Smart Hydrogels: Preparation, Characterization, and Determination of Transition Points of Crosslinked N-Isopropyl Acrylamide/Acrylamide/Carboxylic Acids Polymers
Abstract
:1. Introduction
2. Results and Discussion
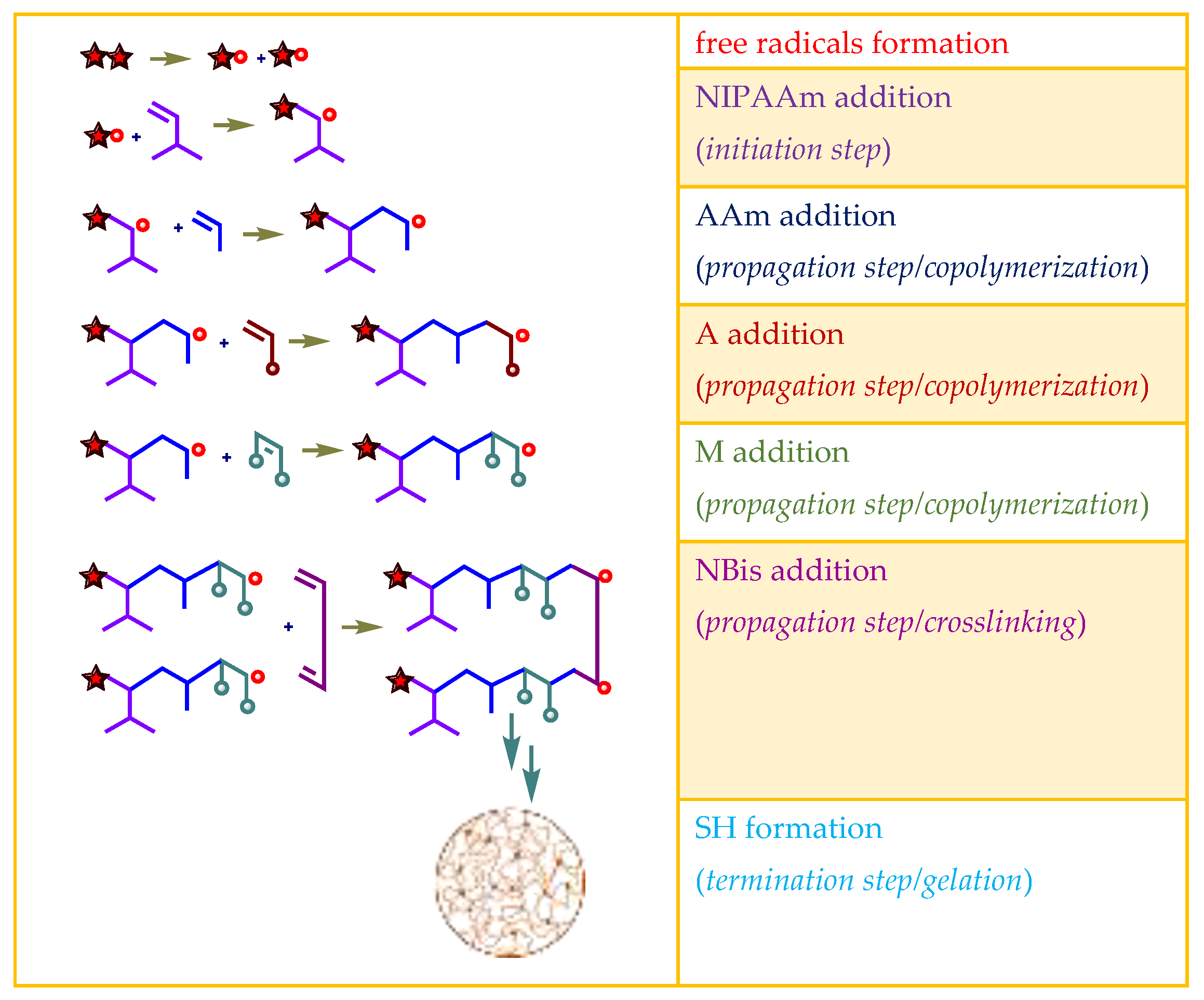
