Challenges for Natural Hydrogels in Tissue Engineering
Abstract
1. Introduction
2. Sources of Protein-Based Biopolymers and Their Isolation Methods
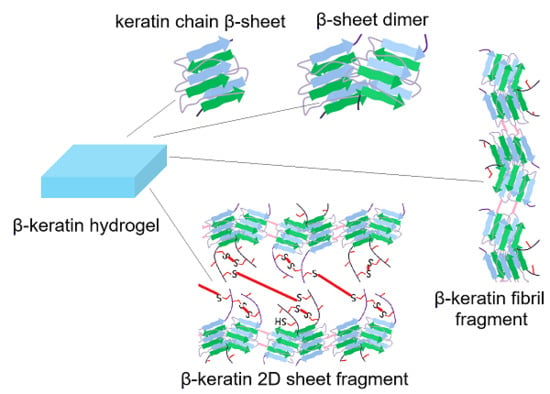
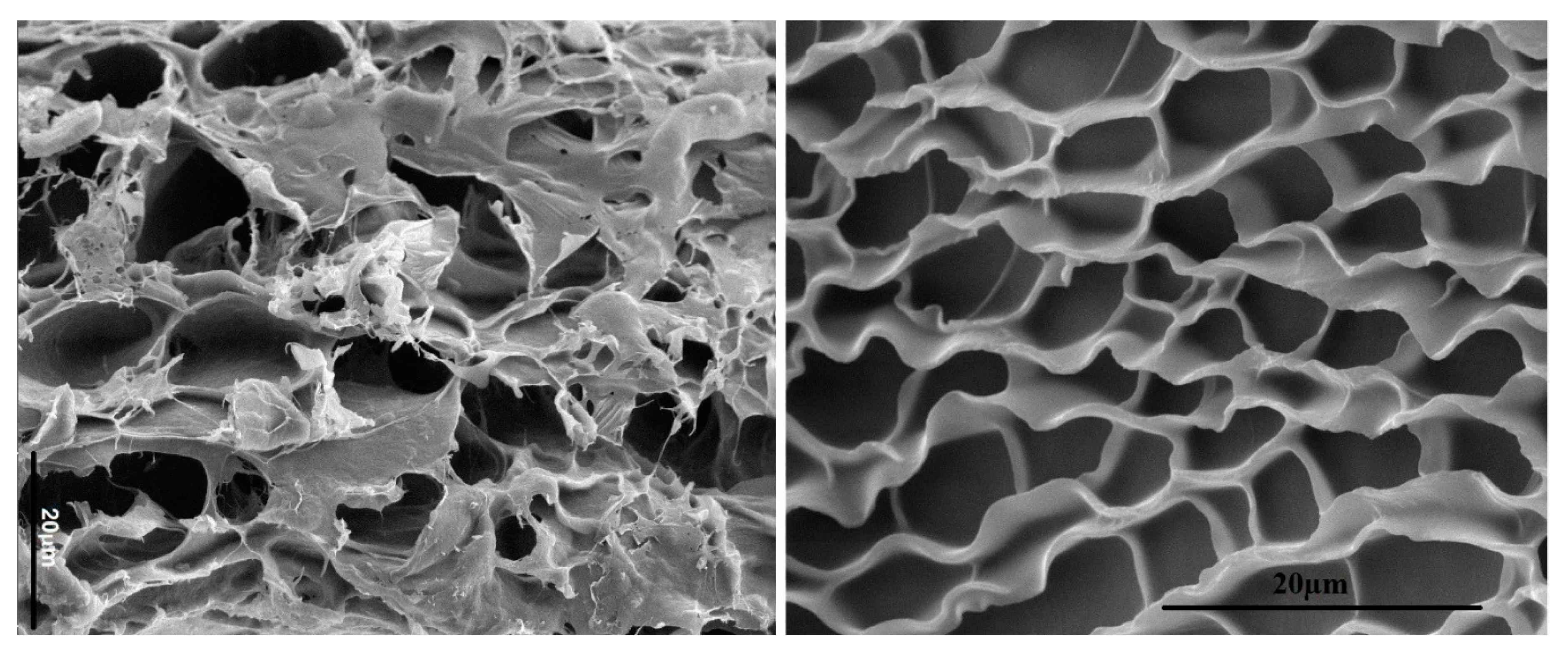
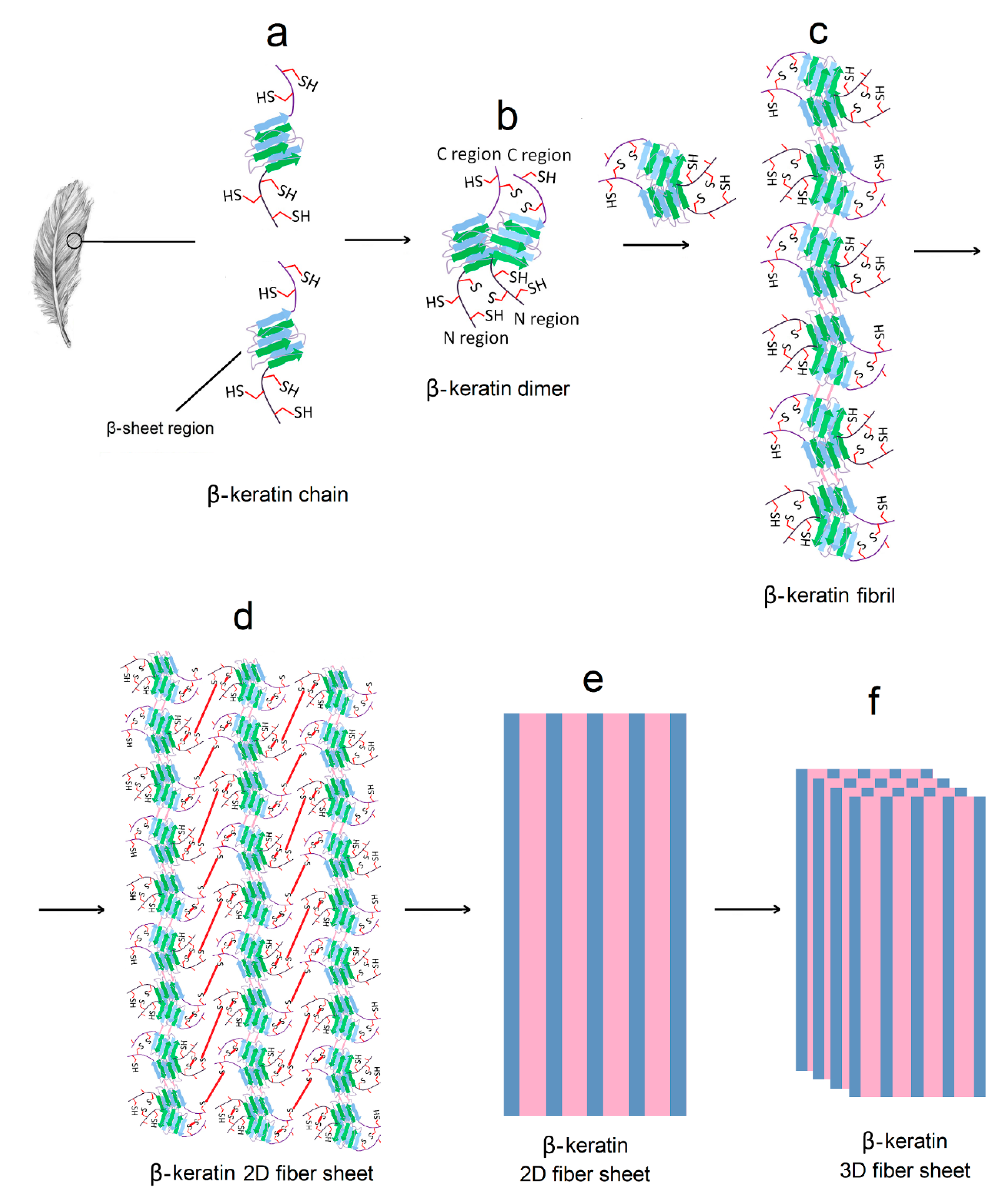
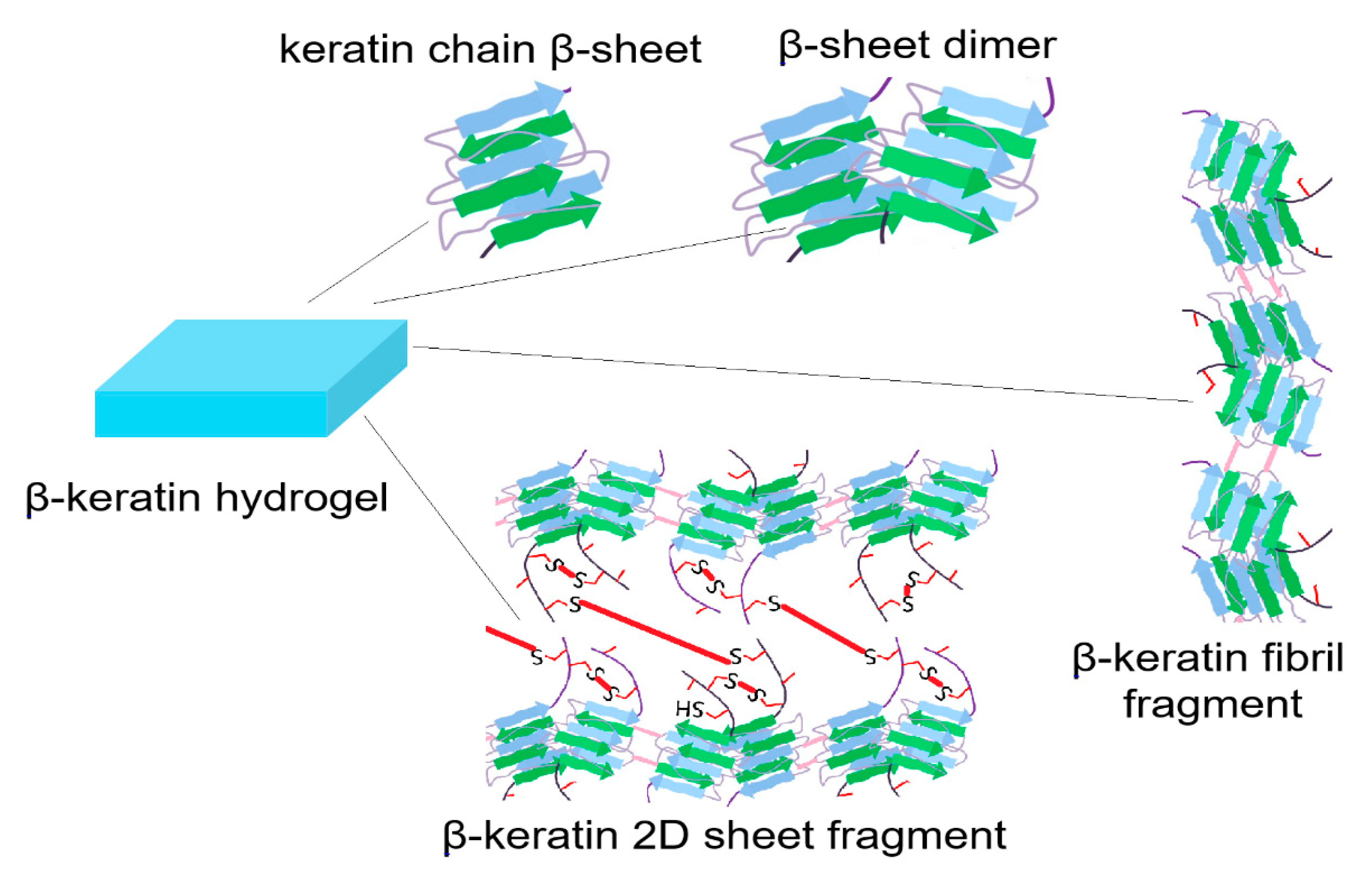
3. Structure of Protein-Based Hydrogels
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, X.; Yang, F. Recent progress in developing injectable matrices for enhancing cell delivery and tissue regeneration. Adv. Healthc. Mater. 2018, 7, e1701065. [Google Scholar] [CrossRef] [PubMed]
- Flegeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, D.A. Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering. Tissue Eng. B Rev. 2017, 23, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shim, K.Y.; Kim, B.; Sung, J.H. Hydrogel-based three-dimensional cell culture for organ-on-a-chip applications. Biotechnol. Prog. 2017, 33, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.A.; Ramos, D.N.; Lopez, R.F. Hydrogel increases localized transport regions and skin permeability during low frequency ultrasound treatment. Sci. Rep. 2017, 7, 44236. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.A. Effects of permeability and living space on cell fate and neo-tissue development in hydrogel-based scaffolds: A study with cartilaginous model. Macromol. Biosci. 2015, 15, 535–545. [Google Scholar] [CrossRef]
- Aurora, A.; Wrice, N.; Walters, T.J.; Christy, R.J.; Natesan, S. A PEGylated platelet free plasma hydrogel based composite scaffold enables stable vascularization and targeted cell delivery for volumetric muscle loss. Acta Biomater. 2018, 65, 150–162. [Google Scholar] [CrossRef]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (epsilon-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef]
