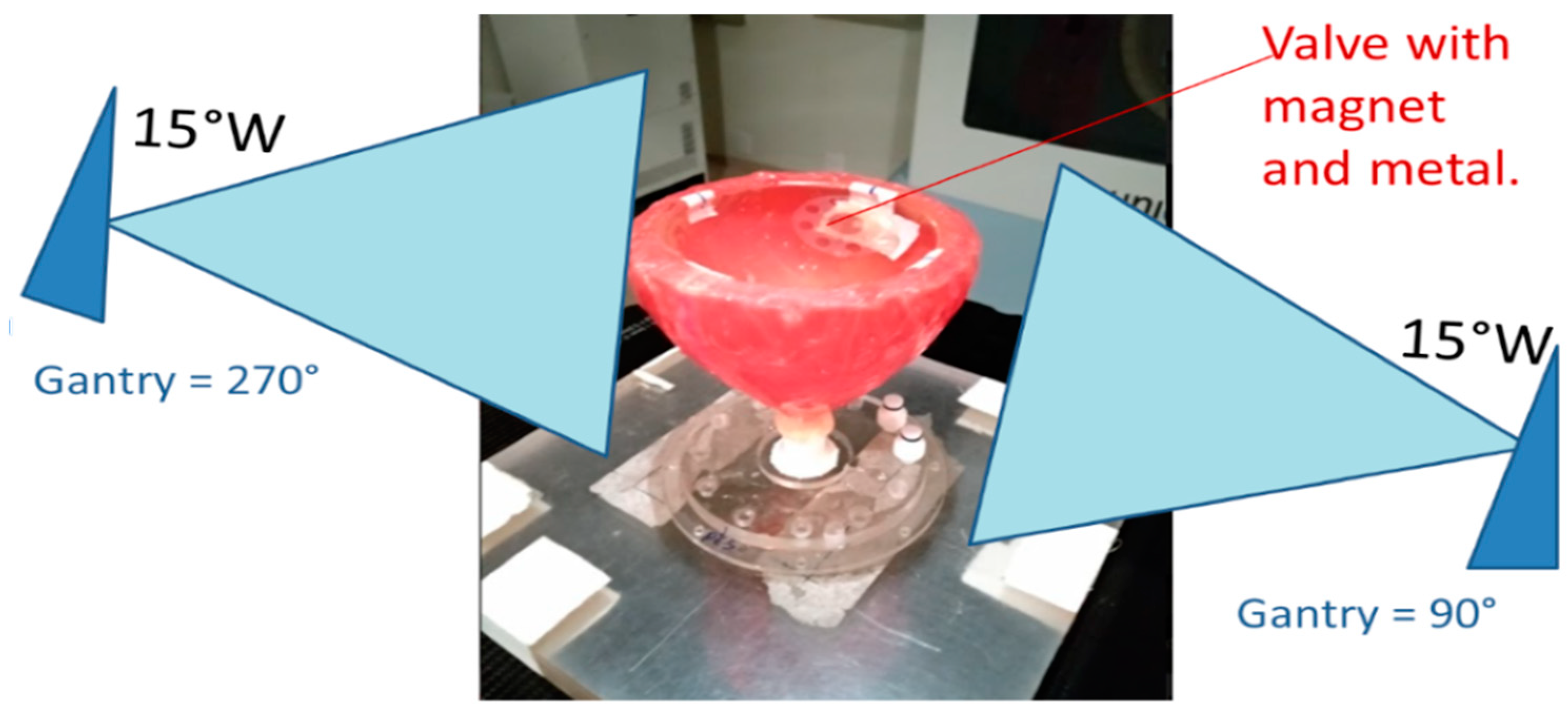
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Song, D.; Xie, L.; Zhan, N.; Xie, W.; Zhang, J. Postmastectomy Radiotherapy in Breast Reconstruction: Current Controversies and Trends. Cancer Innov. 2024, 3, e104. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.; Hu, Z.I.; Mehrara, B.J.; Wilkins, E.G. Radiotherapy in the Setting of Breast Reconstruction: Types, Techniques, and Timing. Lancet Oncol. 2017, 18, e742–e753. [Google Scholar] [CrossRef]
- Piroth, M.D.; Krug, D.; Baumann, R.; Strnad, V.; Borm, K.; Combs, S.; Corradini, S.; Duma, M.N.; Dunst, J.; Fastner, G.; et al. Implant-Based Reconstruction and Adjuvant Radiotherapy in Breast Cancer Patients—Current Status and DEGRO Recommendations. Strahlenther. Onkol. 2025, 201, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Odgaard, E.B.; Frid, N.L.; Lauritzen, E.; Damsgaard, T.E. The Impact of Body Mass Index on Direct to Implant and Two-Stage Immediate Breast Reconstruction Procedure: A Systematic Review. Ann. Breast Surg. 2024, 8, 34. [Google Scholar] [CrossRef]
- Ryu, H.; Shin, K.H.; Chang, J.H.; Jang, B.-S. A Nationwide Study of Breast Reconstruction after Mastectomy in Patients with Breast Cancer Receiving Postmastectomy Radiotherapy: Comparison of Complications According to Radiotherapy Fractionation and Reconstruction Procedures. Br. J. Cancer 2024, 131, 290–298. [Google Scholar] [CrossRef]
- Bellini, E.; Pesce, M.; Santi, P.; Raposio, E. Two-Stage Tissue-Expander Breast Reconstruction: A Focus on the Surgical Technique. BioMed Res. Int. 2017, 2017, 1791546. [Google Scholar] [CrossRef] [PubMed]
- Moni, J.; Graves-Ditman, M.; Cederna, P.; Griffith, K.; Krueger, E.A.; Fraass, B.A.; Pierce, L.J. Dosimetry around Metallic Ports in Tissue Expanders in Patients Receiving Postmastectomy Radiation Therapy: An Ex Vivo Evaluation. Med. Dosim. 2004, 29, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.M.; Cardoso, S.C.; Facure, A.; da Silva, A.X.; da Rosa, L.A.R. Influence of the Presence of Tissue Expanders on Energy Deposition for Post-Mastectomy Radiotherapy. PLoS ONE 2013, 8, e55430. [Google Scholar] [CrossRef]
- Perrucci, E.; Marcantonini, M.; Arena, E.; Fulcheri, C.; Reggioli, V.; Dipilato, A.C.; Palumbo, I.; Saldi, S.; Falcinelli, L.; Ingrosso, G.; et al. Effect of Internal Port on Dose Distribution in Post-Mastectomy Radiotherapy for Breast Cancer Patients after Expander Breast Reconstruction. Rep. Pract. Oncol. Radiother. 2023, 28, 1–8. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Morgan, A.M. Investigation into Dosimetric Effect of a MAGNA-SITETM Tissue Expander on Post-Mastectomy Radiotherapy. Med. Phys. 2005, 32, 1640–1646. [Google Scholar] [CrossRef]