2.1. Preparation of Hydrogels
2.2. Characterization of SHs
2.2.1. FTIR Analysis
2.2.2. Thermal Analysis
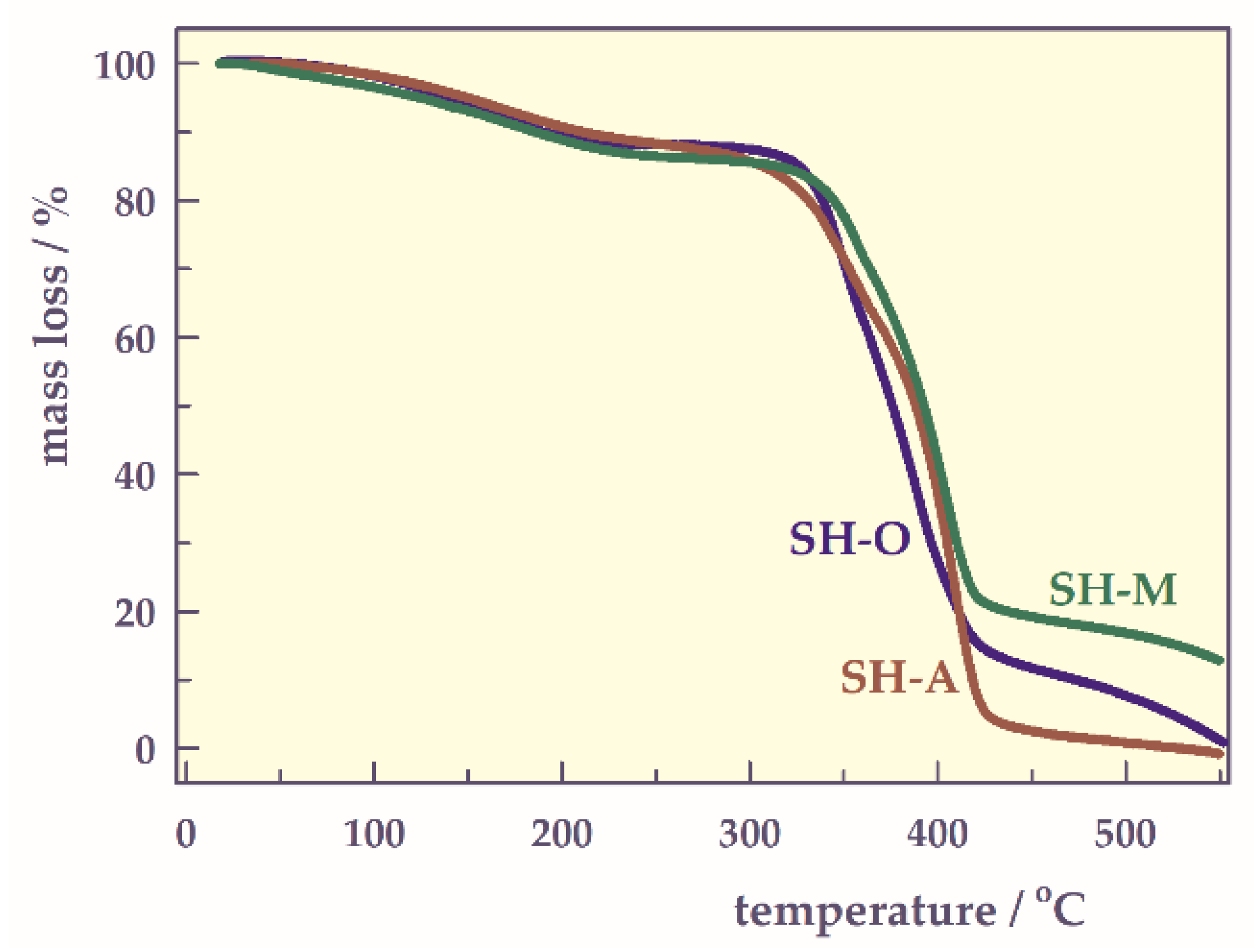
- TG analysis
- The Thermal Degradation Kinetics of the SHs
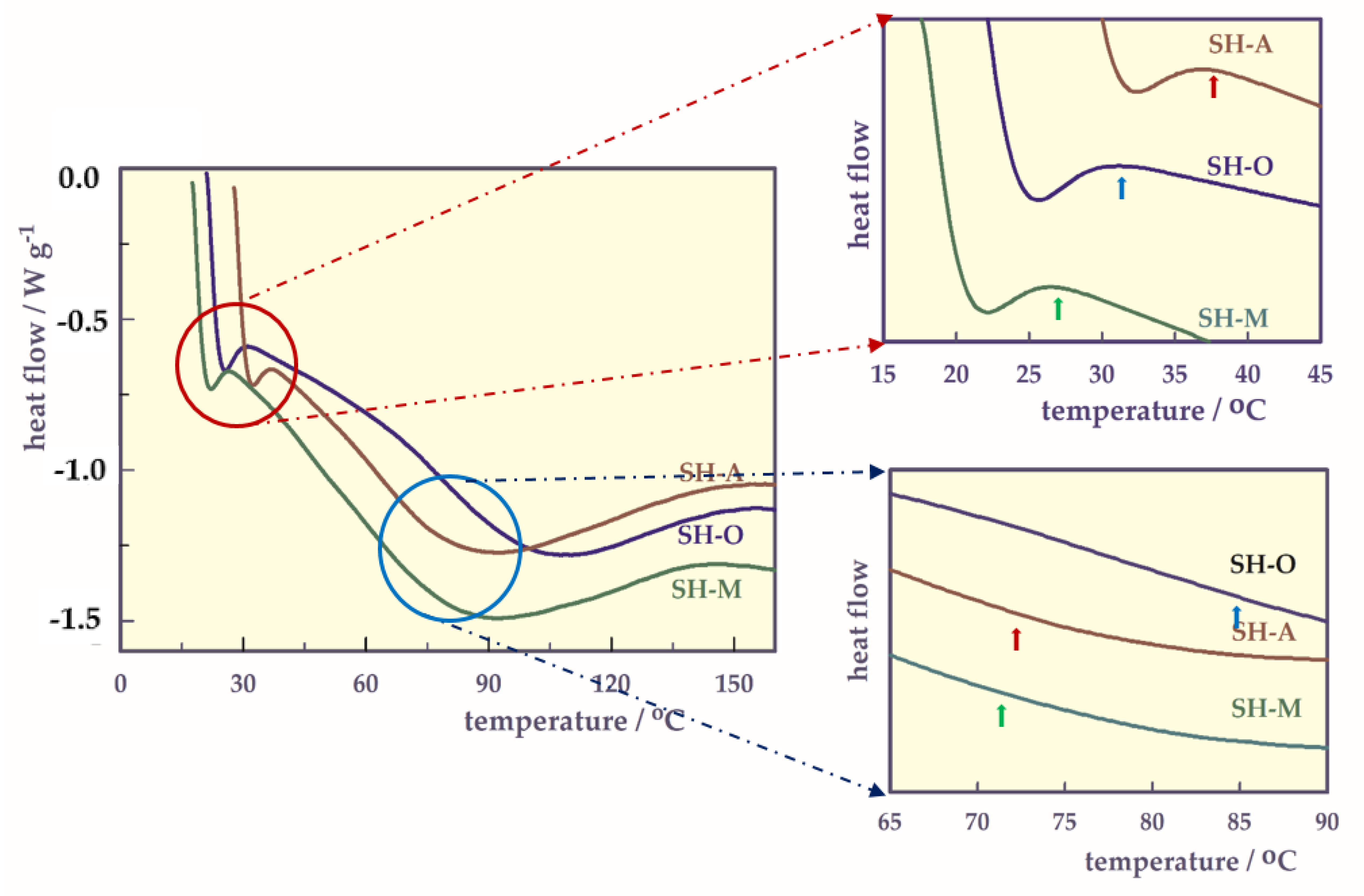
- DSC analysis.