- Ziv, K.; Nuhn, H.; Ben-Haim, Y.; Sasportas, L.S.; Kempen, P.J.; Niedringhaus, T.P.; Hrynyk, M.; Sinclair, R.; Barron, A.E.; Gambhir, S.S. A tunable silk-alginate hydrogel scaffold for stem cell culture and transplantation. Biomaterials 2014, 35, 3736–3743. [Google Scholar] [CrossRef]
- Barati, D.; Shariati, S.R.P.; Moeinzadeh, S.; Melero-Martin, J.M.; Khademhosseini, A.; Jabbari, E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Control. Rel. 2016, 223, 126–136. [Google Scholar] [CrossRef]
- Barati, D.; Kader, S.; Pajoum Shariati, S.R.; Moeinzadeh, S.; Sawyer, R.H.; Jabbari, E. Synthesis and characterization of photo-cross-linkable keratin hydrogels for stem cell encapsulation. Biomacromolecules 2017, 18, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, S.; Pajoum Shariati, S.R.; Jabbari, E. Comparative effect of physicomechanical and biomolecular cues on zone-specific chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2016, 92, 57–70. [Google Scholar] [CrossRef]
- Moeinzadeh, S.; Monavarian, M.; Kader, S.; Jabbari, E. Sequential zonal chondrogenic differentiation of mesenchymal stem cells in cartilage matrices. Tissue Eng. A 2019, 25, 234–247. [Google Scholar] [CrossRef]
- Patel, D.; Sharma, S.; Screen, H.R.C.; Bryant, S.J. Effects of cell adhesion motif, fiber stiffness, and cyclic strain on tenocyte gene expression in a tendon mimetic fiber composite hydrogel. Biochem. Biophys. Res. Commun. 2018, 499, 642–647. [Google Scholar] [CrossRef]
- Komatsu, M.; Konagaya, S.; Egawa, E.Y.; Iwata, H. Maturation of human iPS cell-derived dopamine neuron precursors in alginate-Ca(2+) hydrogel. Biochim. Biophys. Acta 2015, 1850, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical biopolymers, their origin and evolution in biomedical sciences: A systematic review. J. Clin. Diagn. Res. 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef] [PubMed]
