Peptide-Based Physical Gels Endowed with Thixotropic Behaviour
Abstract
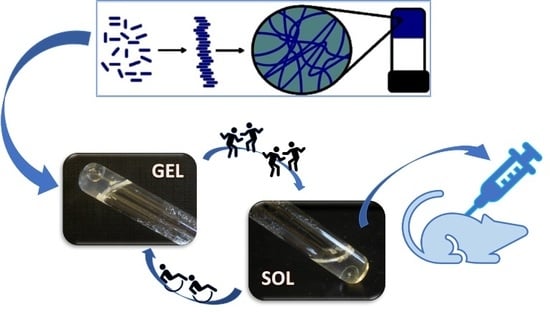
1. Introduction
- (i)
- it is based on viscosity;
- (ii)
- it implies a time-dependent decrease of the viscosity induced by flow;
- (iii)
- the effect is reversible when the flow is decreased or arrested.
2. Thixotropic Hydrogels Prepared Using Biocompatible Low Molecular Weight Gelators (LMWGs)
3. Applications of Thixotropic Peptide Based Physical Hydrogels
4. Conclusions
Conflicts of Interest
References
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Schalek, F.E.; Szegvary, A. Ueber Eisenoxydgallerten—Vorlaufige Mitteilung. Kolloid-Z. 1923, 32, 318–319. [Google Scholar] [CrossRef]
- Schalek, E.; Szegvari, A. Die langsame Koagulation konzentrierter Eisenoxydsole zu reversiblen Gallerten. Kolloid-Z. 1923, 33, 326–334. [Google Scholar] [CrossRef]
- Goodeve, C.F. A general theory of thixotropy and viscosity. Trans. Faraday Soc. 1939, 35, 342–358. [Google Scholar] [CrossRef]
- Reiner, M.; Scott Blair, G.W. Chapter 9—Rheological Terminology. In Rheology, Theory and Applications; Elsevier: Amsterdam, The Netherlands, 1967; Volume 4, pp. 461–488. ISBN 9781483229416. [Google Scholar]
- Bauer, W.H.; Collins, E.A. Chapter 8—Thixotropy and Dilatancy. In Rheology, Theory and Applications; Elsevier: Amsterdam, The Netherlands, 1967; Volume 4, pp. 423–459. ISBN 9781483229416. [Google Scholar]
- Mewis, J. Thixotropy—A general review. J. Non-Newton. Fluid Mech. 1979, 6, 1–20. [Google Scholar] [CrossRef]
- Barnes, H.H.A.; Barnes, A. Thixotropy—A review. J. Non-Newton. Fluid Mech. 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.D.; Kumar, S.S.; Chang, Y.; Kao, T.C.; Munusamy, M.A.; Alarfaj, A.A.; Hsu, S.T.; Umezawa, A. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, IPS, and adult stem cells. Prog. Polym. Sci. 2014, 39, 1585–1613. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Feng, Y.; Yan, Z.-C.; He, Y.-M.; Liu, C.-Y.; Fan, Q.-H. Multistimuli Responsive Dendritic Organogels Based on Azobenzene-Containing Poly(aryl ether) Dendron. Chem. Mater. 2012, 24, 3751–3757. [Google Scholar] [CrossRef]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. Preface to the Molecular and Polymer Gels; Materials with Self-Assembled Fibrillar Networks Special Issue. Langmuir 2009, 25, 8369. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U.; Kasagi, N.; Yamane, H.; Ojida, A.; Hamachi, I.; Maskos, K.; Reinhoudt, D. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C.; Sommerdijk, N.A.J.M.; Ajayaghosh, A.; Meskers, S.C.J.; Schenning, A.P.H.J.; Silva, C.; Friend, R.H.; Aida, T. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Das, R.K.; Maitra, U. Supramolecular gels “in action”. J. Mater. Chem. 2009, 19, 6649–6687. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.; Shinkai, S. What Kind of “Soft Materials” Can We Design from Molecular Gels? Chem.—Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Luo, Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. A Dual-Responsive Supramolecular Polymer Gel Formed by Crown Ether Based Molecular Recognition. Angew. Chem. Int. Ed. 2011, 50, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, C.; Castellucci, N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, C.; Zanna, N. Oxazolidinone-containing pseudopeptides: Supramolecular materials, fibers, crystals, and gels. Biopolymers 2017, 108, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ishi-i, T.; Shinkai, S. Dye-Based Organogels: Stimuli-Responsive Soft Materials Based on One-Dimensional Self-Assembling Aromatic Dyes. In Supermolecular Dye Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 119–160. [Google Scholar]
