Chlorhexidine Gel Use in the Oral District: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
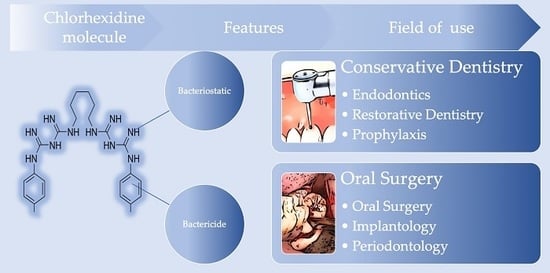
2. Results and Discussion
2.1. Synthesis of Results
2.2. Summary of Evidence
- Powerful bactericide: Chlorhexidine alters the protein structure of the bacterial cell membrane: by exaggeratingly increasing its permeability, the substance promotes the precipitation of cytoplasmic proteins and the consequent cell death by bacterial lysis.
- Bacteriostatic: Once it was believed that chlorhexidine could block the replication of bacteria; today it is well known that it is also able to kill them.
2.3. Additional Analyses
2.4. Limitations
3. Conclusions
4. Materials and Methods
4.1. Protocol and Registration
4.2. Focus Question
4.3. Information Sources
4.4. Search
4.5. Selection of Studies
4.6. Types of Selected Manuscripts
4.7. Types of Studies
4.8. Inclusion and Exclusion Criteria
- Chlorhexidine gel use in randomized clinical trials (RCTs)
- Roles of chlorhexidine gel
- Human RCTs
- Studies involving patients with other specific diseases, immunologic disorders or other oral risk related systemic conditions
- Not enough information regarding the selected topic
- No access to the title and abstract in English language
- Not older than 10 years RCTs
4.9. Sequential Search Strategy
4.10. Data Extraction
4.11. Data Collections
- “Author (Year)”—Revealed the author and year of publication
- “Features”—Results and features evaluated about chlorhexidine gel
- “Field”—Field of use of chlorhexidine gel
- “Statistics”—Significative or not results
- “Type”—Type of article
4.12. Risk of Bias Assessment
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NaOCL | sodium hypochlorite |
| CHX | chlorhexidine |
| DS | Dry socket |
References
- Zhou, W.; Ren, B.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Li, M.; Feng, M.; Li, J.; Xu, X.; Cheng, L. Novel Cavity Disinfectants Containing Quaternary Ammonium Monomer Dimethylaminododecyl Methacrylate. Materials 2016, 9, 674. [Google Scholar] [CrossRef]
- Haseeb, R.; Lau, M.; Sheah, M.; Montagner, F.; Quiram, G.; Palmer, K.; Stefan, M.C.; Rodrigues, D.C. Synthesis and Characterization of New Chlorhexidine-Containing Nanoparticles for Root Canal Disinfection. Materials 2016, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Stanevičiūtė, E.; Builytė, I.U.; Ridziauskas, M.; Besusparis, J.; Kirkliauskienė, A.; Zabulis, V.; Davainis, L.; Valiūnaitė, G.; Triponis, V.; Sirvydis, V. Efficacy of Antiseptic Solutions in Treatment of Staphylococcus Aureus Infected Surgical Wounds with Patches of Vascular Graft: An Experimental Study in Rats. Medicina 2019, 55, 106. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.B.; Souza, M.G.M.D.; Moreira, M.A.; Moreira, M.R.; Furtado, N.A.J.C.; Martins, C.H.G.; Bastos, J.K.; Santos, R.A.D.; Heleno, V.C.G.; Ambrosio, S.R.; et al. Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria. Molecules 2011, 16, 9611–9619. [Google Scholar] [CrossRef] [PubMed]
- Severiano, M.E.; Simao, M.R.; Porto, T.S.; Martins, C.H.G.; Veneziani, R.C.S.; Furtado, N.A.J.C.; Arakawa, N.S.; Said, S.; Oliveira, D.C.R.D.; Cunha, W.R.; et al. Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation. Molecules 2010, 15, 8553–8566. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Hidalgo-Bastida, L.A.; Verran, J.; Williams, D.; Malic, S. Antifungal Activity of Commercial Essential Oils and Biocides against Candida Albicans. Pathogens 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Oteri, G.; Procopio, R.M.; Cicciù, M. Giant salivary gland calculi (GSGC): Report of two cases. Open Dent. J. 2011, 5, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Gostemeyer, G.; Schulze, F.; Paris, S.; Schwendicke, F. Arrest of Root Carious Lesions via Sodium Fluoride, Chlorhexidine and Silver Diamine Fluoride In Vitro. Materials 2017, 11, 9. [Google Scholar] [CrossRef]