- Damast, S.; Beal, K.; Ballangrud, Å.; Losasso, T.J.; Cordeiro, P.G.; Disa, J.J.; Hong, L.; McCormick, B.L. Do Metallic Ports in Tissue Expanders Affect Postmastectomy Radiation Delivery? Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 305–310. [Google Scholar] [CrossRef]
- Chatzigiannis, C.; Lymperopoulou, G.; Sandilos, P.; Dardoufas, C.; Yakoumakis, E.; Georgiou, E.; Karaiskos, P. Dose Perturbation in the Radiotherapy of Breast Cancer Patients Implanted with the Magna-Site: A Monte Carlo Study. J. Appl. Clin. Med. Phys. 2011, 12, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Cheng, C.-W.; Andrews, J.; Das, I.J. Dose Perturbation Due to Metallic Breast Expander in Electron and Photon Beam Treatment of Breast Cancer. J. Radiat. Oncol. 2014, 3, 65–72. [Google Scholar] [CrossRef]
- Trombetta, D.M.; Cardoso, S.C.; Alves, V.G.L.; Facure, A.; Batista, D.V.S.; da Silva, A.X. Evaluation of the Radiotherapy Treatment Planning in the Presence of a Magnetic Valve Tissue Expander. PLoS ONE 2015, 10, e0117548. [Google Scholar] [CrossRef] [PubMed]
- Strang, B.; Murphy, K.; Seal, S.; Dal Cin, A. Does the Presence of an Implant Including Expander with Internal Port Alter Radiation Dose? An Ex Vivo Model. Can. J. Plast. Surg. 2013, 21, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Zabihzadeh, M.; Ghahfarokhi, M.; Razmjoo-Ghalaei, S.; Arvandi, S.; Mashayekhi, Z. Dose Perturbation Due to the Magnetic Port of Tissue Breast Expander in Patient Undergoing the Postmastectomy Radiation Therapy. Biomed. Pharmacol. J. 2016, 9, 285–291. [Google Scholar] [CrossRef]
- Gee, H.E.; Bignell, F.; Odgers, D.; Gill, S.; Martin, D.; Toohey, J.; Carroll, S. In Vivo Dosimetric Impact of Breast Tissue Expanders on Post-Mastectomy Radiotherapy. J. Med. Imaging Radiat. Oncol. 2016, 60, 138–145. [Google Scholar] [CrossRef]
- da Silva, M.F.; de Oliveira, H.F.; Borges, L.F.; Carrara, H.H.A.; Farina, J.A. Effects of the Metallic Port in Tissue Expanders on Dose Distribution in Postmastectomy Radiotherapy. Ann. Plast. Surg. 2018, 80, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric Analysis of the Effects of a Temporary Tissue Expander on the Radiotherapy Technique. Radiol. Med. 2021, 126, 437–444. [Google Scholar] [CrossRef]