- Lloyd, G.O.; Steed, J.W. Anion-tuning of supramolecular gel properties. Nat. Chem. 2009, 1, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Chemically Reversible Organogels: Aliphatic Amines as “Latent” Gelators with Carbon Dioxide. J. Am. Chem. Soc. 2001, 123, 10393–10394. [Google Scholar] [CrossRef] [PubMed]
- John, G.; Zhu, G.; Li, J.; Dordick, J.S. Enzymatically Derived Sugar-Containing Self-Assembled Organogels with Nanostructured Morphologies. Angew. Chem. Int. Ed. 2006, 45, 4772–4775. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Maset, M.D.; Nebot, V.J.; Miravet, J.F.; Escuder, B.; Guggenheim, S.; van Esch, J.H.; Gradzielski, M.; Schalley, C.A.; Sefcik, J.; Boekhoven, J.; et al. Control of molecular gelation by chemical stimuli. Chem. Soc. Rev. 2013, 42, 7086–7098. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular Hydrogels Based on DNA Self-Assembly. Acc. Chem. Res. 2017, 50, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, T.; Nie, J.; Yang, D. Preparation and characterization of a photocrosslinkable bioadhesive inspired by marine mussel. J. Photochem. Photobiol. B Biol. 2013, 119, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Shigemitsu, H.; Fujisaku, T.; Onogi, S.; Yoshii, T.; Ikeda, M.; Hamachi, I. Preparation of supramolecular hydrogel–enzyme hybrids exhibiting biomolecule-responsive gel degradation. Nat. Protoc. 2016, 11, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Pé, B.; Ulijn, R.V.; Dalby, M.J. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016, 1, 298–319. [Google Scholar] [CrossRef]
- Singh, N.; Zhang, K.; Angulo-Pachón, C.A.; Mendes, E.; van Esch, J.H.; Escuder, B. Tandem reactions in self-sorted catalytic molecular hydrogels. Chem. Sci. 2016, 7, 5568–5572. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction. Angew. Chem. Int. Ed. 2016, 55, 9984–9987. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Ximenis, M.; Orvay, F.; Rotger, C.; Costa, A. Supramolecular Hydrogels Based on Minimalist Amphiphilic Squaramide–Squaramates for Controlled Release of Zwitterionic Biomolecules. Chem.—Eur. J. 2017, 23, 7590–7594. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Shao, Y.; Zhou, X.; Wu, Y.; Yang, Z.; Li, Z.; Weil, T.; Liu, D. A writable polypeptide-DNA hydrogel with rationally designed multi-modification sites. Small 2015, 11, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Matsumura, K. Thixotropic injectable hydrogel using a polyampholyte and nanosilicate prepared directly after cryopreservation. Mater. Sci. Eng. C 2016, 69, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Adv. Mater. 2017, 29, 1605021. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.M.; Shanmugam, G.; Duraipandy, N.; Kiran, M.S.; Mandal, A.B. An additional fluorenylmethoxycarbonyl (Fmoc) moiety in di-Fmoc-functionalized L-lysine induces pH-controlled ambidextrous gelation with significant advantages. Soft Matter 2015, 11, 8126–8140. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Adams, D.J.; Cameron, P.J. Peptide based low molecular weight gelators. J. Mater. Chem. 2011, 21, 2024–2027. [Google Scholar] [CrossRef]
- Tao, K.; Levin, A.; Adler-Abramovich, L.; Gazit, E. Fmoc-modified amino acids and short peptides: Simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016, 45, 3935–3953. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Brahmachari, S.; Das, P.K. In situ synthesised silver nanoparticle-infused L-lysine-based injectable hydrogel: Development of a biocompatible, antibacterial, soft nanocomposite. Chempluschem 2014, 79, 1733–1746. [Google Scholar]
- Haines-Butterick, L.; Rajagopal, K.; Branco, M.; Salick, D.; Rughani, R.; Pilarz, M.; Lamm, M.S.; Pochan, D.J.; Schneider, J.P. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, F.; Wen, Y.; Liu, K.; Chen, L.; Mao, Y.; Yang, S.; Yi, T. (−)-Menthol based thixotropic hydrogel and its application as a universal antibacterial carrier. Soft Matter 2014, 10, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Van Esch, J.H.; Feringa, B.L. New Functional Materials Based on Self-Assembling Organogels: From Serendipity towards Design. Angew. Chem. Int. Ed. 2000, 39, 2263–2266. [Google Scholar] [CrossRef]
