Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update
Abstract
:1. Introduction
2. Hydrogel-Based Drug Delivery Systems
2.1. Macrogels
2.1.1. In Situ-Forming Hydrogels
2.1.2. Macroporous Hydrogels
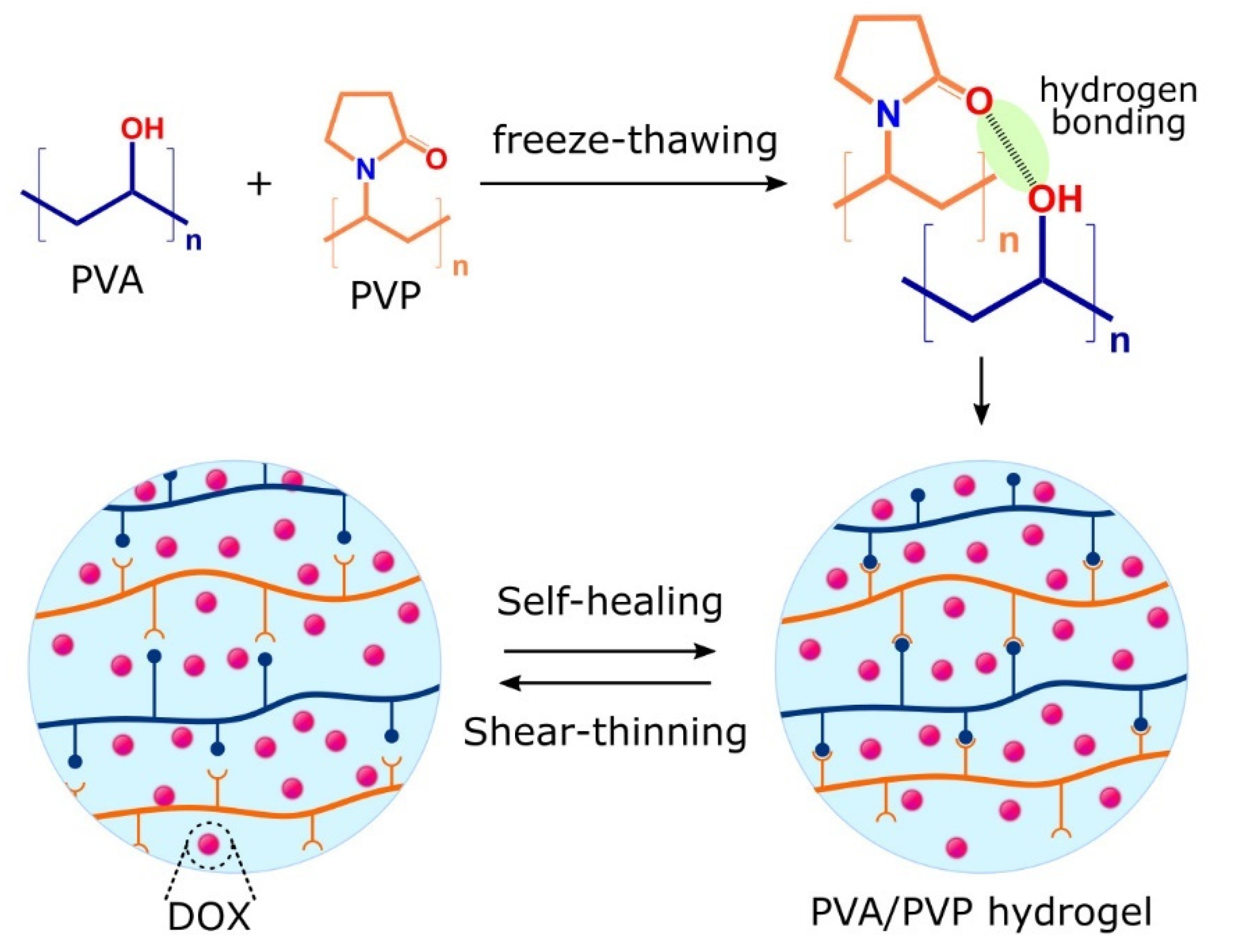
2.1.3. Shear-Thinning Hydrogels
2.2. Microgels and Nanogels
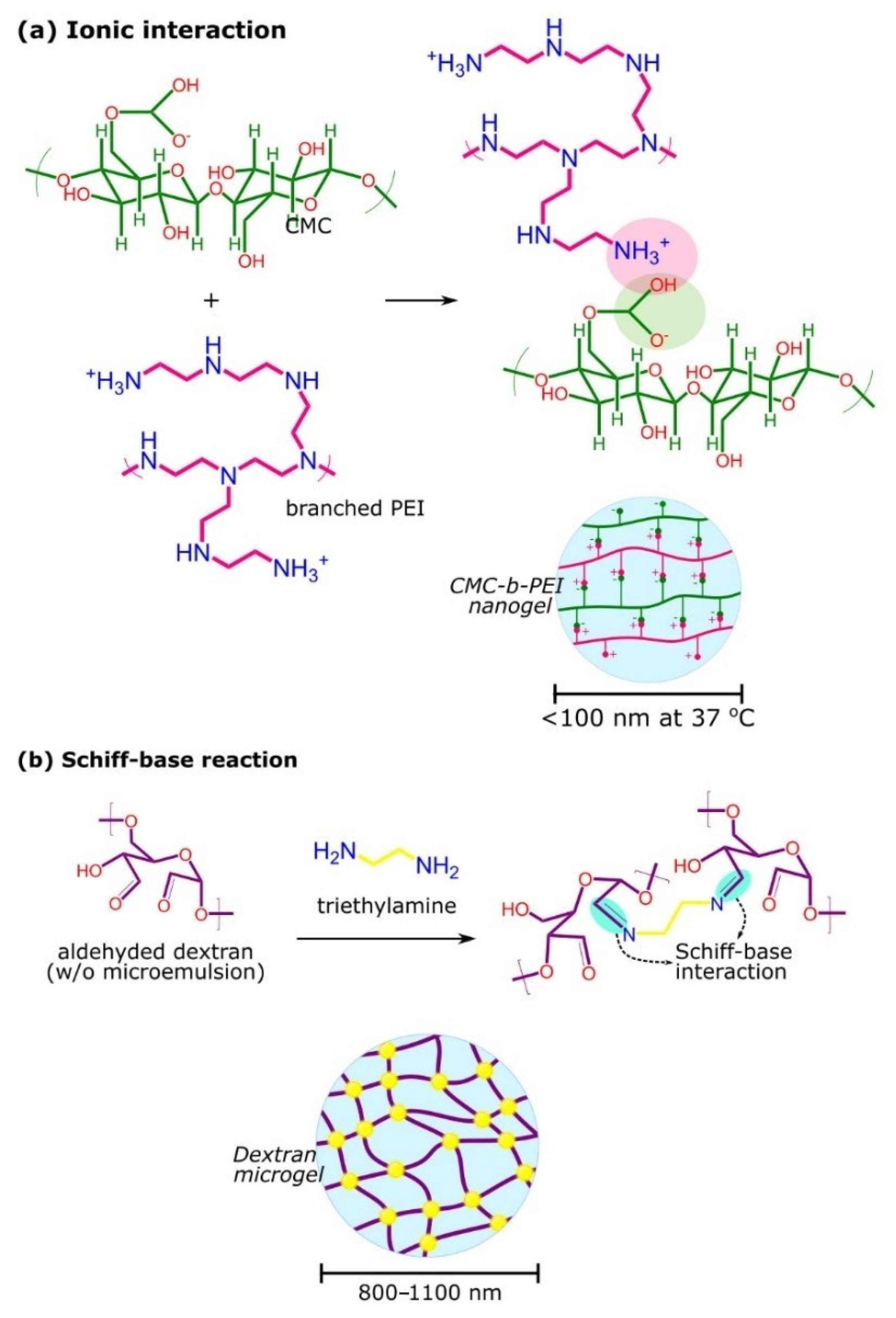
2.2.1. Synthesis and Characteristics of Microgels and Nanogels
2.2.2. Use of Microgels or Nanogels as Drug Delivery Systems
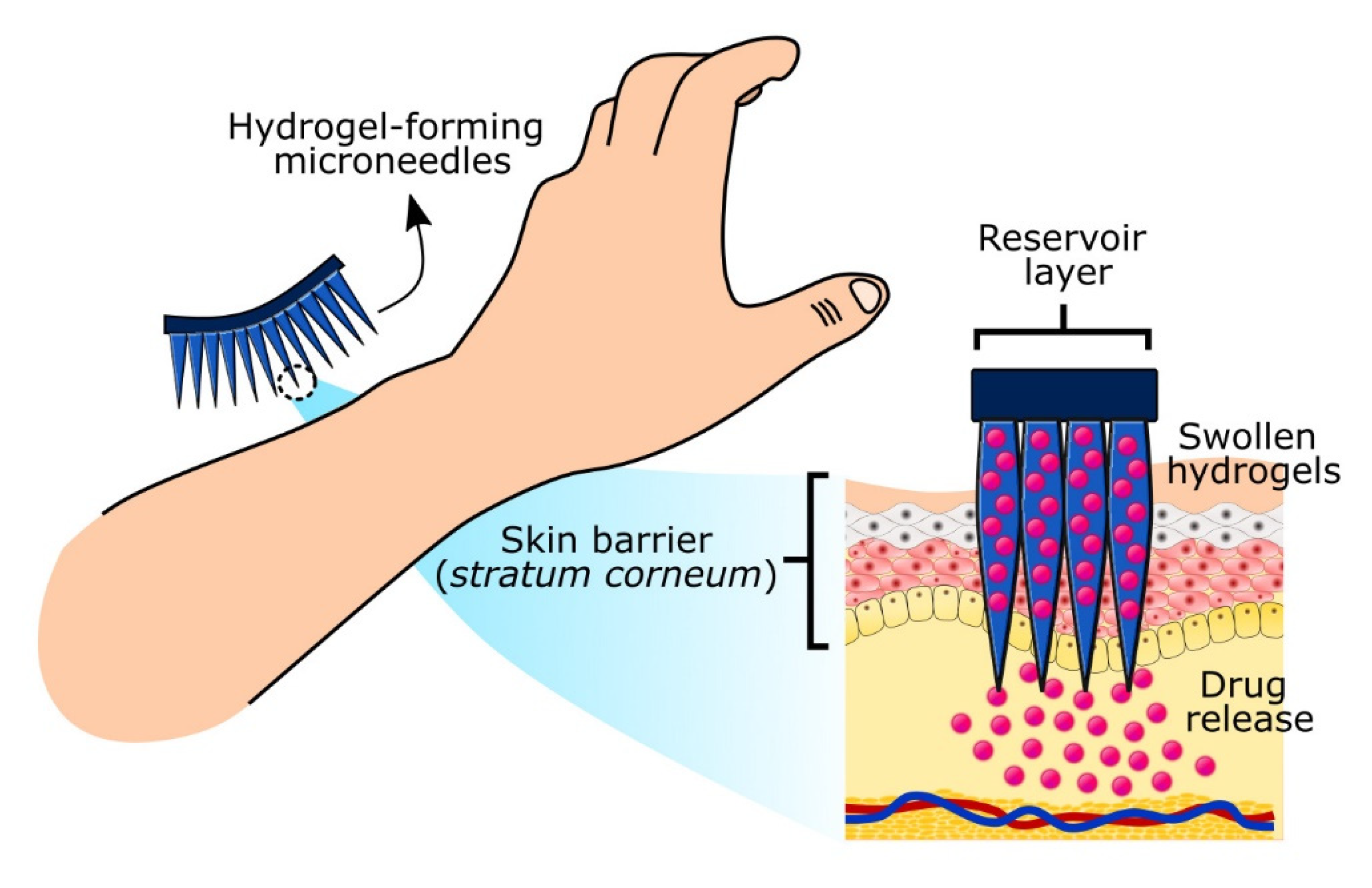
2.3. Hydrogel-Forming Microneedles
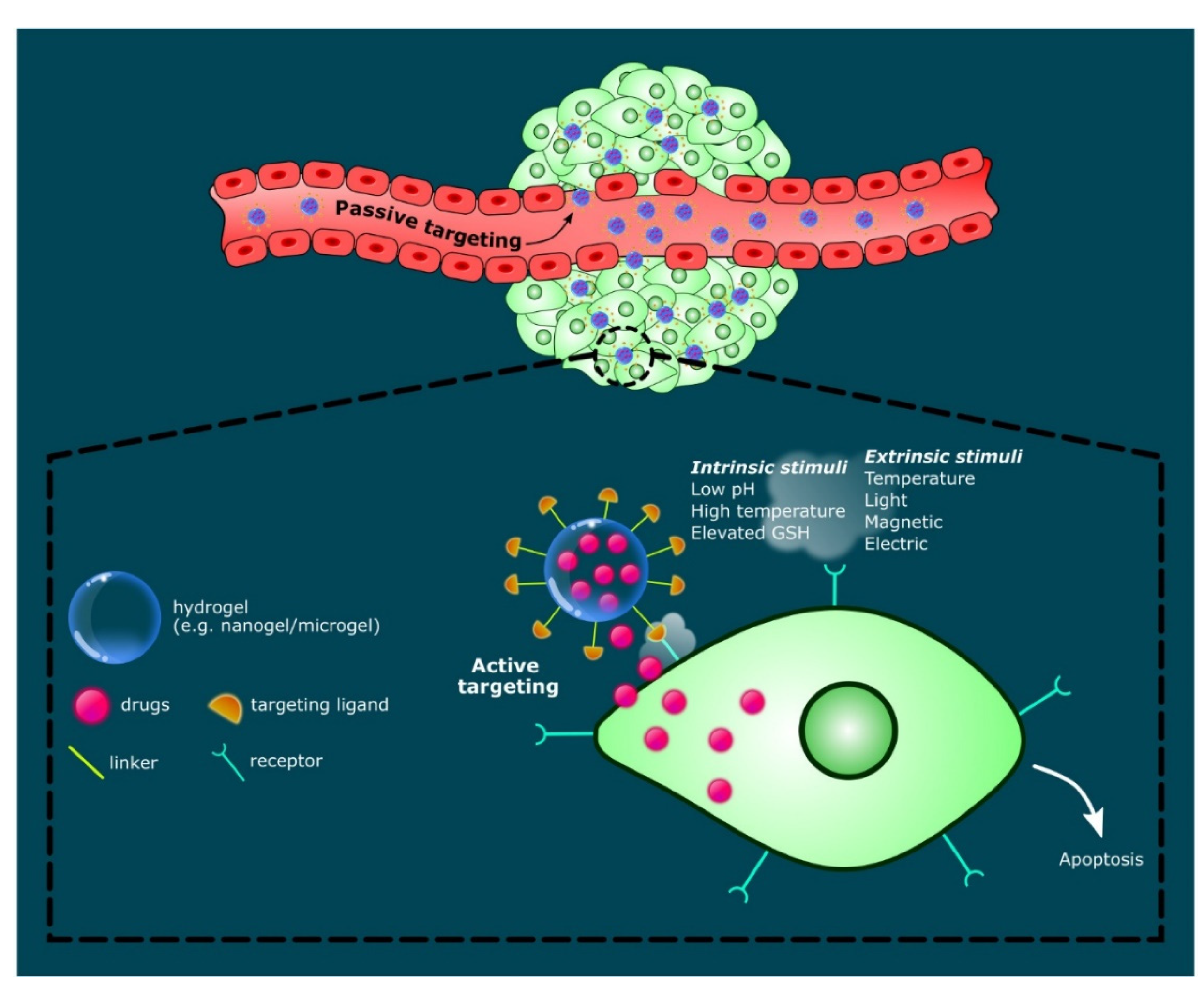
2.4. Stimuli-Responsive Hydrogels
3. Drugs and Inhibitors Delivered by Hydrogel-Based Delivery Systems
3.1. Delivery of Genetic Materials
3.2. Delivery of Peptides or Proteins Using Stimuli-Responsive Hydrogels
3.3. Delivery of Drugs
4. Factors Affecting the Efficacy of Hydrogel Drug Delivery Systems
4.1. Size of Nanoparticles
4.2. Shape of Nanoparticles
4.3. Hydrogel Swelling Ratio
5. Challenges in Hydrogel Drug Delivery System Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, H.; Abribat, T. The rise and rise of drug delivery. Nat. Rev. Drug Discov. 2005, 4, 381–385. [Google Scholar] [CrossRef]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Yang, J.; Han, S.; Zheng, H.; Dong, H.; Liu, J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015, 123, 53–66. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable polysaccharides for controlled drug delivery. Chem. Plus. Chem. 2016, 81, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Shaabani, A. Encapsulation of graphene quantum dot-crosslinked chitosan by carboxymethylcellulose hydrogel beads as a pH-responsive bio-nanocomposite for the oral delivery agent. Int. J. Biol. Macromol. 2019, 123, 389–397. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Advan. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, K.J.; Deshmane, S.V.; Biyani, K.R. Polymers in pharmaceutical drug delivery system: A review. Int. J. Pharm. Sci. Rev. Res. 2012, 14, 57–66. [Google Scholar]
- Ganguly, K.; Chaturvedi, K.; More, U.A.; Nadagouda, M.N.; Aminabhavi, T.M. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J. Control. Release 2014, 193, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Akbal, Ö.; Erdal, E.; Vural, T.; Kavaz, D.; Denkbaş, E.B. Comparison of protein-and polysaccharide-based nanoparticles for cancer therapy: Synthesis, characterization, drug release, and interaction with a breast cancer cell line. Artif. Cell Nanomed. B 2017, 45, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.-Y. Development of polymer-assisted nanoparticles and nanogels for cancer therapy: An update. Gels 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Microparticles drug delivery system: A review. World J. Pharm. Pharm. Sci. 2016, 5, 543–566. [Google Scholar]
- Abdo, G.G.; Zagho, M.M.; Khalil, A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emergent Mater. 2020, 3, 407–425. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. App. 2021, 2, 100067. [Google Scholar]
- Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An updated review of macro, micro, and nanostructured hydrogels for biomedical and pharmaceutical applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Sengel, S.B.; Rees, D.J.; Reed, W.F.; Sahiner, N. 17-Responsive biopolymer-based microgels/nanogels for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, UK, 2018; Volume 1. [Google Scholar]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of nanogels: Current trends and future outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Seyfoori, A.; Koshkaki, M.R.; Majidzadeh-A, K. 26-Nanohybrid stimuli-responsive microgels: A new approach in cancer therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: New York, NY, USA, 2016; pp. 715–742. [Google Scholar]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro- and nanogels with labile crosslinks–from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef] [Green Version]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Guo, X.; Sun, R.; Liu, H.; Tang, L.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. Intra-articular injection of indomethacin–methotrexate in situ hydrogel for the synergistic treatment of rheumatoid arthritis. J. Mater. Chem. B 2020, 8, 993–1007. [Google Scholar] [CrossRef]
- Hoang, H.T.; Jo, S.-H.; Phan, Q.-T.; Park, H.; Park, S.-H.; Oh, C.-W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using “click” chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C 2018, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. pH-responsive UV crosslinkable chitosan hydrogel via “thiol-ene” click chemistry for active modulating opposite drug release behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Santschi, M.; Ferguson, S.J. A Biomimetic Macroporous Hybrid Scaffold with Sustained Drug Delivery for Enhanced Bone Regeneration. Biomacromolecules 2021, 22, 2460–2471. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, D.; Ma, Z.; Liu, K.; Xue, K.; Li, H. An effective strategy for preparing macroporous and self-healing bioactive hydrogels for cell delivery and wound healing. Chem. Eng. Sci. 2021, 425, 130677. [Google Scholar] [CrossRef]
- Goel, S.; Kaur, T.; Singh, N.; Jacob, J. Tunable macroporous D-galactose based hydrogels for controlled release of a hydrophilic drug. Eur. Polym. J. 2021, 150, 110409. [Google Scholar] [CrossRef]
- Ehsanipour, A.; Nguyen, T.; Aboufadel, T.; Sathialingam, M.; Cox, P.; Xiao, W.; Walthers, C.M.; Seidlits, S.K. Injectable, hyaluronic acid-based scaffolds with macroporous architecture for gene delivery. Cell Mol. Bioeng. 2019, 12, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable macroporous hydrogel formed by enzymatic cross-linking of gelatin microgels. ACS Appl. Bio. Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef]
- Wang, L.; Deng, F.; Wang, W.; Li, A.; Lu, C.; Chen, H.; Wu, G.; Nan, K.; Li, L. Construction of injectable self-healing macroporous hydrogels via a template-free method for tissue engineering and drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 36721–36732. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, N.K.; Shome, R.; Biswas, G.; Ghosh, S.S.; Dalal, A. Discerning the self-healing, shear-thinning characteristics and therapeutic efficacy of hydrogel drug carriers migrating through constricted microchannel resembling blood microcapillary. Colloids Surf. A Physiochem. Eng. Asp. 2021, 626, 127070. [Google Scholar] [CrossRef]
- Gharaie, S.; Dabiri, S.M.H.; Akbari, M. Smart shear-thinning hydrogels as injectable drug delivery systems. Polymers 2018, 10, 1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Newby, B.-M.Z. Layer-by-layer polyelectrolyte coating of alginate microgels for sustained release of sodium benzoate and zosteric acid. J. Drug Deliv. Sci. Technol. 2018, 46, 46–54. [Google Scholar] [CrossRef]