- Chen, S.A.; Ogunleye, T.; Dhabbaan, A.; Huang, E.H.; Losken, A.; Gabram, S.; Davis, L.; Torres, M.A. Impact of Internal Metallic Ports in Temporary Tissue Expanders on Postmastectomy Radiation Dose Distribution. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 630–635. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, H.; Tang, W.; Du, X. The Impact of Metal Implants on the Dose and Clinical Outcome of Radiotherapy (Review). Mol. Clin. Oncol. 2024, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Copeland-Halperin, L.R.; Lyatskaya, Y.; Bellon, J.R.; Dey, T.; Carty, M.J.; Barbie, T.; Erdmann-Sager, J. Impact of Prepectoral vs. Subpectoral Tissue Expander Placement on Post-Mastectomy Radiation Therapy Delivery: A Retrospective Cohort Study. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5434. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.-H.; Kim, M.; Lee, N.K.; Lee, S.; Hwang, J. Dosimetric Effect of Injection Ports in Tissue Expanders on Post-Mastectomy Volumetric Modulated Arc Therapy (VMAT) Planning for Left-Sided Breast Cancer. Appl. Sci. 2022, 12, 6461. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Yen, Y.-H.; Tsai, Y.-L.; Tu, P.-C.; Pu, C.-M.; Lin, C.-H.; Lui, L.T.; Shaw, S.; Wu, C.-J.; Nien, H.-H. Critical Factors of Dose Distribution in Breast Cancer Tomotherapy with Metallic Port Breast Tissue Expander: Image Correction, Delivery Mode, and Volume Impact. Technol. Cancer Res. Treat. 2022, 21, 15330338221093148. [Google Scholar] [CrossRef]
- Yoon, J.; Xie, Y.; Heins, D.; Zhang, R. Modeling of the Metallic Port in Breast Tissue Expanders for Photon Radiotherapy. J. Appl. Clin. Med. Phys. 2018, 19, 205–214. [Google Scholar] [CrossRef]
- Wieslander, E.; Knöös, T. Dose Perturbation in the Presence of Metallic Implants: Treatment Planning System Versus Monte Carlo Simulations. Phys. Med. Biol. 2003, 48, 3295–3305. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef]
- Macchione, M.A.; Lechón Páez, S.; Strumia, M.C.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. [Google Scholar] [CrossRef]
- De Deene, Y. Radiation Dosimetry by Use of Radiosensitive Hydrogels and Polymers: Mechanisms, State-of-the-Art and Perspective from 3D to 4D. Gels 2022, 8, 599. [Google Scholar] [CrossRef]
- Sagsoz, M.E.; Korkut, O.; Gallo, S. Advancements in Tissue-Equivalent Gel Dosimeters. Gels 2025, 11, 81. [Google Scholar] [CrossRef]
- De Deene, Y.; Jirasek, A. Gel Dosimetry: An Overview of Dosimetry Systems and Read out Methods. Radiat. Meas. 2024, 179, 107321. [Google Scholar] [CrossRef]