- Steed, J.W. Anion-tuned supramolecular gels: A natural evolution from urea supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Wojciechowski, J.P.; Warren, H.; in het Panhuis, M.; Thordarson, P. Effect of heterocyclic capping groups on the self-assembly of a dipeptide hydrogel. Soft Matter 2016, 12, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Sharma, A.; Shiras, A.; Gopi, H.N. Backbone Engineered γ-Peptide Amphitropic Gels for Immobilization of Semiconductor Quantum Dots and 2D Cell Culture. Langmuir 2017, 33, 7762–7768. [Google Scholar] [CrossRef] [PubMed]
- Hoshizawa, H.; Minemura, Y.; Yoshikawa, K.; Suzuki, M.; Hanabusa, K. Thixotropic Hydrogelators Based on a Cyclo (dipeptide) Derivative. Langmuir 2013, 29, 14666–14673. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Roy, S.; Ghosh, S.; Hermida-Merino, D.; Hamley, I.W.; Banerjee, A. A Peptide-Based Mechano-sensitive, Proteolytically Stable Hydrogel with Remarkable Antibacterial Properties. Langmuir 2016, 32, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Milli, L.; Castellucci, N.; Tomasini, C. Turning Around the L -Phe- D -Oxd Moiety for a Versatile Low-Molecular-Weight Gelator. Eur. J. Org. Chem. 2014, 2014, 5954–5961. [Google Scholar] [CrossRef]
- Zanna, N.; Merlettini, A.; Tatulli, G.; Milli, L.; Focarete, M.L.; Tomasini, C. Hydrogelation Induced by Fmoc-Protected Peptidomimetics. Langmuir 2015, 31, 12240–12250. [Google Scholar] [CrossRef] [PubMed]
- Milli, L.; Zanna, N.; Merlettini, A.; Di Giosia, M.; Calvaresi, M.; Focarete, M.L.; Tomasini, C. Pseudopeptide-Based Hydrogels Trapping Methylene Blue and Eosin Y. Chem.—A Eur. J. 2016, 22, 12106–12112. [Google Scholar] [CrossRef] [PubMed]
- Zanna, N.; Merlettini, A.; Tomasini, C. Self-healing hydrogels triggered by amino acids. Org. Chem. Front. 2016, 3, 1699–1704. [Google Scholar] [CrossRef]
- Zanna, N.; Iaculli, D.; Tomasini, C. The effect of L-DOPA hydroxyl groups on the formation of supramolecular hydrogels. Org. Biomol. Chem. 2017, 15, 5797–5804. [Google Scholar] [CrossRef] [PubMed]
- Zanna, N.; Focaroli, S.; Merlettini, A.; Gentilucci, L.; Teti, G.; Falconi, M.; Tomasini, C. Thixotropic Peptide-Based Physical Hydrogels Applied to Three-Dimensional Cell Culture. ACS Omega 2017, 2, 2374–2381. [Google Scholar] [CrossRef]
- Ramin, M.A.; Latxague, L.; Sindhu, K.R.; Chassande, O.; Barthélémy, P. Low molecular weight hydrogels derived from urea based-bolaamphiphiles as new injectable biomaterials. Biomaterials 2017, 145, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. C 2015, 45, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.P.; Wei, Y.; Leach, J.B. Protein-hydrogel interactions in tissue engineering: Mechanisms and applications. Tissue Eng. Part B Rev. 2013, 19, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, W.; Vats, K.; Rajbhandary, A.; Benoit, D.S.W.; Nilsson, B.L. Multicomponent dipeptide hydrogels as extracellular matrix-mimetic scaffolds for cell culture applications. Chem. Commun. 2015, 51, 11260–11263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Battig, M.R.; Chen, N.; Gaddes, E.R.; Duncan, K.L.; Wang, Y. Chimeric Aptamer-Gelatin Hydrogels as an Extracellular Matrix Mimic for Loading Cells and Growth Factors. Biomacromolecules 2016, 17, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Wu, Y.; Chakraborty, N.; Mohanty, P.; Ghosh, G. Impact of alginate concentration on the viability, cryostorage, and angiogenic activity of encapsulated fibroblasts. Mater. Sci. Eng. C 2016, 65, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Focaroli, S.; Teti, G.; Salvatore, V.; Orienti, I.; Falconi, M. Calcium/Cobalt Alginate Beads as Functional Scaffolds for Cartilage Tissue Engineering. Stem Cells Int. 2016, 2016, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.F.