- Krug, C.; Beer, A.; Hartmann, B.; Prein, C.; Clausen-Schaumann, H.; Holzbach, T.; Aszodi, A.; Giunta, R.E.; Saller, M.M.; Volkmer, E. Fibrin glue displays promising in vitro characteristics as a potential carrier of adipose progenitor cells for tissue regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 359–368. [Google Scholar] [CrossRef]
- Lee, S.J.; Wang, H.J.; Kim, T.H.; Choi, J.S.; Kulkarni, G.; Jackson, J.D.; Atala, A.; Yoo, J.J. In situ tissue regeneration of renal tissue induced by collagen hydrogel injection. Stem Cells Transl. Med. 2018, 7, 241–250. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk fibroin-based scaffold for bone tissue engineering. Adv. Exp. Med. Biol. 2018, 1077, 371–387. [Google Scholar]
- Zhao, Y.H.; Yang, Q.; Xia, Q.; Peng, J.; Lu, S.B.; Guo, Q.Y.; Ma, X.L.; Xu, B.S.; Hu, Y.C.; Zhao, B.; et al. In vitro cartilage production using an extracellular matrix-derived scaffold and bone marrow-derived mesenchymal stem cells. Chin. Med. J. 2013, 126, 3130–3137. [Google Scholar]
- Mahn, I.; Krell, W.; Muller-Berghaus, G. Separation of human des-AB fibrin and fibrinogen by sepharose-plasma chromatography at 20 °C and 37 °C. Thromb. Res. 1979, 14, 651–663. [Google Scholar] [CrossRef]
- Santos, M.H.; Silva, R.M.; Dumont, V.C.; Neves, J.S.; Mansur, H.S.; Heneine, L.G. Extraction and characterization of highly purified collagen from bovine pericardium for potential bioengineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 790–800. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gelling properties of gelatin from goat skin as affected by drying methods. J. Food Sci. Technol. 