2.2.3. Swelling Experiments
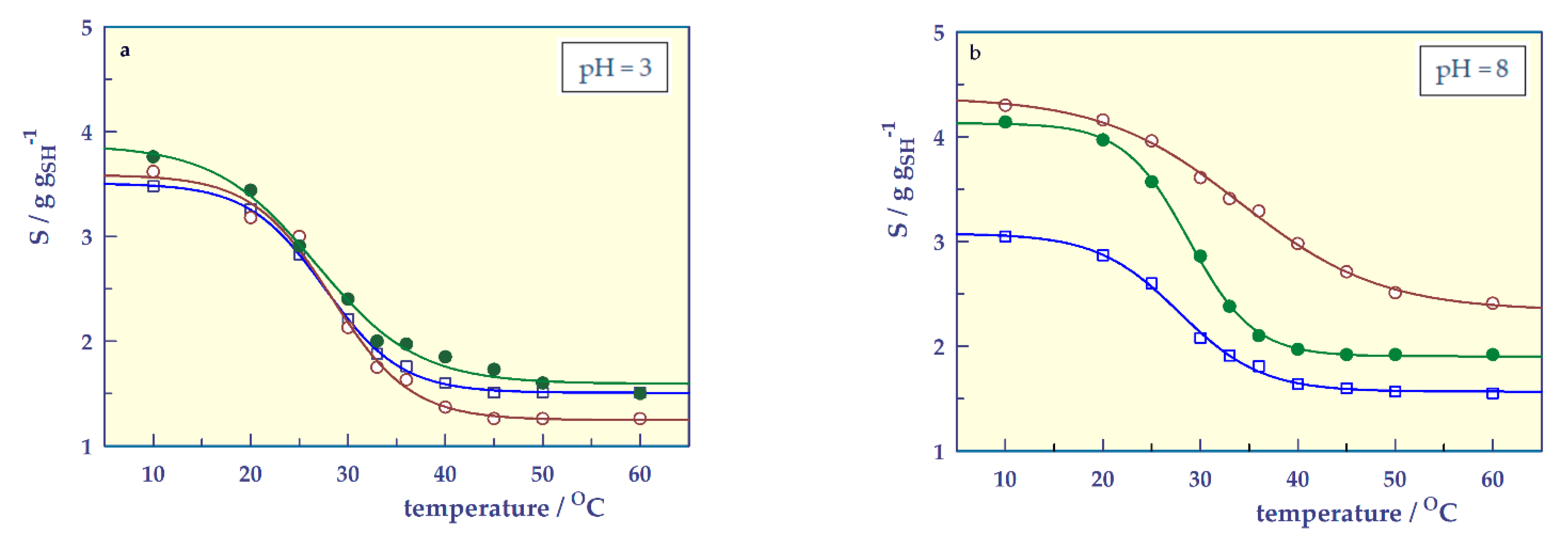
- pH-responsive swelling
- Temperature-responsive swelling behavior
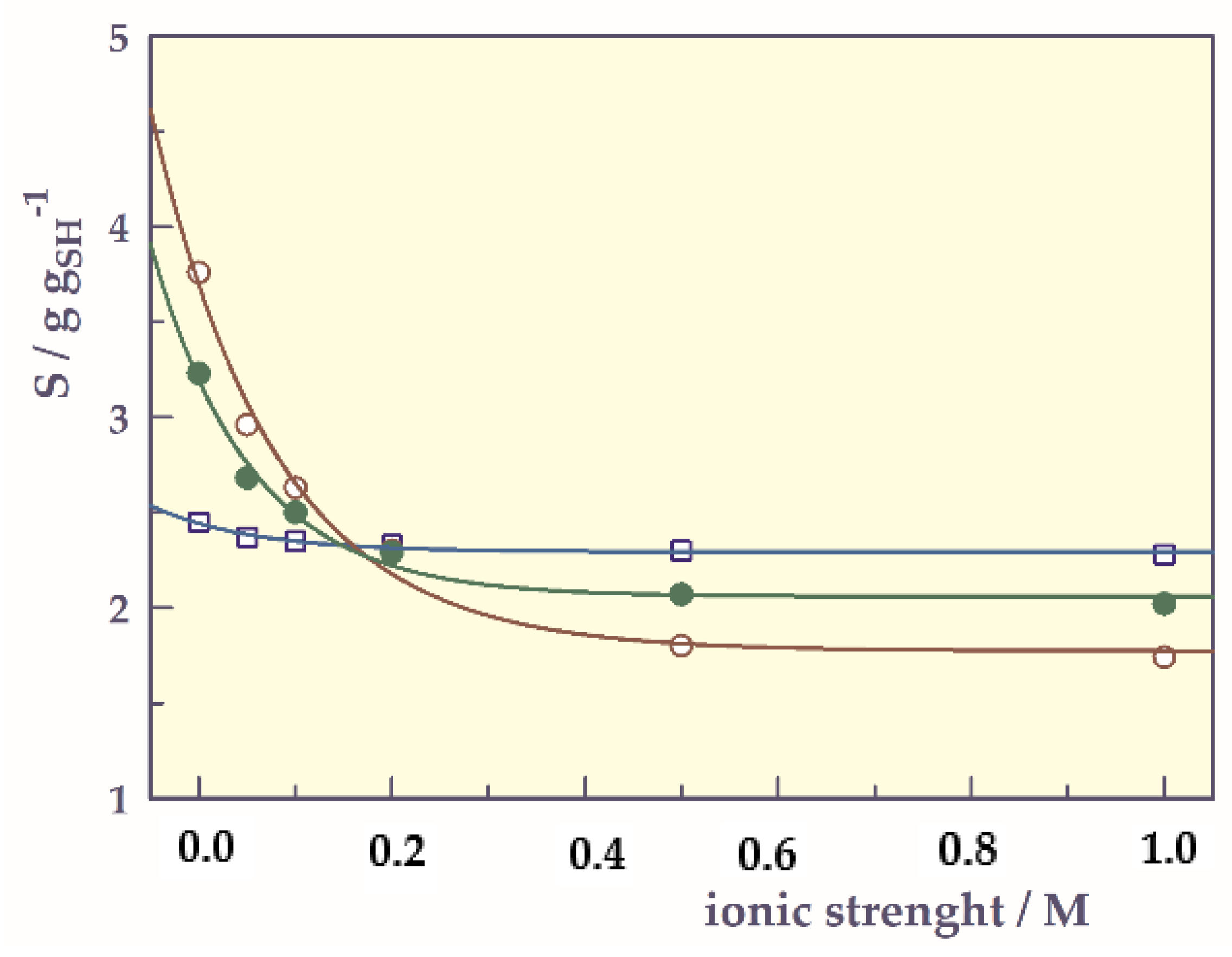
- Ionic strength-responsive swelling behavior
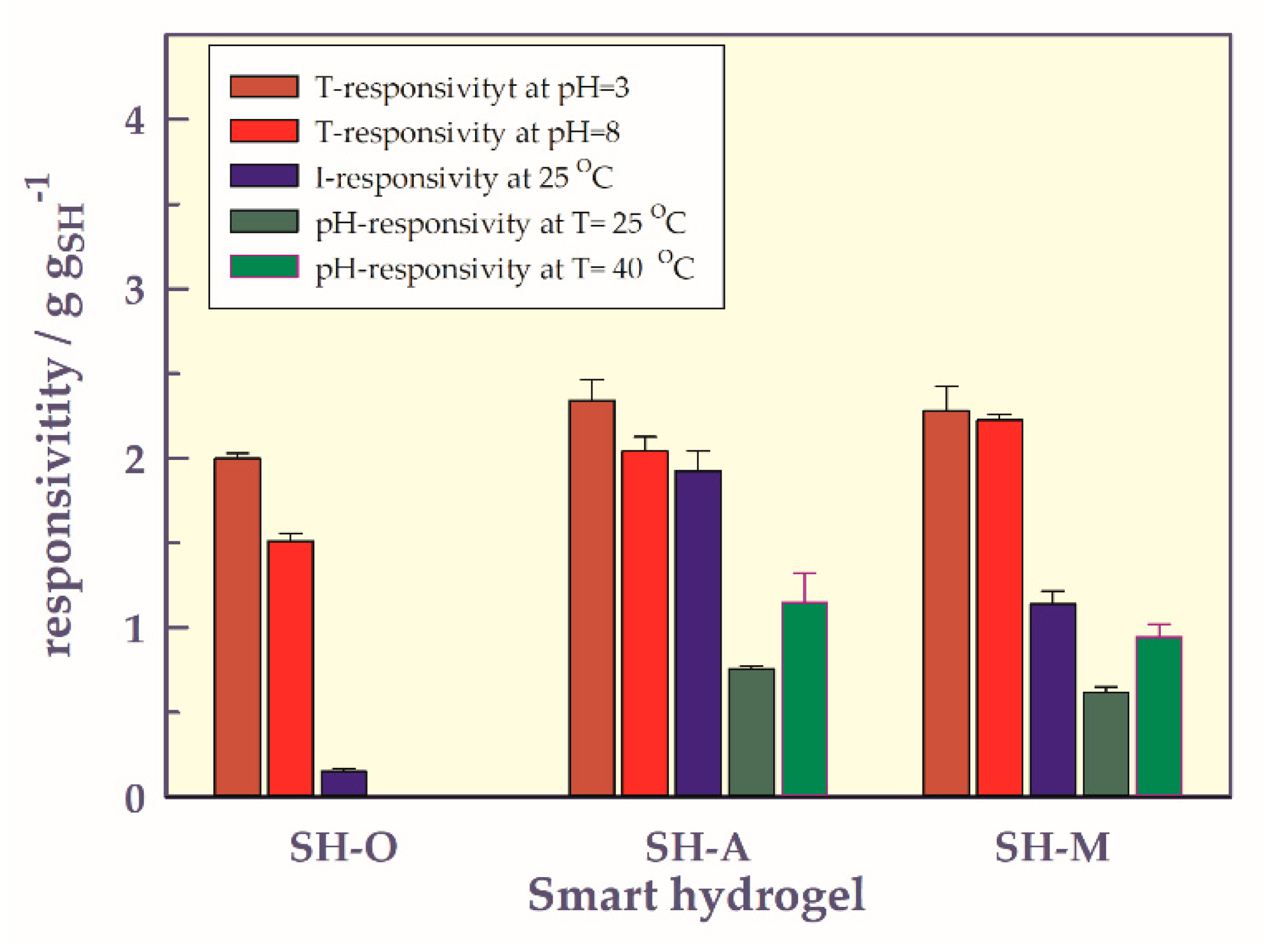
- Comparison of stimuli responsivities
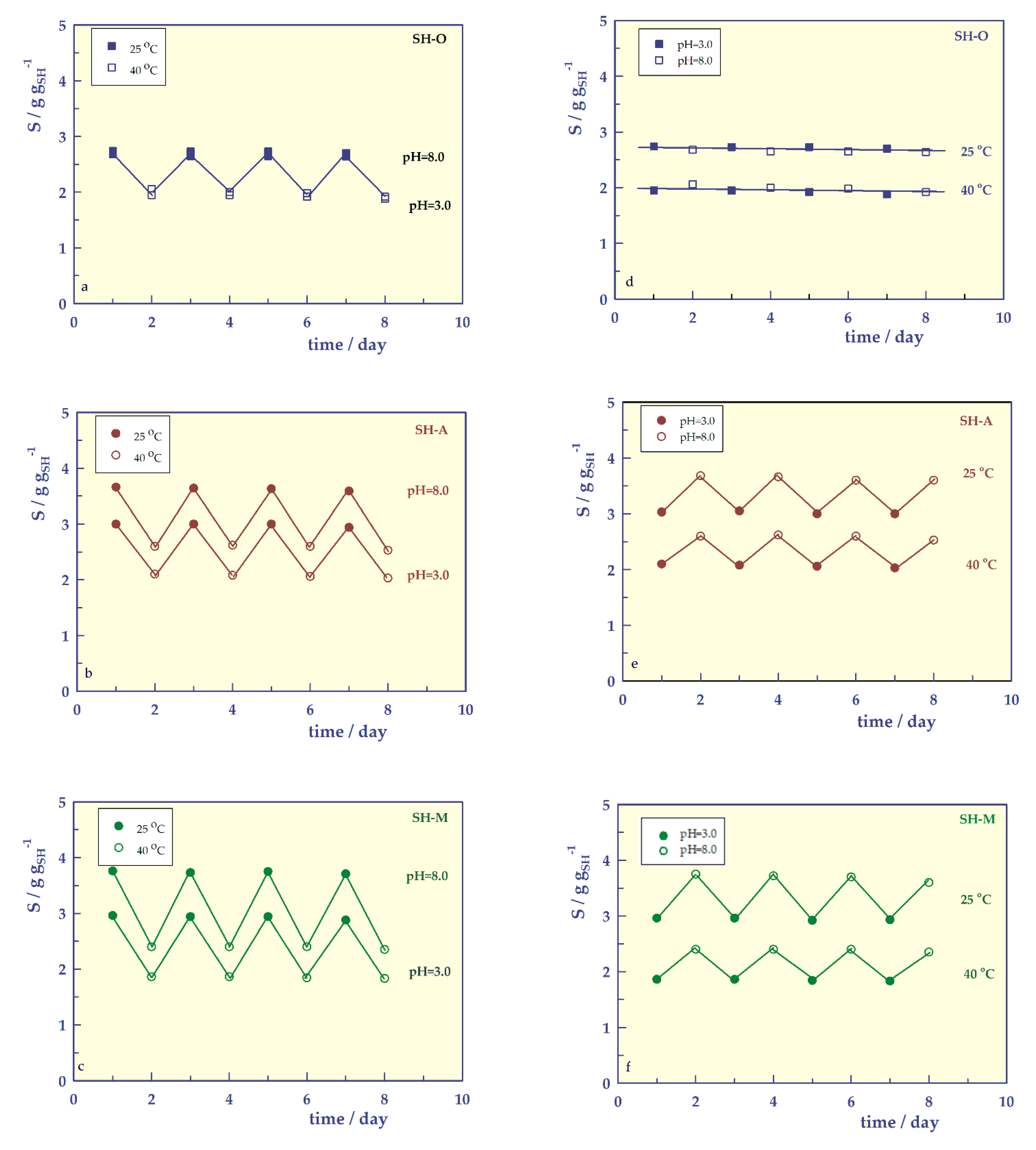
- Reversible swelling
- Solvent concentration-responsive swelling
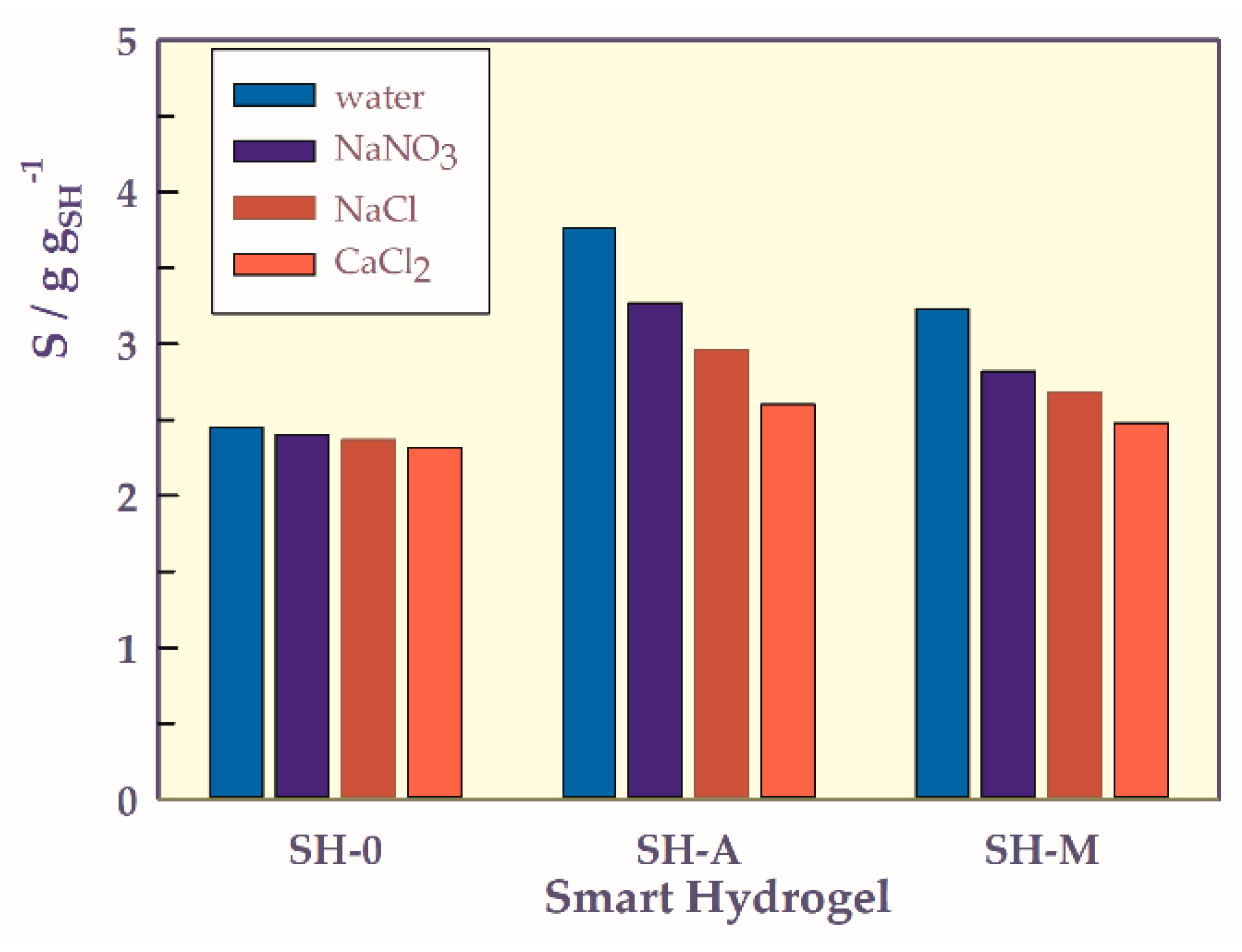
- Solvent type-responsive swellings
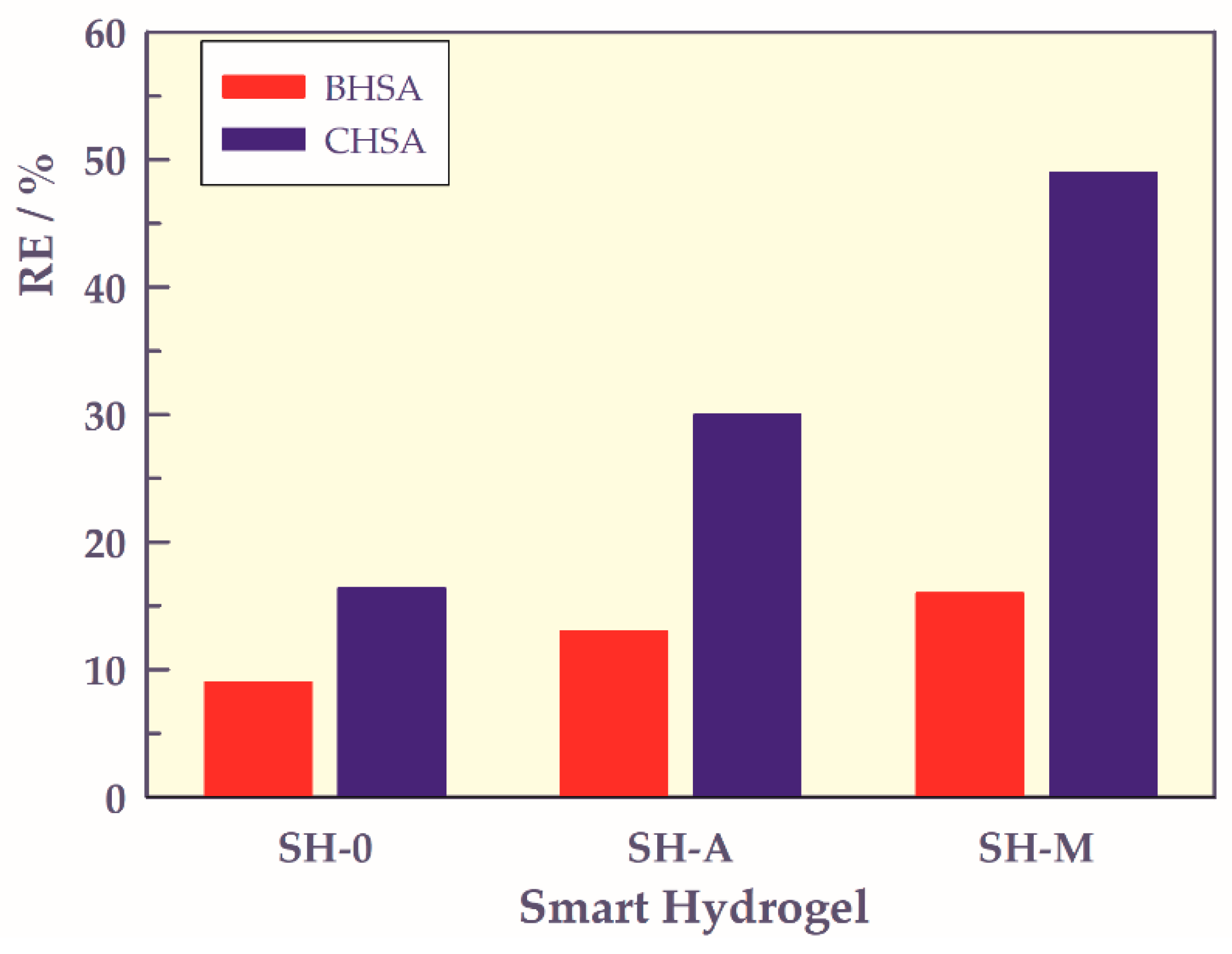
2.3. Adsorption of Human Serum Albumin
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Hydrogels
4.3. Characterization
4.4. Swelling
4.5. Calculations
4.6. Adsorption of Human Serum Albumin
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, in press. [Google Scholar] [CrossRef]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Faré, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Kuckling, D. Stimuli-Responsive