- Khan, A.; Othman, M.B.H.; Chang, B.P.; Akil, H.M. Preparation, physicochemical and stability studies of chitosan-PNIPAM based responsive microgels under various pH and temperature conditions. Iran. Polym. J. 2015, 24, 317–328. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M. Nanoarchitectonics for Smart Delivery and Drug Targeting; William Andrew Publishing: New York, NY, USA, 2016. [Google Scholar]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable hydrogel for sustained protein release by salt-induced association of hyaluronic acid nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Jamard, M.; Hoare, T.; Sheardown, H. Nanogels of methylcellulose hydrophobized with N-tert-butylacrylamide for ocular drug delivery. Drug Deliv. Transl. Res. 2016, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wang, X.; Chen, D.; Yuan, Y.; Wang, S.; Li, C.; Yan, Y.; Liu, Q.; Shao, L.; Huang, L.; et al. Enhanced treatment effects of Tilmicosin against Staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Lin, S.; Nune, K.C.; Misra, R.D.K. Chitosan-gelatin-based microgel for sustained drug delivery. J. Biomater. Sci. Polym. Ed. 2016, 27, 441–453. [Google Scholar] [CrossRef]
- Klinger, D.; Landfester, K. Stimuli-responsive microgels for the loading and release of functional compounds: Fundamental concepts and applications. Polymer 2012, 53, 5209–5231. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Jia, Q.; Shan, S. Synthesis and characterization of Schiff base contained dextran microgels in water-in-oil inverse microemulsion. Carbohydr. Polym. 2016, 152, 156–162. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, B.; Hu, X.; Jin, Z.; Xu, X.; Tian, Y. Preparation and characterization of carboxymethyl starch microgel with different crosslinking densities. Carbohydr. Polym. 2015, 124, 245–253. [Google Scholar] [CrossRef]
- Mejías, J.C.; Roy, K. In-vitro and in-vivo characterization of a multi-stage enzyme-responsive nanoparticle-in-microgel pulmonary drug delivery system. J. Control. Release 2019, 316, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, Z.; Mohammadnejad, J.; Razavi Bazaz, S.; Abouei Mehrizi, A.; Saidijam, M.; Dinarvand, R.; Ebrahimi Warkiani, M.; Soleimani, M. Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels. Carbohydr. Polym. 2020, 229, 115551. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.; Khashab, N.M. Hybrid iron oxide–graphene oxide–polysaccharides microcapsule: A micro-matryoshka for on-demand drug release and antitumor therapy in vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurkshtovich, T.; Golub, N.; Yurkshtovich, N.; Bychkovskii, P.; Kosterova, R.; Alinovskaya, V. Starch phosphate microgels for controlled release of biomacromolecules. Appl. Biochem. Microbiol. 2017, 53, 814–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-stabilized hyaluronate nanogel for intracellular codelivery of Doxorubicin and Cisplatin to osteosarcoma. Adv. Sci. 2018, 5, 1700821. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi. Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, S.-I.; Yukawa, H.; Takeda, S.; Sasaki, Y.; Akiyoshi, K. Self-assembled nanogel of cholesterol-bearing xyloglucan as a drug delivery nanocarrier. J. Biomater. Sci. Polym. Ed. 2017, 28, 1183–1198. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.; Chen, H.; Qin, A.; Zhu, P. Anti-tumor study of chondroitin sulfate-methotrexate nanogels. Nanoscale Res. Lett. 2017, 12, 572. [Google Scholar] [CrossRef]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-network-structured graphene oxide-containing nanogels as photothermal agents for the treatment of colorectal cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef]