2017, 54, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Levett, P.A.; te Moller, N.C.; Besems, J.; Boere, K.W.; van Rijen, M.H.; de Grauw, J.C.; Dhert, W.J.; van Weeren, P.R.; Malda, J. Crosslinkable hydrogels derived from cartilage, meniscus, and tendon tissue. Tissue Eng. Part A 2015, 21, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Cervantes, S.D.; Vicente-Cervantes, D.; Meseguer-Olmo, L.; Cenis, J.L.; Lozano-Perez, A.A. Influence of the protocol used for fibroin extraction on the mechanical properties and fiber sizes of electrospun silk mats. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Siritientong, T.; Bonani, W.; Motta, A.; Migliaresi, C.; Aramwit, P. The effects of Bombyx mori silk strain and extraction time on the molecular and biological characteristics of sericin. Biosci. Biotechnol. Biochem. 2016, 80, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Pacak, C.A.; MacKay, A.A.; Cowan, D.B. An improved method for the preparation of type I collagen from skin. J. Vis. Exp. 2014, e51011. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Khatibi, A.H.; Verma, P.; Jamshidi, D.; Nevins, A.J.; Torabinejad, M. Clinical, radiographic, and histologic outcome of regenerative endodontic treatment in human teeth using a novel collagen-hydroxyapatite scaffold. J. Endod. 2019, 45, 136–143. [Google Scholar] [CrossRef]
- Farkash, U.; Avisar, E.; Volk, I.; Slevin, O.; Shohat, N.; El Haj, M.; Dolev, E.; Ashraf, E.; Luria, S. First clinical experience with a new injectable recombinant human collagen scaffold combined with autologous platelet-rich plasma for the treatment of lateral epicondylar tendinopathy (tennis elbow). J. Shoulder Elbow Surg. 2019, 28, 503–509. [Google Scholar] [CrossRef]