Gels. Gels 2018, 4, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wan, Y.; Carvalho, W.; Hu, L.; Serpe, M.J. Stimuli-Responsive Polymers for Sensing and Reacting to Environmental Conditions. Prog. Polym. Sci. 2021, 116, 101386. [Google Scholar] [CrossRef]
- Jingcheng, L.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent Polymers, Fibers and Applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef]
- Rivera-Tarazona, L.K.; Campbell, Z.T.; Ware, T.H. Stimuli-responsive engineered living materials. Soft Matter. 2021, 17, 785–809. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Li, L.; Urban, M.W. Recent Advances in Stimuli-Responsive Commodity Polymers. Macromol. Rapid Commun. 2021, 2100054. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Ortega-Zamora, C.; Carrillo, R.; Hernández-Borges, J. Application of stimuli-responsive materials for extraction purposes. J. Chromatogr. A 2021, 1636, 461764. [Google Scholar] [CrossRef]
- Gelmi, A.; Schutt, C.E. Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control. Adv. Healthc. Mater. 2020, 10, 2001125. [Google Scholar] [CrossRef]
- Kaynak, A.; Zolfagharian, A. Stimuli-Responsive Polymer Systems—Recent Manufacturing Techniques and Applications. Materials 2019, 12, 2380. [Google Scholar] [CrossRef] [Green Version]
- Drozdov, A.D. Equilibrium Swelling of Biocompatible ThermoResponsive Copolymer Gels. Gels 2021, 7, 40. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-Responsive Polymers and Their Applications in Nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 1288. [Google Scholar] [CrossRef] [PubMed]
- WOS. Available online: http://apps.webofknowledge.com (accessed on 22 June 2021).