- Şanlı, O.; Kahraman, A.; Kondolot Solak, E.; Olukman, M. Preparation of magnetite-chitosan/methylcellulose nanospheres by entrapment and adsorption techniques for targeting the anti-cancer drug 5-fluorouracil. Artif. Cells Nanomed. Biotechnol. 2016, 44, 950–959. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Permana, A.D.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Tekko, I.A.; Larrañeta, E.; Vora, L.K.; Ramadon, D.; Donnelly, R.F. Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs. Eur. J. Pharm. Biopharm. 2021, 158, 294–312. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Huang, S.; Fu, T.; Xue, W.; Guo, R. Dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohydr. Polym. 2020, 246, 116650. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Yu, H.; Wang, L.; Chen, X.; Feng, J.; Li, C.; Xiong, W.; Zhang, Q. Glucose-responsive hydrogel-based microneedles containing phenylborate ester bonds and N-isopropylacrylamide moieties and their transdermal drug delivery properties. Eur. Polym. J. 2021, 148, 110348. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Chang, J.Y.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart microneedle fabricated with silk fibroin combined semi-interpenetrating network hydrogel for glucose-responsive insulin delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- Drude, N.; Singh, S.; Winz, O.H.; Möller, M.; Mottaghy, F.M.; Morgenroth, A. Multistage passive and active delivery of radiolabeled nanogels for superior tumor penetration efficiency. Biomacromolecules 2017, 18, 2489–2498. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Lu, H.; Kong, L.; Zhang, S.; Zhang, W.; Nie, J.; Du, B.; Wang, X. Ionic microgel loaded with gold nanoparticles for the synergistic dual-drug delivery of doxorubicin and diclofenac sodium. Ind. Eng. Chem. Res. 2019, 58, 10922–10930. [Google Scholar] [CrossRef]
- Karzar Jeddi, M.; Mahkam, M. Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery. Int. J. Biol. Macromol. 2019, 135, 829–838. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [Green Version]
- Raemdonck, K.; Van Thienen, T.G.; Vandenbroucke, R.E.; Sanders, N.N.; Demeester, J.; De Smedt, S.C. Dextran microgels for time-controlled delivery of siRNA. Adv. Funct. Mater. 2008, 18, 993–1001. [Google Scholar] [CrossRef]
- Pereira, P.; Morgado, D.; Crepet, A.; David, L.; Gama, F.M. Glycol chitosan-based nanogel as a potential targetable carrier for siRNA. Macromol. Biosci. 2013, 13, 1369–1378. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.N.; Park, J.S.; Jeon, S.Y.; Park, K.H. Carboxymethylcellulose (CMC) formed nanogels with branched poly(ethyleneimine) (bPEI) for inhibition of cytotoxicity in human MSCs as a gene delivery vehicles. Carbohydr. Polym. 2015, 122, 265–275. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Y.; Zhao, Y.; Xie, R.; Yodsanit, N.; Johnston, K.; Gong, S. Double-network nanogel as a nonviral vector for DNA delivery. ACS Appl. Mater. Interfaces 2019, 11, 42865–42872. [Google Scholar] [CrossRef] [PubMed]