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl-dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Liang, G.; Yang, Z.; Zhang, R.; Li, L.; Fan, Y.; Kuang, Y.; Gao, Y.; Wang, T.; Lu, W.W.; Xu, B. Supramolecular hydrogel of a D-amino acid dipeptide for controlled drug release in vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.F.; Devgun, J.M.; Collier, J.H. Fibrillized peptide microgels for cell encapsulation and 3D cell culture. Soft Matter 2011, 7, 6005–6011. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.P.; Nagaraj, A.K.; Fox, E.K.; Rudra, J.S.; Devgun, J.M.; Collier, J.H. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 2009, 30, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.T.; Shek, P.N. Novel wound sealants: Biomaterials and applications. Expert Rev. Med. Devices 2010, 7, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.V.; Kumar, R.A.; Sivashanmugam, A.; Nair, S.V.; Jayakumar, R. Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications. J. Funct. Biomater. 2015, 6, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Laurenti, M.; Al Subaie, A.; Abdallah, M.N.; Cortes, A.R.G.; Ackerman, J.L.; Vali, H.; Basu, K.; Zhang, Y.L.; Murshed, M.; Strandman, S.; et al. Two-Dimensional Magnesium Phosphate Nanosheets Form Highly Thixotropic Gels That Up-Regulate Bone Formation. Nano Lett. 2016, 16, 4779–4787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Liu, Q.; Wickremasinghe, N.C.; Shi, S.; Cornwright, T.T.; Deng, Y.; Azares, A.; Moore, A.N.; Acevedo-Jake, A.M.; Agudo, N.R.; et al. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials 2016, 98, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Maity, I.; Parmar, H.S.; McDonald, T.O.; Konda, M. Lipase-Catalyzed Dissipative Self-Assembly of a Thixotropic Peptide Bolaamphiphile Hydrogel for Human Umbilical Cord Stem-Cell Proliferation. Biomacromolecules 2015, 16, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ling, S.; Wang, S.; Chen, X.; Shao, Z. Thixotropic silk nanofibril-based hydrogel with extracellular matrix-like structure. Biomater. Sci. 2014, 2, 1338–1342. [Google Scholar] [CrossRef]
- Wu, H.; Liu, S.; Xiao, L.; Dong, X.; Lu, Q.; Kaplan, D.L. Injectable and pH-Responsive Silk Nanofiber Hydrogels for Sustained Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17118–17126. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Han, H.; Fan, Z.; Lu, H.; Sang, Y.; Yao, Y.; Cheng, Q.; Lu, Q.; Kaplan, D.L. Nanoscale Silk-Hydroxyapatite Hydrogels for Injectable Bone Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 16913–16921. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Liu, Y.; Chen, X.; Shao, Z. Structure and properties of various hybrids fabricated by silk nanofibrils and nanohydroxyapatite. Nanoscale 2016, 8, 20096–20102. [Google Scholar] [CrossRef] [PubMed]






















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanna, N.; Tomasini, C. Peptide-Based Physical Gels Endowed with Thixotropic Behaviour. Gels 2017, 3, 39. https://doi.org/10.3390/gels3040039
Zanna N, Tomasini C. Peptide-Based Physical Gels Endowed with Thixotropic Behaviour. Gels. 2017; 3(4):39. https://doi.org/10.3390/gels3040039
Chicago/Turabian StyleZanna, Nicola, and Claudia Tomasini. 2017. "Peptide-Based Physical Gels Endowed with Thixotropic Behaviour" Gels 3, no. 4: 39. https://doi.org/10.3390/gels3040039
APA StyleZanna, N., & Tomasini, C. (2017). Peptide-Based Physical Gels Endowed with Thixotropic Behaviour. Gels, 3(4), 39. https://doi.org/10.3390/gels3040039