- Silveira, M.A.; Pavoni, J.F.; Baffa, O. Three-Dimensional Quality Assurance of IMRT Prostate Plans Using Gel Dosimetry. Phys. Med. 2017, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, J.F.; Neves-Junior, W.F.P.; da Silveira, M.A.; Haddad, C.M.K.; Baffa, O. Evaluation of a Composite Gel-Alanine Phantom on an End-to-End Test to Treat Multiple Brain Metastases by a Single Isocenter VMAT Technique. Med. Phys. 2017, 44, 4869–4879. [Google Scholar] [CrossRef]
- da Silveira, M.A.; Pavoni, J.F.; Bruno, A.C.; Arruda, G.V.; Baffa, O. Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure. Gels 2022, 8, 582. [Google Scholar] [CrossRef]
- Pavoni, J.F.; Pike, T.L.; Snow, J.; DeWerd, L.; Baffa, O. Tomotherapy Dose Distribution Verification Using MAGIC-f Polymer Gel Dosimetry. Med. Phys. 2012, 39, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.M.C.; Pavoni, J.F.; Arruda, G.V.; Baffa, O. Gel and Thermoluminescence Dosimetry for Dose Verifications of a Real Anatomy Simulated Prostate Conformal Radiation Treatment in the Presence of Metallic Femoral Prosthesis. J. Appl. Clin. Med. Phys. 2021, 22, 278–287. [Google Scholar] [CrossRef]
- Lizar, J.C.; Volpato, K.C.; Brandão, F.C.; da Silva Guimarães, F.; Arruda, G.V.; Pavoni, J.F. Three-Dimensional Dose Evaluation of the Respiratory Motion Influence on Breast Radiotherapy Treatments Using Conformal Radiotherapy, Forward IMRT, and Inverse IMRT Planning Techniques. Phys. Med. 2021, 81, 60–68. [Google Scholar] [CrossRef]
- Mizukami, S.; Watanabe, Y.; Mizoguchi, T.; Gomi, T.; Hara, H.; Takei, H.; Fukunishi, N.; Ishikawa, K.L.; Fukuda, S.; Maeyama, T. Whole Three-Dimensional Dosimetry of Carbon Ion Beams with an MRI-Based Nanocomposite Fricke Gel Dosimeter Using Rapid T1 Mapping Method. Gels 2021, 7, 233. [Google Scholar] [CrossRef]
- Zirone, L.; Bonanno, E.; Borzì, G.R.; Cavalli, N.; D’Anna, A.; Galvagno, R.; Girlando, A.; Gueli, A.M.; Pace, M.; Stella, G.; et al. HyperArcTM Dosimetric Validation for Multiple Targets Using Ionization Chamber and RT-100 Polymer Gel. Gels 2022, 8, 481. [Google Scholar] [CrossRef]
- Kunkyab, T.; Lakrad, K.; Jirasek, A.; Oldham, M.; Quinn, B.; Hyde, D.; Adamson, J. Clinical Applicability of Linac-Integrated CBCT Based NIPAM 3D Dosimetry: A Dual-Institutional Investigation. Phys. Med. Biol. 2024, 69, 155002. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Jaszczak-Kuligowska, M.; Maras, P. Measurement of Ionising Radiation Dose Absorbed by Bones by Using a Bone-Imitating Polymer Gel Dosimeter. Measurement 2025, 240, 115633. [Google Scholar] [CrossRef]
- De Deene, Y. Essential characteristics of polymer gel dosimeters. J. Phys. Conf. Ser. 2004, 3, 34–57. [Google Scholar] [CrossRef]