- Yavvari, P.S.; Verma, P.; Mustfa, S.A.; Pal, S.; Kumar, S.; Awasthi, A.K.; Ahuja, V.; Srikanth, C.V.; Srivastava, A.; Bajaj, A. A nanogel based oral gene delivery system targeting SUMOylation machinery to combat gut inflammation. Nanoscale 2019, 11, 4970–4986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ding, F.; Liu, X.; Shen, J.; Su, Y.; Qian, J.; Zhu, X.; Zhang, C. Nanobody-guided targeted delivery of microRNA via nucleic acid nanogel to inhibit the tumor growth. J. Control. Release 2020, 328, 425–434. [Google Scholar] [CrossRef]
- Theune, L.E.; Charbaji, R.; Kar, M.; Wedepohl, S.; Hedtrich, S.; Calderón, M. Critical parameters for the controlled synthesis of nanogels suitable for temperature-triggered protein delivery. Mater. Sci. Eng. C 2019, 100, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ma, Y.; Xu, X.; Ji, Q.; Feng, M.; Cheng, C.; Feng, Y.; He, B.; Mo, R. Enzyme-instructed hybrid nanogel/nanofiber oligopeptide hydrogel for localized protein delivery. Acta Pharm. Sin. B 2020, 11, 2070–2079. [Google Scholar] [CrossRef]
- Si, X.; Song, W.; Yang, S.; Ma, L.; Yang, C.; Tang, Z. Glucose and pH dual-responsive nanogels for efficient protein delivery. Macromol. Biosci. 2019, 19, 1900148. [Google Scholar] [CrossRef]
- Massi, L.; Najer, A.; Chapman, R.; Spicer, C.D.; Nele, V.; Che, J.; Booth, M.A.; Doutch, J.J.; Stevens, M.M. Tuneable peptide cross-linked nanogels for enzyme-triggered protein delivery. J. Mater. Chem. B 2020, 8, 8894–8907. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, L.; Li, D.; Wang, X.; Zhang, P.; Wang, J.; Yan, G.; Tang, R. Carboxymethyl chitosan-based nanogels via acid-labile ortho ester linkages mediated enhanced drug delivery. Int. J. Biol. Macromol. 2019, 129, 477–487. [Google Scholar] [CrossRef]
- Sagbas Suner, S.; Ari, B.; Onder, F.C.; Ozpolat, B.; Ay, M.; Sahiner, N. Hyaluronic acid and hyaluronic acid: Sucrose nanogels for hydrophobic cancer drug delivery. Int. J. Biol. Macromol. 2019, 126, 1150–1157. [Google Scholar] [CrossRef]
- Peng, S.; Ouyang, B.; Xin, Y.; Zhao, W.; Shen, S.; Zhan, M.; Lu, L. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm. Sin. B 2021, 11, 560–571. [Google Scholar] [CrossRef]
- She, D.; Huang, H.; Li, J.; Peng, S.; Wang, H.; Yu, X. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem. Eng. J. 2021, 408, 127359. [Google Scholar] [CrossRef]
- Huang, G.; Xie, J.; Shuai, S.; Wei, S.; Chen, Y.; Guan, Z.; Zheng, Q.; Yue, P.; Wang, C. Nose-to-brain delivery of drug nanocrystals by using Ca2+ responsive deacetylated gellan gum based in situ-nanogel. Int. J. Pharm. 2021, 594, 120182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Zhang, C.; Pich, A.; Xing, L.; Shi, X. Intelligent nanogels with self-adaptive responsiveness for improved tumor drug delivery and augmented chemotherapy. Bioact. Mater. 2021, 6, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chu, B.; Wei, X.; Lei, M.; Hu, D.; Zha, R.; Zhong, L.; Wang, M.; Wang, F.; Qian, Z. Redox/pH dual-stimuli responsive camptothecin prodrug nanogels for “on-demand” drug delivery. J. Control. Release 2019, 296, 93–106. [Google Scholar] [CrossRef]
- Gao, L.; Zabihi, F.; Ehrmann, S.; Hedtrich, S.; Haag, R. Supramolecular nanogels fabricated via host–guest molecular recognition as penetration enhancer for dermal drug delivery. J. Control. Release 2019, 300, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, G.; Shojaosadati, S.A.; Abdollahi, M. Synthesis and characterization of Schiff base containing bovine serum albumin-gum arabic aldehyde hybrid nanogels via inverse miniemulsion for delivery of anticancer drug. Int. J. Biol. Macromol. 2021, 170, 222–231. [Google Scholar] [CrossRef]