- Wright, P.P.; Kahler, B.; Walsh, L.J. Alkaline Sodium Hypochlorite Irrigant and Its Chemical Interactions. Materials 2017, 10, 1147. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Spagnuolo, G.; Bramanti, E.; Laino, L.; Lauritano, F.; Cicciu, M. Interface Between MTA and Dental Bonding Agents: Scanning Electron Microscope Evaluation. J. Int. Soc. Prev. Community Dent. 2017, 7, 64–68. [Google Scholar]
- Cicciu, M.; Cervino, G.; Herford, A.S.; Fama, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Maridati, P.; Stoffella, E.; Speroni, S.; Cicciu, M.; Maiorana, C. Alveolar antral artery isolation during sinus lift procedure with the double window technique. Open Dent. J. 2014, 8, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Beretta, M.; Cicciù, M.; Bramanti, E.; Maiorana, C. Schneider membrane elevation in presence of sinus septa: Anatomic features and surgical management. Int. J. Dent. 2012, 2012, 261905. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.M.; Selvan, S.T.; Narayanan, K.; Fawzy, A.S. Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material. Bioengineering 2017, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yang, H.; Li, K.; Yu, J.; Huang, C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules 2017, 22, 1225. [Google Scholar] [CrossRef] [PubMed]
- Giammarinaro, E.; Marconcini, S.; Genovesi, A.; Poli, G.; Lorenzi, C.; Covani, U. Propolis as an adjuvant to non-surgical periodontal treatment: A clinical study with salivary anti-oxidant capacity assessment. Minerva Stomatol. 2018, 67, 183–188. [Google Scholar]
- Lobo, P.L.; Fonteles, C.S.; Marques, L.A.; Jamacaru, F.V.; Fonseca, S.G.; de Carvalho, C.B.; de Moraes, M.E. The efficacy of three formulations of Lippia sidoides Cham. essential oil in the reduction of salivary Streptococcus mutans in children with caries: A randomized, double-blind, controlled study. Phytomed. Int. J. Phytoth. Phytopharmacol. 2014, 21, 1043–1047. [Google Scholar] [CrossRef]
- Sundell, A.L.; Ullbro, C.; Koch, G. Evaluation of preventive programs in high caries active preschool children. Swedish Dent. J. 2013, 37, 23–29. [Google Scholar]
- Coello-Gomez, A.; Navarro-Suarez, S.; Diosdado-Cano, J.M.; Azcarate-Velazquez, F.; Bargiela-Perez, P.; Serrera-Figallo, M.A.; Torres-Lagares, D.; Gutierrez-Perez, J.L. Postoperative effects on lower third molars of using mouthwashes with super-oxidized solution versus 0.2% chlorhexidine gel: A randomized double-blind trial. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e716–e722. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-Implant Bone Resorption during Healing Abutment Placement: The Effect of a 0.20% Chlorhexidine Gel vs. Placebo-A Randomized Double Blind Controlled Human Study. Biomed. Res. Int. 2018, 2018, 532634. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Stratul, S.I.; Sarbu, C.; Roman, A.; Anghel, A.; Didilescu, A.; Jentsch, H. Evaluation of a hydrophobic gel adhering to the gingiva in comparison with a standard water-soluble 1% chlorhexidine gel after scaling and root planing in patients with moderate chronic periodontitis. A randomized clinical trial. Int. J. Dent. Hyg. 2017, 15, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Palau, J.; Garcia-Linares, J.; Hueto-Madrid, J.A.; Gonzalez-Lagunas, J.; Raspall-Martin, G.; Mareque-Bueno, J. Effect of intra-alveolar placement of 0.2% chlorhexidine bioadhesive gel on the incidence of alveolar osteitis following the extraction of mandibular third molars. A double-blind randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e117–e222. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Frankenthal, S.; Joseph, L.; Rozitsky, D.; Levi, G.; Machtei, E.E. Water jet with adjunct chlorhexidine gel for nonsurgical treatment of peri-implantitis. Quintessence Int. 2015, 46, 133–137. [Google Scholar] [PubMed]