- Saraydin, D.; Karadağ, E.; Güven, O. Super Water-Retainer Hydrogels: Crosslinked Acrylamide/Succinic Acid Copolymers. Polym. J. 1997, 29, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Işikver, Y.; Saraydin, D. Environmentally sensitive hydrogels: N-isopropyl acrylamide/Acrylamide/Mono-, Di-, Tricarboxylic acid crosslinked polymers. Polym. Eng. Sci. 2014, 55, 843–851. [Google Scholar] [CrossRef]
- Kubilay, Ş.; Selçuk, K.; Saraydin, D. Synthesis and characterization of p (N-isopropylacrylamide) hydrogels with tunable swelling behavior using different crosslinkers. Hacet. J. Biol. Chem. 2021, 49, 92–106. [Google Scholar] [CrossRef]
- Işikver, Y.; Saraydin, D. Stimuli Responsive Hydrogels: NIPAM/AAm/Carboxylic Acid Polymers. Acta Chem. Iasi 2019, 27, 155–184. [Google Scholar] [CrossRef] [Green Version]
- Saraydin, D.; Koptagel, E.; Ünver-Saraydin, S.; Karadağ, E.; Güven, O. In vivo biocompatibility of radiation induced acrylamide and acrylamide/maleic acid hydrogels. J. Mater. Sci. 2001, 36, 2473–2481. [Google Scholar] [CrossRef]
- Öztop, H.N.; Saraydın, D.; Karadağ, E.; Çaldıran, Y.; Güven, O. Influence of some aromatic amino acids on the swelling behavior of acrylamide/maleic acid hydrogel. Polym. Bull. 1998, 40, 575–581. [Google Scholar] [CrossRef]
- Işıkver, Y.; Saraydın, D.; Aydın, H. In vitro swelling studies in simulated physiological solutions and biocompatibility of NIPAM-based hydrogels with some biochemical parameters of human sera. J. Macromol. Sci. Part A 2017, 54, 452–457. [Google Scholar] [CrossRef]
- Lai, E.P.; Wang, Y.X.; Wei, Y.; Li, G. Preparation of uniform-sized and dual stimuli-responsive microspheres of poly(N-isopropylacrylamide)/poly(acrylic acid) with semi-IPN structure by one-step method. Polymers 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Jerez, A. A modification to the Freeman and Carroll method for the analysis of the kinetics of non-isothermal processes. J. Therm. Anal. 1983, 26, 315–318. [Google Scholar] [CrossRef]
- Hosseini, H.; Shirkavand Hadavand, B. Synthesis and Viscoelastic Properties of Smart Hydrogel. Polym. Sci. Ser. B 2020, 62, 394–399. [Google Scholar] [CrossRef]
- Leibovich-Raveh, T.; Lewis, D.J.; Al-Rubaiey Kadhim, S.; Ansari, D. A new method for calculating individual subitizing ranges. J. Numer. Cogn. 2018, 4, 429–447. [Google Scholar] [CrossRef] [Green Version]
- Mégevand, P.; Molholm, S.; Nayak, A.; Foxe, J.J. Recalibration of the Multisensory Temporal Window of Integration Results from Changing Task Demands. PLoS ONE 2013, 8, e71608. [Google Scholar] [CrossRef] [Green Version]
- Myhrvold, N.P. Revisiting the Estimation of Dinosaur Growth Rates. PLoS ONE 2013, 8, e81917. [Google Scholar] [CrossRef] [Green Version]
- Scott Harris, S.R.; Hess, D.R.; Venegas, J.G. An Objective Analysis of the Pressure-Volume Curve in the Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2000, 161, 432–439. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Chung, T.; Han, I.K.; Han, J.; Ahn, K.; Kim, Y.S. Fast and Large Shrinking of Thermoresponsive Hydrogels with Phase-Separated Structures. Gels 2021, 7, 18. [Google Scholar] [CrossRef]
- Shang, J.; Theato, P. Smart composite hydrogel with pH-, ionic strength- and temperature-induced actuation. Soft Matter 2018, 14, 8401–8407. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tong, Z.; Hu, O. Swelling equilibria of hydrogels with sulfonate groups in water and in aqueous salt solutions. Macromolecules 1995, 28, 3813–3817. [Google Scholar] [CrossRef]
- Atta, A.M. Swelling behaviors of polyelectrolyte hydrogels containing sulfonate groups. Polym. Adv. Technol. 2002, 13, 567–576. [Google Scholar] [CrossRef]
- Yang, H.; Song, W.; Zhuang, Y.; Deng, X. Synthesis of strong electrolyte temperature-sensitive hydrogels by radiation polymerization and application in protein separation. Macromol. Biosci. 2003, 3, 400–403. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Reversible adsorption by a pH- and temperature-sensitive acrylic hydrogel. J. Control. Release 2002, 80, 247–257. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Shi, Z.; Huang, X.; Zhao, W.; Zhao, C. Multi-responsive, tough and reversible hydrogels with tunable swelling property. J. Hazard. Mater. 2017, 322, 499–507. [Google Scholar] [CrossRef]
