Polydopamine Antioxidant Hydrogels for Wound Healing Applications
Abstract
1. Introduction
2. Results and Discussion
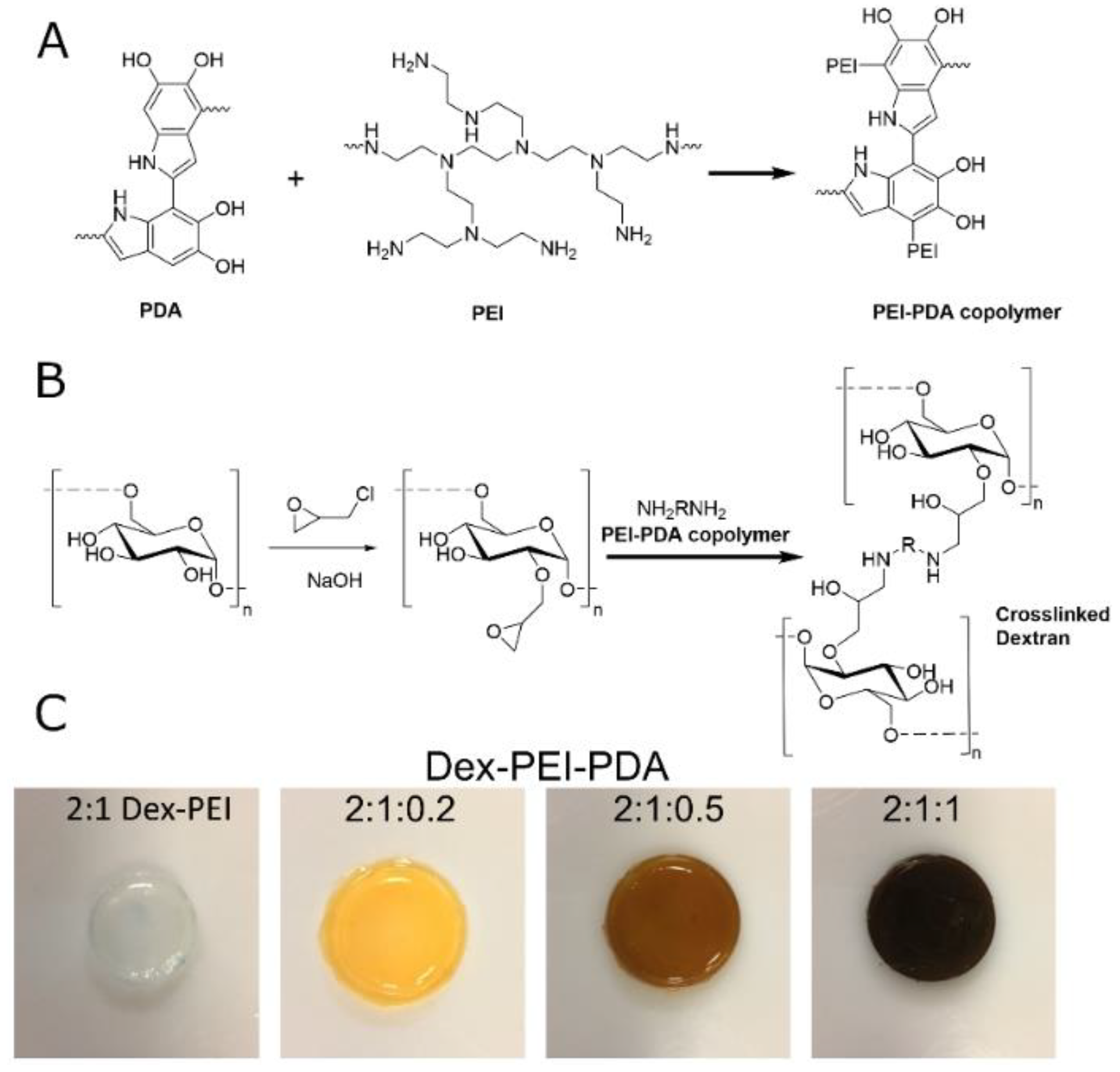
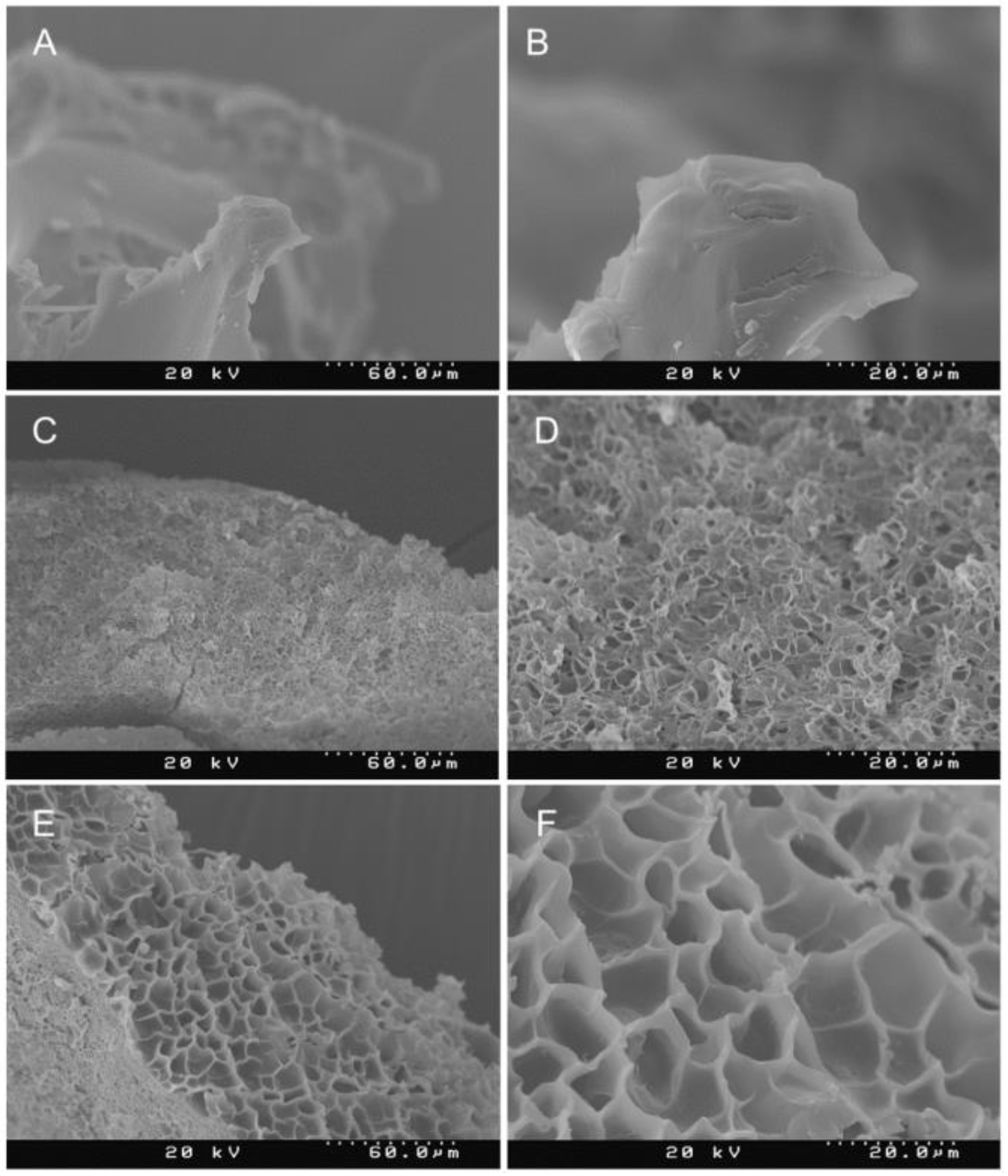
2.1. Synthesis and Structure
2.2. Swelling, Antioxidant and Compression Studies
2.3. Wound Healing Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Dextran:Polydopamine (Dex-PDA) and Dextran:PEI:Polydopamine (Dex-PEI-PDA) Hydrogels
4.3. Swelling Ratio
4.4. Compression Studies
4.5. Antioxidant Studies
4.6. Wound Healing Studies
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murphy, P.S.; Evans, G.R.D. Advances in Wound Healing: A Review of Current Wound Healing Products. Plast. Surg. Int. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Balestrin, L.A.; Bidone, J.; Bortolin, R.C.; Moresco, K.; Moreira, J.C.; Teixeira, H.F. Protective effect of a hydrogel containing Achyrocline satureioides extract-loaded nanoemulsion against UV-induced skin damage. J. Photochem. Photobiol. B 2016, 163, 269–276. [Google Scholar] [CrossRef]
- Cui, H.; Cui, L.; Zhang, P.; Huang, Y.; Wei, Y.; Chen, X. In Situ Electroactive and Antioxidant Supramolecular Hydrogel Based on Cyclodextrin/Copolymer Inclusion for Tissue Engineering Repair. Macromol. Biosci. 2014, 14, 440–450. [Google Scholar] [CrossRef]