- Xu, D.; Zhuang, Q.; Li, Z.; Ren, Z.; Chen, X.; Li, S. A randomized controlled trial on the effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries. J. Orthop. Surg. Res. 2017, 12, 166. [Google Scholar] [CrossRef]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A Novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef]
- Grogan, S.P.; Duffy, S.F.; Pauli, C.; Koziol, J.A.; Su, A.I.; D’Lima, D.D.; Lotz, M.K. Zone-specific gene expression patterns in articular cartilage. Arthritis Rheum. 2013, 65, 418–428. [Google Scholar] [CrossRef]
- Karimi, T.; Barati, D.; Karaman, O.; Moeinzadeh, S.; Jabbari, E. A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration. Integr. Biol. 2015, 7, 112–127. [Google Scholar] [CrossRef]
- Yang, P.J.; Temenoff, J.S. Engineering orthopedic tissue interfaces. Tissue Eng. B Rev. 2009, 15, 127–141. [Google Scholar] [CrossRef]
- Alibardi, L. Review: Cornification, morphogenesis and evolution of feathers. Protoplasma 2017, 254, 1259–1281. [Google Scholar] [CrossRef]
- Belarmino, D.B.D.; Ladchumananandasivam, R.; Belarmino, L.D.; Pimentel, J.R.D.M.; da Rocha, B.G.; Galv, A.O.; de Andrade, S.M.B. Physical and morphological structure of chicken feathers (keratin biofiber) in natural, chemically and thermally modified forms. Mater. Sci. Appl. 2012, 3, 887–893. [Google Scholar] [CrossRef]
- Han, S.; Ham, T.R.; Haque, S.; Sparks, J.L.; Saul, J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater. 2015, 23, 201–213. [Google Scholar] [CrossRef]
- Yamauchi, K.; Yamauchi, A.; Kusunoki, T.; Kohda, A.; Konishi, Y. Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J. Biomed. Mater. Res. 1996, 31, 439–444. [Google Scholar] [CrossRef]