- El-Halah, A.; González, N.; Contreras, J.; López-Carrasquero, F. Effect of the synthesis solvent in swelling ability of polyacrylamide hydrogels. J. Polym. Res. 2019, 27, 21. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, P.; Wei, H.; Halila, S.; Osi, A.R.; Zhou, Y.; Dai, Z.; Wang, R.; Chen, J. Reinforced macromolecular micelle-crosslinked hyaluronate gels induced by water/DMSO binary solvent. Soft Matter 2020, 16, 8647–8654. [Google Scholar] [CrossRef]
- Ledvij, M. Curve fitting made easy. Ind. Phys. 2003, 9, 24–27. [Google Scholar]
- Du, Z.H.; Gao, H.; Cao, X.H. Direct high-precision measurement of the effective optical path length of multi-pass cell with optical frequency domain reflectometer. Opt. Express 2016, 24, 417. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Zimmermann, F.; Drolet, D.; Hansen, C.M.; Chollot, A.; Monta, N. The use of the Hansen solubility parameters in the selection of protective polymeric materials resistant to chemicals. Int. J. Curr. Res. 2017, 9, 47860–47867. [Google Scholar]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels-Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef] [Green Version]
- Longo, G.S.; Pérez-Chávez, N.A.; Szleifer, I. How protonation modulates the interaction between proteins and pH-responsive hydrogel films. Curr. Op. Coll. Int. Sci. 2019, 41, 27–39. [Google Scholar] [CrossRef]
- Gomes, P.F.; Loureiro, J.M.; Rodrigues, A.E. Adsorption of human serum albumin (HSA) on a mixed-mode adsorbent: Equilibrium and kinetics. Adsorption 2017, 23, 491–505. [Google Scholar] [CrossRef]
- Zhu, X.; Gu, X.; Zhang, L.; Kong, X.-Z. Preparation and characterization of nanosized P(NIPAM-MBA) hydrogel particles and adsorption of bovine serum albumin on their surface. Nanoscale Res. Lett. 2012, 7, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, Q.; Griesser, H.J.; Milthorpe, B.K.; Garrett, R.W. Irreversible adsorption of human serum albumin to hydrogel contact lenses: A study using electron spin resonance spectroscopy. Biomaterials 1999, 20, 1345–1356. [Google Scholar] [CrossRef]
- Yamada, Y.; Fichman, G.; Schneider, J.P. Serum Protein Adsorption Modulates the Toxicity of Highly Positively Charged Hydrogel Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 8006–8014. [Google Scholar] [CrossRef]




| Monomer | Abbreviation | Chemical Structure | Illustration | Molar Mass/g mol−1 |
|---|---|---|---|---|
| N-isopropylacrylamide (2-propenamide N-(1-methylethyl)-) | NIPAAm | 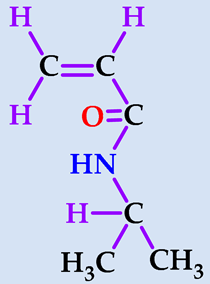 | 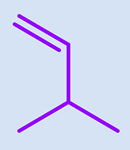 | 113.16 |
| acrylamide (2-propenamide) | AAm | 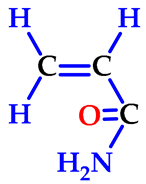 |  | 71.08 |
| acrylic acid (propenoic acid) | AA | 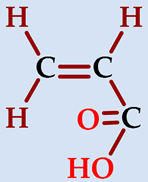 |  | 72.06 |
| maleic acid (cis-butenedioic acid) | MA | 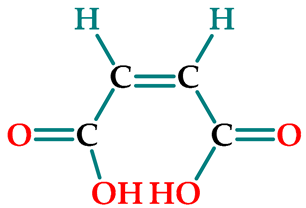 |  | 116.06 |
| N,N′-methylenebisacrylamide | NBis |  |  | 154.17 |
| SH | Ti/°C | Tmax/°C | Tf/°C | Th/°C | Rmax/mg min−1 | Cmax/% |
|---|---|---|---|---|---|---|
| SH-O | 330 | 382 | 415 | 374 | 0.85 | 44 |
| SH-A | 335 | 400 | 436 | 390 | 0.85 | 41 |
| SH-M | 340 | 396 | 427 | 390 | 0.87 | 45 |
| Parameters | SH-0 | SH-A | SH-M |
|---|---|---|---|
| −EFC/R ± SE | 25,911.217 ± 712.623 | 8325.565 ± 207.644 | 19,191.567 ± 718.121 |
| nFC ± SE | 4.181 ± 0.288 | 0.529 ± 0.110 | 2.752 ± 0.404 |
| EFC | 215.43 | 69.287 | 159.392 |
| r | 0.999 | 0.998 | 0.997 |
| nJ | 0.049 | 0.022 | 0.044 |
| EJ | 115.159 | 59.343 | 109.974 |
| SH | LCST/°C | Tg/°C |
|---|---|---|
| SH-O | 31.24 | 84.82 |
| SH-A | 36.58 | 72.20 |
| SH-M | 27.41 | 71.88 |
| T | 25 °C | 40 °C | ||||
|---|---|---|---|---|---|---|
| Parameter | SH-O | SH-A | SH-M | SH-O | SH-A | SH-M |