- Jesudasan, J.S.; Wahab, P.U.; Sekhar, M.R. Effectiveness of 0.2% chlorhexidine gel and a eugenol-based paste on postoperative alveolar osteitis in patients having third molars extracted: A randomised controlled clinical trial. Br. J. Oral Maxillofac. Surg. 2015, 53, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Haraji, A.; Rakhshan, V. Chlorhexidine gel and less difficult surgeries might reduce post-operative pain, controlling for dry socket, infection and analgesic consumption: A split-mouth controlled randomised clinical trial. J. Oral Rehabil. 2015, 42, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Freudenthal, N.; Sternudd, M.; Jansson, L.; Wannfors, K. A double-blind randomized study evaluating the effect of intra-alveolar chlorhexidine gel on alveolar osteitis after removal of mandibular third molars. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2015, 73, 600–605. [Google Scholar] [CrossRef]
- Diaz-Sanchez, R.M.; Pachon-Ibanez, J.; Marin-Conde, F.; Rodriguez-Caballero, A.; Gutierrez-Perez, J.L.; Torres-Lagares, D. Double-blind, randomized pilot study of bioadhesive chlorhexidine gel in the prevention and treatment of mucositis induced by chemoradiotherapy of head and neck cancer. Med. Oral Patol.Oral Cir. Bucal 2015, 20, e378–e385. [Google Scholar] [CrossRef]
- Haraji, A.; Rakhshan, V. Single-dose intra-alveolar chlorhexidine gel application, easier surgeries and younger ages are associated with reduced dry socket risk. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2014, 72, 259–265. [Google Scholar] [CrossRef]
- Singh, R.D.; Khatter, R.; Bal, R.K.; Bal, C.S. Intracanal medications versus placebo in reducing postoperative endodontic pain—A double-blind randomized clinical trial. Braz. Dent. J. 2013, 24, 25–29. [Google Scholar] [CrossRef]
- Pukallus, M.L.; Plonka, K.A.; Barnett, A.G.; Walsh, L.J.; Holcombe, T.F.; Seow, W.K. A randomised, controlled clinical trial comparing chlorhexidine gel and low-dose fluoride toothpaste to prevent early childhood caries. Int. J. Paediatr. Dent. 2013, 23, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.A.; Carvalho, C.B.; Ribeiro, T.R.; Fonteles, C.S. Antimicrobial efficacy of chlorhexidine and calcium hydroxide/camphorated paramonochlorophenol on infected primary molars: A split-mouth randomized clinical trial. Quintessence Int. 2013, 44, 113–122. [Google Scholar] [PubMed]
- De Siena, F.; Francetti, L.; Corbella, S.; Taschieri, S.; Del Fabbro, M. Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri-implant mucositis. An observational study. Int. J. Dent. Hyg. 2013, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- de Lucena, J.M.; Decker, E.M.; Walter, C.; Boeira, L.S.; Lost, C.; Weiger, R. Antimicrobial effectiveness of intracanal medicaments on Enterococcus faecalis: Chlorhexidine versus octenidine. Int. Endod. J. 2013, 46, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.; Marques, E.; De Martin, A.S.; da Silveira Bueno, C.E.; Nowakowski, A.; Cunha, R.S. Influence of irrigating solution on postoperative pain following single-visit endodontic treatment: Randomized clinical trial. J. Can. Dent. Assoc. 2012, 78, c84. [Google Scholar] [PubMed]
- Heitz-Mayfield, L.J.; Salvi, G.E.; Botticelli, D.; Mombelli, A.; Faddy, M.; Lang, N.P. Anti-infective treatment of peri-implant mucositis: A randomised controlled clinical trial. Clin. Oral Implant. Res. 2011, 22, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lagares, D.; Gutierrez-Perez, J.L.; Hita-Iglesias, P.; Magallanes-Abad, N.; Flores-Ruiz, R.; Basallote-Garcia, M.; Gonzalez-Martin, M. Randomized, double-blind study of effectiveness of intra-alveolar application of chlorhexidine gel in reducing incidence of alveolar osteitis and bleeding complications in mandibular third molar surgery in patients with bleeding disorders. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2010, 68, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Laino, L.; Cicciù, M.; Fiorillo, L.; Crimi, S.; Bianchi, A.; Amoroso, G.; Monte, I.P.; Herford, A.S.; Cervino, G. Surgical Risk on Patients with Coagulopathies: Guidelines on Hemophiliac Patients for Oro-Maxillofacial Surgery. Int. J. Environ. Res. Public Health 2019, 16, 1386. [Google Scholar] [CrossRef]