- Lin, Y.A.; Chalker, J.M.; Floyd, N.; Bernardes, G.A.J.L.; Davis, B.G. Allyl sulfides are privileged substrates in aqueous cross-metathesis: Application to site-selective protein modification. J. Am. Chem. Soc. 2008, 130, 9642–9643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta Prot. Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B.; Shanmugam, G. Extraction and characterization of keratin from bovine hoof: A potential material for biomedical applications. Springerplus 2014, 3, 596. [Google Scholar] [CrossRef]
- Hached, F.; Vinatier, C.; Le Visage, C.; Gonde, H.; Guicheux, J.; Grimandi, G.; Billon-Chabaud, A. Biomaterial-assisted cell therapy in osteoarthritis: From mesenchymal stem cells to cell encapsulation. Best Pract. Res. Clin. Rheumatol. 2017, 31, 730–745. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, X.; Ngai, T. Measurements of long-range interactions between protein-functionalized surfaces by total internal reflection microscopy. Langmuir 2015, 31, 3101–3107. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Chen, C.S. Measuring cell-generated forces: A guide to the available tools. Nat. Methods 2016, 13, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, R.A. Theoretical principles and practical considerations for fluorescence resonance energy transfer microscopy. Methods Cell Biol. 2007, 81, 479–494. [Google Scholar]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbari, E. Challenges for Natural Hydrogels in Tissue Engineering. Gels 2019, 5, 30. https://doi.org/10.3390/gels5020030
Jabbari E. Challenges for Natural Hydrogels in Tissue Engineering. Gels. 2019; 5(2):30. https://doi.org/10.3390/gels5020030
Chicago/Turabian StyleJabbari, Esmaiel. 2019. "Challenges for Natural Hydrogels in Tissue Engineering" Gels 5, no. 2: 30. https://doi.org/10.3390/gels5020030
APA StyleJabbari, E. (2019). Challenges for Natural Hydrogels in Tissue Engineering. Gels, 5(2), 30. https://doi.org/10.3390/gels5020030