| α ± SE | - | 0.757 ± 0.016 | 0.617 ± 0.034 | - | 1.149 ± 0.172 | 0.945 ± 0.073 |
| β ± SE | - | 0.484 ± 0.034 | 0.356 ± 0.080 | - | 1.177 ± 0.284 | 0.838 ± 0.165 |
| IP ± SE | - | 3.964 ± 0.036 | 4.626 ± 0.112 | - | 4.198 ± 0.355 | 5.170 ± 0.168 |
| So ± SE | - | 2.920 ± 0.013 | 2.954 ± 0.027 | - | 2.785 ± 0.141 | 2.859 ± 0.049 |
| r | - | 0.999 | 0.996 | - | 0.996 | 0.996 |
| Smax | - | 3.571 | 3.677 | - | 3.934 | 3.804 |
| IPS | - | 3.262 | 3.080 | - | 3.360 | 3.332 |
| Si (at pH = 3) | 2.70 | 2.90 | 2.94 | 2.62 | 2.96 | 2.90 |
| Sf (at pH = 9) | 2.84 | 3.58 | 3.69 | 2.88 | 3.95 | 3.83 |
| pH | 3 | 8 | ||||
|---|---|---|---|---|---|---|
| Parameter | SH-0 | SH-A | SH-M | SH-0 | SH-A | SH-M |
| ϕ ± SE | 1.999 ± 0.031 | 2.342 ± 0.122 | 2.281 ± 0.145 | 1.510 ± 0.047 | 2.042 ± 0.083 | 2.226 ± 0.031 |
| Ψ ± SE | –3.929 ± 0.152 | –4.077 ± 0.535 | –5.192 ± 0.707 | –4.219 ± 0.319 | –7.152 ± 0.576 | –3.321 ± 0.133 |
| LCST ± SE | 27.601 ± 0.191 | 28.303 ± 0.653 | 26.745 ± 0.853 | 27.835 ± 0.392 | 34.215 ± 0.535 | 28.798 ± 0.164 |
| So ± SE | 1.508 ± 0.014 | 1.247 ± 0.056 | 1.593 ± 0.057 | 1.567 ± 0.021 | 2.340 ± 0.047 | 1.904 ± 0.015 |
| r | 1.000 | 0.996 | 0.996 | 0.999 | 0.999 | 1.000 |
| Smax | 3.507 | 3.589 | 3.874 | 3.077 | 4.382 | 4.130 |
| LCSTS | 2.508 | 2.419 | 2.730 | 2.321 | 3.362 | 3.017 |
| Si (at T = 10 °C) | 1.51 | 1.26 | 1.50 | 1.55 | 2.41 | 1.92 |
| Sf (at T = 60 °C) | 3.48 | 3.62 | 3.76 | 3.05 | 4.30 | 4.14 |
| Parameters | SH-O | SH-A | SH-M |
|---|---|---|---|
| So ± SE | 2.293 ± 0.011 | 1.774 ± 0.081 | 2.059 ± 0.048 |
| ξ ± SE | 0.150 ± 0.018 | 1.923 ± 0.121 | 1.138 ± 0.077 |
| λ ± SE | 9.714 ± 2.677 | 7.806 ± 1.186 | 9.723 ± 1.525 |
| r | 0.980 | 0.994 | 0.993 |
| Smax (So + ξ) | 2.443 | 3.196 | 3.696 |
| Si (at I = 0 M) | 2.28 | 2.02 | 1.74 |
| Sf (at I = 1 M) | 2.45 | 3.23 | 3.76 |
| Parameters | SH-O | SH-A | SH-M |
|---|---|---|---|
| φ ± SE | 4.052 ± 1.264 | 4.550 ± 0.341 | 4.609 ± 0.674 |
| ω ± SE | −3.002 ± 1.225 | −2.916 ± 0.312 | −3.328 ± 0.644 |
| RP ± SE | 49.300 ± 1.041 | 48.174 ± 0.831 | 47.983 ± 0.903 |
| σ ± SE | 39.771 ± 12.151 | 27.679 ± 3.281 | 32.693 ± 5.650 |
| r | 0.986 | 0.992 | 0.990 |
| RPS | 1.051 | 1.635 | 1.281 |
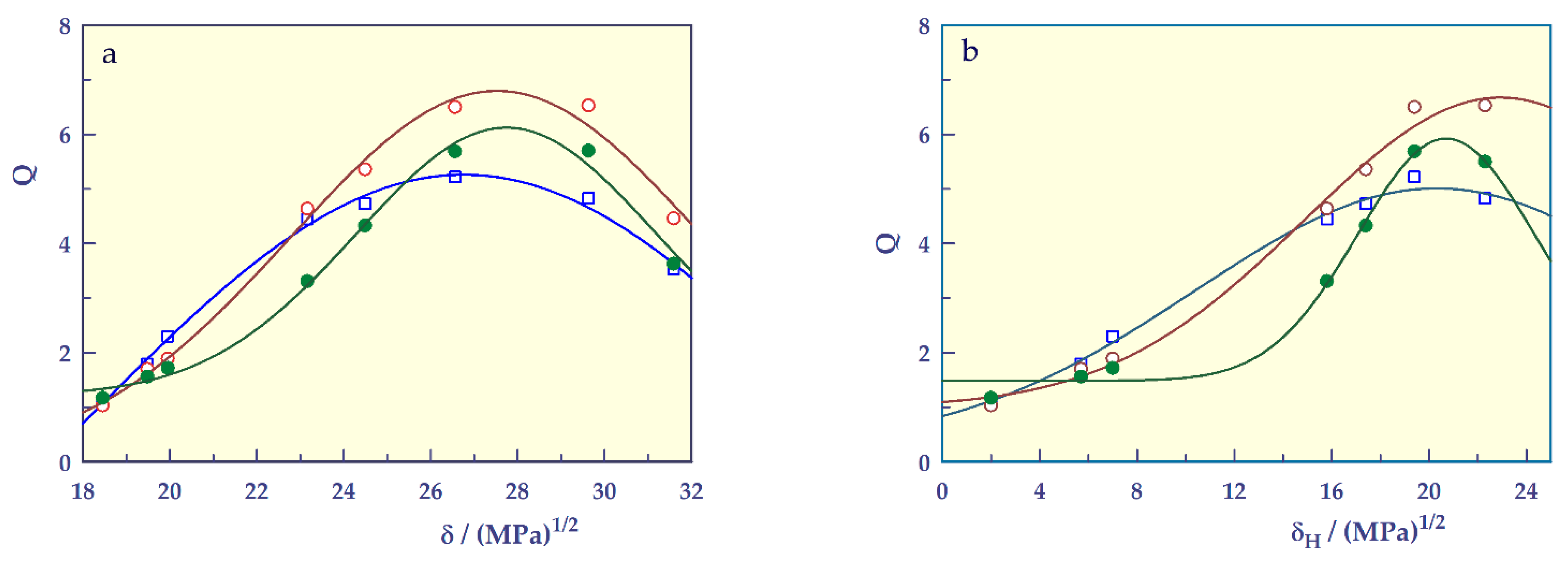
| (a) | |||
| Parameters | SH-O | SH-A | SH-M |
| ψ ± SE | –6.503 ± 7.271 | –0.005 ± 0.808 | 1.208 ± 0.201 |
| Υ ± SE | 11.765 ± 7.205 | 6.797 ± 0.700 | 4.914 ± 0.257 |
| δSH ± SE | 26.757 ± 0.140 | 27.524 ± 0.176 | 27.742 ± 0.135 |
| σ ± SE | 8.849 ± 3.316 | 4.742 ± 0.637 | 3.441 ± 0.257 |
| r | 0.997 | 0.995 | 0.996 |
| (b) | |||
| Parameters | SH-O | SH-A | SH-M |
| ψ ± SE | 0.254 ± 0.744 | 0.998 ± 0.489 | 1.484 ± 0.138 |
| Υ ± SE | 4.758 ± 0.733 | 5.676 ± 0.682 | 4.439 ± 0.248 |
| δSH,H ± SE | 20.307 ± 0.962 | 22.937 ± 1.941 | 20.703 ± 0.324 |
| σ ± SE | 9.914 ± 1.892 | 8.036 ± 2.149 | 3.620 ± 0.415 |
| r | 0.997 | 0.996 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Işıkver, Y.; Saraydın, D. Smart Hydrogels: Preparation, Characterization, and Determination of Transition Points of Crosslinked N-Isopropyl Acrylamide/Acrylamide/Carboxylic Acids Polymers. Gels 2021, 7, 113. https://doi.org/10.3390/gels7030113
Işıkver Y, Saraydın D. Smart Hydrogels: Preparation, Characterization, and Determination of Transition Points of Crosslinked N-Isopropyl Acrylamide/Acrylamide/Carboxylic Acids Polymers. Gels. 2021; 7(3):113. https://doi.org/10.3390/gels7030113
Chicago/Turabian StyleIşıkver, Yasemin, and Dursun Saraydın. 2021. "Smart Hydrogels: Preparation, Characterization, and Determination of Transition Points of Crosslinked N-Isopropyl Acrylamide/Acrylamide/Carboxylic Acids Polymers" Gels 7, no. 3: 113. https://doi.org/10.3390/gels7030113
APA StyleIşıkver, Y., & Saraydın, D. (2021). Smart Hydrogels: Preparation, Characterization, and Determination of Transition Points of Crosslinked N-Isopropyl Acrylamide/Acrylamide/Carboxylic Acids Polymers. Gels, 7(3), 113. https://doi.org/10.3390/gels7030113