- Fiorillo, L.; De Stefano, R.; Cervino, G.; Crimi, S.; Bianchi, A.; Campagna, P.; Herford, A.S.; Laino, L.; Cicciù, M. Oral and Psychological Alterations in Haemophiliac Patients. Biomedicines 2019, 7, 33. [Google Scholar] [CrossRef]
- Slot, D.E.; Rosema, N.A.; Hennequin-Hoenderdos, N.L.; Versteeg, P.A.; Van Der Velden, U.; Van Der Weijden, G.A. The effect of 1% chlorhexidine gel and 0.12% dentifrice gel on plaque accumulation: A 3-day non-brushing model. Int. J. Dent. Hyg. 2010, 8, 294–300. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Martinez-Canovas, A. Clinical evaluation of polyvinylpyrrolidone sodium hyaluronate gel and 0.2% chlorhexidine gel for pain after oral mucosa biopsy: A preliminary study. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2010, 68, 2159–2163. [Google Scholar] [CrossRef]
- Cabov, T.; Macan, D.; Husedzinovic, I.; Skrlin-Subic, J.; Bosnjak, D.; Sestan-Crnek, S.; Peric, B.; Kovac, Z.; Golubovic, V. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: A randomized placebo-controlled study. Wien. Klinische Wochenschr. 2010, 122, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; D’Ercole, S.; Pilloni, A.; D’Archivio, D.; Lisanti, L.; Graziani, F.; Femminella, B.; Sammartino, G.; Perillo, L.; Tete, S.; et al. Clinical, microbiologic and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J. Periodontol. 2009, 80, 1479–1492. [Google Scholar] [CrossRef]
- Malkhassian, G.; Manzur, A.J.; Legner, M.; Fillery, E.D.; Manek, S.; Basrani, B.R.; Friedman, S. Antibacterial efficacy of MTAD final rinse and two percent chlorhexidine gel medication in teeth with apical periodontitis: A randomized double-blinded clinical trial. J. Endod. 2009, 35, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Gerspach, I.; Pfaffli-Savtchenko, V.; Dahnhardt, J.E.; Meyer, J.; Lussi, A. Comparison of the immediate effects of gaseous ozone and chlorhexidine gel on bacteria in cavitated carious lesions in children in vivo. Clin. Oral Investig. 2009, 13, 287–291. [Google Scholar] [CrossRef]
- Gomes, B.P.; Martinho, F.C.; Vianna, M.E. Comparison of 2.5% sodium hypochlorite and 2% chlorhexidine gel on oral bacterial lipopolysaccharide reduction from primarily infected root canals. J. Endod. 2009, 35, 1350–1353. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and Periodontal Disease: The Panacea to Problem Solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Sortino, F.; Cicciù, M. Strategies used to inhibit postoperative swelling following removal of impacted lower third molar. Dent. Res. J. 2011, 8, 162–171. [Google Scholar]
- Cicciù, M.; Risitano, G.; Lo Giudice, G.; Bramanti, E. Periodontal health and caries prevalence evaluation in patients affected by Parkinson’s disease. Parkinsons Dis. 2012, 541908. [Google Scholar] [CrossRef]
- Lombardi, T.; Bernardello, F.; Berton, F.; Porrelli, D.; Rapani, A.; Camurri Piloni, A.; Fiorillo, L.; Di Lenarda, R.; Stacchi, C. Efficacy of Alveolar Ridge Preservation after Maxillary Molar Extraction in Reducing Crestal Bone Resorption and Sinus Pneumatization: A Multicenter Prospective Case-Control Study. Biomed. Res. Int. 2018, 2018, 9352130. [Google Scholar] [CrossRef]