- Obuobi, S.; Julin, K.; Fredheim, E.G.A.; Johannessen, M.; Škalko-Basnet, N. Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections. J. Control. Release 2020, 324, 620–632. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.H.; Mahdavinia, G.R.; Massoumi, B. pH-controlled sunitinib anticancer release from magnetic chitosan nanoparticles crosslinked with κ-carrageenan. Mater. Sci. Eng. C 2018, 91, 705–714. [Google Scholar] [CrossRef]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Dadalt Souto, G.; Garrastazu Pereira, G.; Bianchera, A.; Tiozzo Fasiolo, L.; Colombo, G.; Marques, M.; Raffin Pohlmann, A.; et al. Chitosan-coated nanoparticles: Effect of chitosan molecular weight on nasal transmucosal delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.; Nguyen, M.; Tan, Y.; Chew, J.; Khan, S.; Hadinoto, K. Millifluidic synthesis of amorphous drug-polysaccharide nanoparticle complex with tunable size intended for supersaturating drug delivery applications. Eur. J. Pharm. Biopharm. 2017, 112, 196–203. [Google Scholar] [CrossRef]
- Chen, J.; Clay, N.; Kong, H. Non-spherical particles for targeted drug delivery. Chem. Eng. Sci. 2015, 125, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef]
- Williford, J.-M.; Santos, J.L.; Shyam, R.; Mao, H.-Q. Shape control in engineering of polymeric nanoparticles for therapeutic delivery. Biomater. Sci. 2015, 3, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of shape, size, and aspect ratio on nanoparticle penetration and distribution inside solid tissues using 3D spheroid models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Tan, H.L.; Kai, D.; Pasbakhsh, P.; Teow, S.Y.; Lim, Y.Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces. 2020, 188, 110713. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Jahangirian, H.; Teow, S.Y.; Umakoshi, H.; Saleh, B.; Rafiee-Moghaddam, R.; Webster, T.J. The potential anticancer activity of 5-fluorouracil loaded in cellulose fibers isolated from rice straw. Int. J. Nanomed. 2020, 15, 5417. [Google Scholar] [CrossRef]
- Yusefi, M.; Soon, M.L.K.; Shameli, K.; Teow, S.-Y.; Ali, R.R.; Siew, K.-K.; Chan, H.-Y.; Wong, M.M.-T.; Lim, W.-L.; Kuca, K. 5-fluorouracil loaded magnetic cellulose bionanocomposites for potential colorectal cancer treatment. Carbohydr. Polym. 2021, 273, 118523. [Google Scholar] [CrossRef]
- Yusefi, M.; Chan, H.Y.; Teow, S.Y.; Kia, P.; Soon, M.L.K.; Che Sidik, N.A.; Shameli, K. 5-fluorouracil encapsulated chitosan-cellulose fiber bionanocomposites: Synthesis, characterization and in vitro analysis towards colorectal cancer cells. Nanomaterials 2021, 11, 1691. [Google Scholar] [CrossRef]
- Dash, R.; Ragauskas, A.J. Synthesis of a novel cellulose nanowhisker-based drug delivery system. RSC Adv. 2012, 2, 3403–3409. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, W.; Liu, Y.; Zhao, Y.; Zhang, J.; Hou, M. Hygroscopicity modulation of hydrogels based on carboxymethyl chitosan/Alginate polyelectrolyte complexes and its application as pH-sensitive delivery system. Carbohydr. Polym. 2018, 198, 86–93. [Google Scholar] [CrossRef]