- Kang, B.; Vales, T.P.; Cho, B.-K.; Kim, J.-K.; Kim, H.-J. Development of gallic acid-modified hydrogels using interpenetrating chitosan network and evaluation of their antioxidant activity. Molecules 2017, 22, 1976. [Google Scholar] [CrossRef]
- Kim, B.; Kang, B.; Vales, T.P.; Yang, S.K.; Lee, J.; Kim, H.-J. Polyphenol-functionalized hydrogels using an interpenetrating chitosan network and investigation of their antioxidant activity. Macromol. Res. 2018, 26, 35–39. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Altimari, I.; Puoci, F.; Parisi, O.I.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Yen, G.-C.; Hsieh, C.-L. Antioxidant Effects of Dopamine and Related Compounds. Biosci. Biotechnol. Biochem. 2014, 61, 1646–1649. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, R.; Lu, M.; You, G.; Wang, Y.; Chen, G.; Zhao, C.; Wang, Z.; Song, X.; Wu, Y.; et al. Bioinspired Polydopamine-Coated Hemoglobin as Potential Oxygen Carrier with Antioxidant Properties. Biomacromolecules 2017, 18, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zuo, F.; Liao, Z.; Qin, Z.; Du, S.; Zhao, Z. Mussel-Inspired One-Pot Synthesis of a Fluorescent and Water-Soluble Polydopamine-Polyethyleneimine Copolymer. Macromol. Rapid Commun. 2015, 36, 909–915. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Abugharbieh, A.; Yasmeen, F.; Buabeng, E.; Mathew, S.; Samaroo, D.; Cheng, H.P. The crosslinking of polysaccharides with polyamines and dextran-polyallylamine antibacterial hydrogels. Int. J. Biol. Macromol. 2015, 72, 88–93. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Jitianu, M.; Nunez, G.; Picard, Q.; Wong, M.; Akpatsu, D.; Negrin, A.; Gharbaran, R.; Lugo, D.; Shaker, S.; et al. Dextran hydrogels by crosslinking with amino acid diamines and their viscoelastic properties. Int. J. Biol. Macromol. 2018, 111, 370–378. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels 2020, 6, 39. https://doi.org/10.3390/gels6040039
O’Connor NA, Syed A, Wong M, Hicks J, Nunez G, Jitianu A, Siler Z, Peterson M. Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels. 2020; 6(4):39. https://doi.org/10.3390/gels6040039
Chicago/Turabian StyleO’Connor, Naphtali A., Abdulhaq Syed, Madeline Wong, Josiah Hicks, Greisly Nunez, Andrei Jitianu, Zach Siler, and Marnie Peterson. 2020. "Polydopamine Antioxidant Hydrogels for Wound Healing Applications" Gels 6, no. 4: 39. https://doi.org/10.3390/gels6040039
APA StyleO’Connor, N. A., Syed, A., Wong, M., Hicks, J., Nunez, G., Jitianu, A., Siler, Z., & Peterson, M. (2020). Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels, 6(4), 39. https://doi.org/10.3390/gels6040039