- Cervino, G.; Terranova, A.; Briguglio, F.; De Stefano, R.; Famà, F.; D’Amico, C.; Amoroso, G.; Marino, S.; Gorassini, F.; Mastroieni, R.; et al. Diabetes: Oral health related quality of life and oral alterations. Biomed. Res. Int. 2019, 2019, 5907195. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Romeo, U.; Bianchi, A.; Crimi, S.; D’Amico, C.; De Stefano, R.; Troiano, G.; Santoro, R.; et al. Molecular Biomarkers Related to Oral Carcinoma: Clinical Trial Outcome Evaluation in a Literature Review. Dis. Markers 2019, 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Ramaglia, L.; Cordasco, G.; Lucchese, A.; Fiorillo, L.; Matarese, G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017, 66, 1–8. [Google Scholar] [PubMed]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Lauritano, F.; D’Amico, C.; Lo Giudice, R.; Laino, L.; Troiano, G.; Crimi, S.; Cicciu, M. Interferon Crevicular Fluid Profile and Correlation with Periodontal Disease and Wound Healing: A Systemic Review of Recent Data. Int. J. Mol. Sci. 2018, 19, 1908. [Google Scholar] [CrossRef] [PubMed]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Laino, L.; Cicciu, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Zhurakivska, K.; Lo Muzio, L. Comparison of Two Routes of Administration of Dexamethasone to Reduce the Postoperative Sequelae After Third Molar Surgery: A Systematic Review and Meta-Analysis. Open Dent. J. 2018, 12, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.-F.; Seneviratne, C.J.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wong, C.-H.; Pang, K.Y.; Wong, C.W.; Wat, E.; Jin, L. Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms. Nanomaterials 2016, 6, 61. [Google Scholar] [CrossRef]
- Iordanskii, A.; Zhulkina, A.; Olkhov, A.; Fomin, S.; Burkov, A.; Stilman, M. Characterization and Evaluation of Controlled Antimicrobial Release from Petrochemical (PU) and Biodegradable (PHB) Packaging. Polymers 2018, 10, 817. [Google Scholar] [CrossRef]
- Gupta, T.T.; Karki, S.B.; Fournier, R.; Ayan, H. Mathematical Modelling of the Effects of Plasma Treatment on the Diffusivity of Biofilm. Appl. Sci. 2018, 8, 1729. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef]
- Castellucci, N.; Falini, G.; Angelici, G.; Tomasini, C. Formation of gels in the presence of metal ions. Amino Acids 2011, 41, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Mensi, M.; Cochis, A.; Sordillo, A.; Uberti, F.; Rimondini, L. Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks. Materials 2018, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’Amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.; Conway, B.; Worthington, T. Digluconate and Isopropyl Alcohol Biocide Formulation. Int. J. Mol. Sci. 2012, 13, 14016–14025. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Muangman, P.; Namviriyachote, N.; Srichana, T. In Vitro Evaluation of the Antimicrobial Effectiveness and Moisture Binding Properties of Wound Dressings. Int. J. Mol. Sci. 2010, 11, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Melok, A.L.; Lee, L.H.; Mohamed Yussof, S.A.; Chu, T. Green Tea Polyphenol Epigallocatechin-3-Gallate-Stearate Inhibits the Growth of Streptococcus mutans: A Promising New Approach in Caries Prevention. Dent. J. 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Kucher, M.; Dannemann, M.; Modler, N.; Hannig, C.; Weber, M.-T. Effects of Endodontic Irrigants on Material and Surface Properties of Biocompatible Thermoplastics. Dent. J. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Antibiotic Resistance Can Be Enhanced in Gram-Positive Species by Some Biocidal Agents Used for Disinfection. Antibiotics 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A. IgE and Drug Allergy: Antibody Recognition of ‘Small’ Molecules of Widely Varying Structures and Activities. Antibodies 2014, 3, 56–91. [Google Scholar] [CrossRef]
- Cervino, G.; Cicciù, M.; Biondi, A.; Bocchieri, S.; Herford, A.S.; Laino, L.; Fiorillo, L. Antibiotic Prophylaxis on Third Molar Extraction: Systematic Review of Recent Data. Antibiotics 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’Amico, C.; Nastro Siniscalchi, E.; et al. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar. Drugs 2018, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- European Federation of Periodontology. Available online: https://www.efp.org/patients/what-is-periodontology.html (accessed on 27 May 2019).
- SICMF. Available online: https://www.sicmf.org/ (accessed on 27 May 2019).