- Selvakumaran, S.; Muhamad, I.I.; Razak, S.I.A. Evaluation of kappa carrageenan as potential carrier for floating drug delivery system: Effect of pore forming agents. Carbohydr. Polym. 2016, 135, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Bertram, U.; Bodmeier, R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: In vitro characterization of a new nasal dosage form. Eur. J. Pharm. Sci. 2006, 27, 62–71. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings. A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness and biodegradability. Polymer 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Sharma, S.K. Rececent advances in guar gum based drug delivery systems and their administrative routes. Int. J. Biomed. Macromol. 2021, 181, 653–671. [Google Scholar] [CrossRef]
- Lima, A.C.; Sher, P.; Mano, J.F. Production methodologies of polymeric and hydrogel particles for drug delivery applications. Exp. Opin. Drug Deliv. 2012, 9, 231–248. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Yan, F.; Zheng, Y.; Mei, L.; Tang, L.; Song, C.; Huang, L. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nanomedicine 2010, 6, 170–178. [Google Scholar] [CrossRef]
- Water, J.J.; Kim, Y.; Maltesen, M.J.; Franzyk, H.; Foged, C.; Nielsen, H.M. Hyaluronic acid-based nanogels produced by microfluidics-facilitated self-assembly improves the safety profile of the cationic host defense peptide novicidin. Pharm. Res. 2015, 32, 2727–2735. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Pellá, M.C.G.; de Lima, H.H.C.; Rinaldi, A.W.; Fajardo, A.E.; Tenório-Neto, E.T.; Guilherme, M.R.; Lima-Tenório, M.K. Chitosan-based hydrogels for drug delivery. In Functional Chitosan; Springer: Singapore, 2019. [Google Scholar]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Modeling the drug release from hydrogel-based matrices. Mol. Pharm. 2015, 12, 474–483. [Google Scholar] [CrossRef]
| Polymer Name | Polysaccharides and Additives | Delivered Molecule | Functions of Polysaccharides | Year | References | |
|---|---|---|---|---|---|---|
| Genes | siRNA-loaded microgels | Cationic biodegradable dextran microgels | siRNA | Increase encapsulation amount, Controlled release of materials | 2008 | [74] |
| Folate conjugated nanogel | Glycol chitosan-based nanogel | siRNA | Improve delivery of material | 2013 | [75] | |
| Plasmid DNA-loaded nanogel | CMC complex with bPEI nanogel | Plasmid DNA | Improve delivery of materials, Increase uptake and gene transfection | 2015 | [76] | |
| Double-network nanogels | Silane-crosslinked PEI with pH-responsive poly(2-(hexamethyleneimino)ethyl methacrylate) | DNA | pH-responsive delivery, Improve stability of genetic material | 2019 | [77] | |
| TAC6-derived nanogel | TAC6 polymer | PIAS1 | Decrease gut inflammation by repressing NF-KB | 2020 | [78] | |
| Nanobody-functionalized nanogel | Nanobody conjugated DNA strands with Nb-DNA conjugate | miRNA | Increase accumulation of miRNA to desired site, Increase anti-tumor effect | 2020 | [79] | |
| Peptides and Proteins | Hydrophillic thermo-responsive nanogels | NIPAM, poly(N-Isopropylmethacrylamide) | Protein | High protein encapsulation, Release protein at desired temperature | 2019 | [80] |
| Nisin-loaded antimicrobial dual responsive nanogel | PLLA-g-CS | Peptide | Release nisin at specific pH and temperature | 2019 | [81] | |