- Miften, M.; Olch, A.; Mihailidis, D.; Moran, J.; Pawlicki, T.; Molineu, A.; Li, H.; Wijesooriya, K.; Shi, J.; Xia, P.; et al. Tolerance Limits and Methodologies for IMRT Measurement-Based Verification QA: Recommendations of AAPM Task Group No. 218. 2018, 45, e53–e83. [Google Scholar] [CrossRef]
- ICRU. Prescribing, Recording, and Reporting Photon Beam Therapy; ICRU Report 50; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1993. [Google Scholar]
- Park, J.B.; Jang, B.-S.; Chang, J.H.; Kim, J.H.; Choi, C.H.; Hong, K.Y.; Jin, U.S.; Chang, H.; Myung, Y.; Jeong, J.H.; et al. The Impact of the New ESTRO-ACROP Target Volume Delineation Guidelines for Postmastectomy Radiotherapy After Implant-Based Breast Reconstruction on Breast Complications. Front. Oncol. 2024, 14, 1373434. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Yang, Y.-J.; Sung, S.; Choi, Y.; Yang, E.-J. Postoperative Complications of Hypofractionated and Conventional Fractionated Radiation Therapy in Patients with Implant-Based Breast Reconstruction: A Systematic Review and Meta-Analysis. Breast 2024, 77, 103782. [Google Scholar] [CrossRef] [PubMed]


| Components | Mass Concentration (%) |
|---|---|
| Ultra-pure deionized water (Labtools, Ribeirão Preto, SP, Brazil) | 82.31 |
| Bovine gelatin—250 bloom (Gelita AG, Cotia, SP, Brazil) | 8.33 |
| Ascorbic acid (Sigma-Aldrich, Saint Louis, MO, USA) | 0.03 |
| Cooper sulfate (Sigma-Aldrich, Saint Louis, MO, USA) | 0.02 |
| Formaldehyde (Sigma-Aldrich, Saint Louis, MO, USA) | 3.32 |
| Methacrylic acid (Sigma-Aldrich, Saint Louis, MO, USA) | 5.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavoni, J.F.; Lizar, J.C.; Borges, L.F.; Nicolucci, P.; Krutman, Y.; Baffa, O. Three-Dimensional Gel Dosimetry in a Simulated Postmastectomy with Expandable Prosthesis Radiotherapy. Gels 2025, 11, 335. https://doi.org/10.3390/gels11050335
Pavoni JF, Lizar JC, Borges LF, Nicolucci P, Krutman Y, Baffa O. Three-Dimensional Gel Dosimetry in a Simulated Postmastectomy with Expandable Prosthesis Radiotherapy. Gels. 2025; 11(5):335. https://doi.org/10.3390/gels11050335
Chicago/Turabian StylePavoni, Juliana Fernandes, Jessica Caroline Lizar, Leandro Frederiche Borges, Patricia Nicolucci, Yanai Krutman, and Oswaldo Baffa. 2025. "Three-Dimensional Gel Dosimetry in a Simulated Postmastectomy with Expandable Prosthesis Radiotherapy" Gels 11, no. 5: 335. https://doi.org/10.3390/gels11050335
APA StylePavoni, J. F., Lizar, J. C., Borges, L. F., Nicolucci, P., Krutman, Y., & Baffa, O. (2025). Three-Dimensional Gel Dosimetry in a Simulated Postmastectomy with Expandable Prosthesis Radiotherapy. Gels, 11(5), 335. https://doi.org/10.3390/gels11050335