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]



| Author (Year) | Features | Field | Statistics | Type |
|---|---|---|---|---|
| Coello-Gomez et al. (2018) [20] | Chlorhexidine gel vs. mouthwash with super-oxidized solution (SOS) | Oral surgery | Not significative | RCT double blinded |
| Sinjari et al. (2018) [21] | Chlorhexidine gel vs. placebo gel for periimplantitis prevention | Implantology; Periodontology | Significative | RCT blinded |
| Rusu et al (2017) [22] | Chlorhexidine-based gingiva-adhering gel containing herbal ingredients vs. 1% chlorhexidine water-soluble gel for periodontitis prevention after scaling | Periodontology | Not significative | RCT blinded |
| Rubio-Palau et al. (2015) [23] | 0.2% Chlorhexidine gel vs. placebo for alveolar osteitis prevention after third molar surgery | Oral surgery | Not Significative | RCT double blind |
| Levin et al. (2015) [24] | Chlorhexidine gel adjunct water jet for periimplantitis prevention | Implantology; periodontology | Not Significative | RCT |
| Jesudasan et al. (2015) [25] | 0.2% Chlorhexidine gel vs. eugenol based paste vs. control for alveolar osteitis prevention after third molar surgery | Oral surgery | P = 0.002 Eugenol paste has better results | RCT |
| Haraji et al. (2015) [26] | 0.2% Chlorhexidine after third molar extraction for alveolitis prevention | Oral surgery | Significative, chlorhexidine can reduce pain | RCT split mouth |
| Freudhental et al. (2015) [27] | 0.2% Chlorhexidine vs. placebo for alveolar osteitis prevention | Oral surgery | Not significative | RCT double blinded |
| Diaz-Sanchez et al. [28] | Bioadhesive 0.2% chlorhexidine gel vs. placebo for mucositis radio and chemotherapy induced prevention | Oral surgery; periodontology | Not significative | RCT double blinded |
| Haraji et al. (2014) [29] | 0.2% Chlorhexidine gel vs. control for dry socket (DS) prevention after third molar surgery | Oral surgery | P = 0.004 Use of chlorhexidine lowered DS | RCT split mouth |
| Singh et al. (2013) [30] | Calcium hydroxide paste mixed with 2% chlorhexidine gel vs. 2% chlorhexidine gel, vs. calcium hydroxide paste vs. control (no dressing) for intracanal medications | Endodontics | Group I and II P < 0.05 | RCT double blinded |
| Pukallus et al. (2013) [31] | 0.12% Chlorhexidine (CHX) gel vs. 304% fluoride toothpaste to prevent early childhood caries | Prophylaxis | Not significative | RCT |
| Lima et al. (2013) [32] | 1% Chlorhexidine gel vs. calcium hydroxide/camphorated paramonochlorophenol (Callen PMCC) vs. a one-visit endodontic treatment to bacterial proliferation | Endodontics | Chlorhexidine vs. mutans streptococci P = 0.10; PMCC vs. anaerobic bacteria P = 0.002 | RCT split mouth |
| De Siena et al. (2013) [33] | 1% Chlorhexidine gel vs. 0.2% chlorhexidine for peri-implant mucositis treatment | Implantology; Periodontology | Not significative | Observational study |
| De Lucena et al. (2013) [34] | Calcium hydroxide paste (CH) vs. chlorhexidine gel (CHX-gel) (5.0%) vs. chlorhexidine/gutta-percha points (CHX-GP) vs. octenidine gel (OCT-gel) (5.0%) for dentin E. faecalis contamination preventing | Endodontics; Restorative dentistry | CHX-gel and OCT-gel significative | RCT |
| Almeida et al. (2012) [35] | 5.25% Sodium hypochlorite (NaOCl) or 2% chlorhexidine gel (CHX) for apical periodontitis preventing | Endodontics; Periodontology | Not significative | RCT |
| Heitz-Mayfield et al. (2011) [36] | 0.5% Chlorhexidine gel vs. placebo gel for peri-implant mucositis managing | Implantology; Periodontology | Significative | RCT double blinded |