| CytoC-embedded hydrogel | Acrylamide (AAm), 2-(dime- thylamino)ethyl methacrylate (DMAEMA) monomer and glycerol dimethacrylate (GDA) | Protein | Promoted delivery of CytoC to target sites, Improve tumor growth inhibition | 2020 | [82] | |
| pH and glucose dual-responsive nanogels | Dextran and poly(L-glutamic acid)-g-methoxy poly-(-ethylene glycol)/phenyl boronic acid (PLG-g-mPEG/PBA) | Protein | Mediates the delivery and release of protein | 2019 | [83] | |
| Temperature-sensitive nanogel | PEG, N-cyclopropylacrylamide (NCPAM), N-isopropylacrylamide (NIPAM), methacrylic acid (MAA) and trimethylsilylpropargylmethacrylate (TMSPMA) | Protein | Mediates the release of protein when triggered by enzyme and at specific temperature | 2020 | [84] | |
| Acid-degradable nanogels | Carboxymethyl chitosan | DOX | Decrease degradation of drug, improve cellular uptake of drug, improve stability of drug in physiological conditions | 2019 | [85] | |
| Drugs | Hyaluronic acid nanogel | Hyaluronic acid, sucrose, glycerol diglycidyl ether (GDE) | [3-((E)-3-(4-hydroxyphenyl) acryloil)-2H-chromen-2-on | Improve delivery of drug, improve long-term delivery of drug | 2019 | [86] |
| Zwitterionic phosphorylcholine-based nanogel | Poly(phosphorylcholine) (HPMPC) | DOX | Longer blood circulation of nanogel, improve tumor inhibition, effective degradability to release drug to desired site | 2021 | [87] | |
| Zwitterionic phosphorylcholine-based nanogel Ca2+ responsive deacetylated gellan gum based in situ-nanogel | Poly(phosphorylcholine) (HPMPC) Deacetylated gellan gum (DGG) | DOX Harmine nanocrystals (HAR-NC) | Able to deliver drugs passing through BBB, release drug in hypoxic environment | 2021 | [88] | |
| Improve intranasal drug delivery, increase bioavailability of drug in brain, | 2021 | [89] | ||||
| Chitosan-polypyrrole nanogels | Chitosan, pyrrole | DOX | Highly adaptive to surrounding pH, compatible at physiological pH, high loading of drug, prolonged circulation, increase tumor accumulation of nanogel | 2021 | [90] | |
| Tumor microenvironment (TME)-responsive P(CPT-MAA) prodrug nanogel | Methacrylic acid (MAA), CPT monomer (CTPM), and N-N’-methylenebisacrylamide (Bis) | Campthothecin (CPT) | Improve drug delivery, releases drug at specific conditions | 2019 | [91] | |
| Supramolecular polymer nanogel | Hyperbranced polyglycerol, polyglycerylamine, carboxylic-substituted copillar[5]arene, carbonyldiimidazole, sodium 6-chlorohexyl sulfate, DMF and triethylamine | Dexamethasone | Increase drug loading capacity, improve skin penetration | 2019 | [92] | |
| Hybrid bovine serum albumin-gum arabic aldehyde (BSA-GAA) nanogels | Gum arabic aldehyde, bovine serum albumin | 5-FU | Increase drug release at acidic pH, no toxicity of drug when loaded into nanogel | 2021 | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.-T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153. https://doi.org/10.3390/gels7040153
Pushpamalar J, Meganathan P, Tan HL, Dahlan NA, Ooi L-T, Neerooa BNHM, Essa RZ, Shameli K, Teow S-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels. 2021; 7(4):153. https://doi.org/10.3390/gels7040153
Chicago/Turabian StylePushpamalar, Janarthanan, Puviarasi Meganathan, Hui Li Tan, Nuraina Anisa Dahlan, Li-Ting Ooi, Bibi Noorheen Haleema Mooneerah Neerooa, Raahilah Zahir Essa, Kamyar Shameli, and Sin-Yeang Teow. 2021. "Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update" Gels 7, no. 4: 153. https://doi.org/10.3390/gels7040153