| Torres-Lagares et al. (2010) [37] | 0.2% Chlorhexidine gel vs. placebo for postextractive alveolitis prevention after third molar extraction on bleeding disorders patients [38,39] | Oral surgery | Significative | RCT double blinded |
| Slot et al. (2010) [40] | 1% Chlorhexidine gel vs. 0.12% chlorhexidine dentifrice-gel vs. control dentifrice vs. 0.2% chlorhexidine mouthwash for plaque formation prevention | Prophylaxis | 1% Chlorhexidine gel and 0.2% chlorhexidine mouthwash were significative | RCT |
| Lopez-Jornet et al. (2010) [41] | Polyvinylpyrrolidone-sodium hyaluronate (Aloclair) gel vs. 0.2% chlorhexidine digluconate gel vs. control for symptom prevention after mucosa biopsy | Oral surgery | Significative | RCT |
| Cabov et al. (2010) [42] | Chlorhexidine gel vs. control to prevent oral mucosa contamination | Oral surgery; Prophylaxis | Significative | RCT double blinded |
| Paolantonio et al. (2009) [43] | Chlorhexidine gel vs. Xantan base chlorhexidine | Oral surgery; prophylaxis | Significative | RCT |
| Malkhassian et al. (2009) [44] | BioPure MTAD vs. 2% Chlorhexidine gel for root canal treatment | Endodontics | Not significative | RCT double blinded |
| Hauser-Gerspach et al. (2009) [45] | Gaseous ozone and chlorhexidine gel for cavities prevention | Prophylaxis | Not significative | RCT |
| Gomes et al. (2009) [46] | 2.5% Sodium hypochlorite (NaOCl) vs. 2% chlorhexidine (CHX) gel on eliminating oral bacteria | Endodontics; Prophylaxis | Not significative | RCT |
| Author (Year) | Random Sequence Generation | Allocation Concealment | Selective Reporting | Other Sources of Bias | Blinding | Incomplete Outcome Data |
|---|---|---|---|---|---|---|
| Coello-Gomez et al. (2018) [20] | - | - | - | ? | - | - |
| Sinjari et al. (2018) [21] | ? | ? | - | ? | + | - |
| Rusu et al (2017) [22] | - | - | - | - | - | - |
| Rubio-Palau et al. (2015) [23] | - | - | - | ? | - | - |
| Levin et al. (2015) [24] | - | - | - | - | + | - |
| Jesudasan et al. (2015) [25] | + | - | - | - | + | - |
| Haraji et al. (2015) [26] | - | - | - | ? | + | - |
| Freudhental et al. (2015) [27] | - | - | - | ? | - | - |
| Diaz-Sanchez et al. [28] | - | - | - | - | - | - |
| Haraji et al. (2014) [29] | - | + | - | - | + | - |
| Singh et al. (2013) [30] | - | - | - | ? | - | - |
| Pukallus et al. (2013) [31] | - | - | - | - | + | - |
| Lima et al. (2013) [32] | - | - | - | ? | + | - |
| De Siena et al. (2013) [33] | - | - | - | - | + | - |
| De Lucena et al. (2013) [34] | - | - | - | - | + | - |
| Almeida et al. (2012) [35] | - | - | - | - | + | - |
| Heitz-Mayfield et al. (2011) [36] | - | - | - | ? | - | - |
| Torres-Lagares et al. (2010) [37] | - | - | - | - | - | - |
| Slot et al. (2010) [40] | - | - | - | ? | - | - |
| Lopez-Jornet et al. (2010) [41] | + | - | - | - | + | - |
| Cabov et al. (2010) [42] | - | - | - | ? | - | - |
| Paolantonio et al. (2009) [43] | - | - | - | ? | + | - |
| Malkhassian et al. (2009) [44] | - | - | - | ? | - | - |
| Hauser-Gerspach et al. (2009) [45] | - | - | - | ? | + | - |
| Gomes et al. (2009) [46] | - | - | - | ? | + | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, L. Chlorhexidine Gel Use in the Oral District: A Systematic Review. Gels 2019, 5, 31. https://doi.org/10.3390/gels5020031
Fiorillo L. Chlorhexidine Gel Use in the Oral District: A Systematic Review. Gels. 2019; 5(2):31. https://doi.org/10.3390/gels5020031
Chicago/Turabian StyleFiorillo, Luca. 2019. "Chlorhexidine Gel Use in the Oral District: A Systematic Review" Gels 5, no. 2: 31. https://doi.org/10.3390/gels5020031
APA StyleFiorillo, L. (2019). Chlorhexidine Gel Use in the Oral District: A Systematic Review. Gels, 5(2), 31. https://doi.org/10.3390/gels5020031