Locally Injectable Hydrogels for Tumor Immunotherapy
Abstract
:1. Introduction
2. Hydrogels
2.1. Characteristics of Hydrogels
2.2. Classification of Hydrogels
3. Tumor Immunotherapy
3.1. Immunomodulator
3.2. Cellular Immunotherapy
3.3. Cancer Vaccine
4. Injectable Hydrogel for Local Chemo-Immunotherapy Combination
5. Injectable Hydrogel for Local Radio-Immunotherapy Combination
6. Injectable Hydrogel for Local Photo-Immunotherapy Combination
6.1. Combination of PTT and Immunotherapy
6.2. Combination of PDT and Immunotherapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mooney, D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kuang, J.; Li, C.-X.; Zhang, M.; Zheng, D.; Zeng, X.; Liu, C.; Zhang, X.-Z. Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication. ACS Nano 2018, 12, 1978–1989. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Lai, W.-F.; Cui, L.; Zhu, X. How to overcome the side effects of tumor immunotherapy. Biomed. Pharmacother. 2020, 130, 110639. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, G.; Wang, B.; Cao, G.; Li, D.; Wang, Y.; Zhang, Y.; Geng, J.; Li, H.; Li, Y. Reinforcing the Combinational Immuno-Oncotherapy of Switching “Cold” Tumor to “Hot” by Responsive Penetrating Nanogels. ACS Appl. Mater. Interfaces 2021, 13, 36824–36838. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Luo, Z.; Li, Z.; Wang, Y.; Tao, J.; Gong, C.; Liu, X. Improving Cancer Immunotherapy Outcomes Using Biomaterials. Angew. Chem. Int. Ed. 2020, 59, 17332–17343. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Tang, L. Surgery-free injectable macroscale biomaterials for local cancer immunotherapy. Biomater. Sci. 2019, 7, 733–749. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, M.; Tan, Y.; Shen, J.; Jin, Q.; Deng, W.; Sun, J.; Wang, C.; Liu, Z.; Chen, Q. Injectable Reactive Oxygen Species-Responsive SN38 Prodrug Scaffold with Checkpoint Inhibitors for Combined Chemoimmunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 50248–50259. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef] [PubMed]
- Khosravimelal, S.; Mobaraki, M.; Eftekhari, S.; Ahearne, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Hydrogels as Emerging Materials for Cornea Wound Healing. Small 2021, 17, e2006335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Y.-H.; Li, J.-Y.; Tang, Z.-R.; Xu, Y.-J. 3D graphene-based gel photocatalysts for environmental pollutants degradation. Environ. Pollut. 2019, 253, 365–376. [Google Scholar] [CrossRef]
- Singh, A.; Qin, H.; Fernandez, I.; Wei, J.; Lin, J.; Kwak, L.W.; Roy, K. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release 2011, 155, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Weiden, J.; Voerman, D.; Dölen, Y.; Das, R.K.; van Duffelen, A.; Hammink, R.; Eggermont, L.; Rowan, A.E.; Tel, J.; Figdor, C.G. Injectable Biomimetic Hydrogels as Tools for Efficient T Cell Expansion and Delivery. Front. Immunol. 2018, 9, 2798. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; He, C.; Chen, X. Injectable Hydrogels as Unique Platforms for Local Chemotherapeutics-Based Combination Antitumor Therapy. Macromol. Biosci. 2018, 18, e1800240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Wu, K.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Synthesis of cellulose-based double-network hydrogels demonstrating high strength, self-healing, and antibacterial properties. Carbohydr. Polym. 2017, 168, 112–120. [Google Scholar] [CrossRef]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Jin, Y.; Lai, S.; Shi, L.; Fan, W.; Shen, Y. An antibacterial hydrogel with desirable mechanical, self-healing and recyclable properties based on triple-physical crosslinking. Chem. Eng. J. 2019, 370, 1228–1238. [Google Scholar] [CrossRef]
- Guo, J.; Sun, H.; Lei, W.; Tang, Y.; Hong, S.; Yang, H.; Tay, F.; Huang, C. MMP-8-Responsive Polyethylene Glycol Hydrogel for Intraoral Drug Delivery. J. Dent. Res. 2019, 98, 564–571. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Li, X.; He, T.; Gong, S.; Zhu, S.; Zhang, M.; Wu, Q.; Gong, C. A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response. Theranostics 2021, 11, 6936–6949. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, L.; Sigen, S.; Gao, Y.; Zhou, D.; Greiser, U.; Creagh-Flynn, J.; Zhang, H.; Dong, Y.; Cutlar, L.; et al. Injectable hyperbranched poly(β-amino ester) hydrogels with on-demand degradation profiles to match wound healing processes. Chem. Sci. 2018, 9, 2179–2187. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wang, Y.; Yang, H.; Peng, K.; Zhang, X. Injectable self-healing hydrogels formed via thiol/disulfide exchange of thiol functionalized F127 and dithiolane modified PEG. J. Mater. Chem. B 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Kaixuan, R.; He, C.; Zhang, Z.; Ren, K.; Chen, X. Injectable, Biomolecule-Responsive Polypeptide Hydrogels for Cell Encapsulation and Facile Cell Recovery through Triggered Degradation. ACS Appl. Mater. Interfaces 2016, 8, 30692–30702. [Google Scholar] [CrossRef]
- Nada, A.A.; Ali, E.; Soliman, A.A. Biocompatible chitosan-based hydrogel with tunable mechanical and physical properties formed at body temperature. Int. J. Biol. Macromol. 2019, 131, 624–632. [Google Scholar] [CrossRef]
- Yu, S.; Wang, C.; Yu, J.; Wang, J.; Lu, Y.; Zhang, Y.; Zhang, X.; Hu, Q.; Sun, W.; He, C.; et al. Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade. Adv. Mater. 2018, 30, e1801527. [Google Scholar] [CrossRef]
- Huebsch, N.; Kearney, C.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.; Xu, L.; Liang, C.; Feng, L.; Xu, J.; Dong, Z.; Tian, L.; Yi, X.; Yang, K.; Liu, Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018, 2, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics 2019, 9, 6080–6098. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.G.; Duong, H.T.T.; Thambi, T.; Nguyen, T.L.; Turabee, H.; Yin, Y.; Kim, S.H.; Kim, J.; Jeong, J.H.; Lee, D.S. Modularly engineered injectable hybrid hydrogels based on protein-polymer network as potent immunologic adjuvant in vivo. Biomaterials 2019, 195, 100–110. [Google Scholar] [CrossRef]
- Yang, F.; Shi, K.; Jia, Y.; Hao, Y.; Peng, J.; Yuan, L.; Chen, Y.; Pan, M.; Qian, Z. A biodegradable thermosensitive hydrogel vaccine for cancer immunotherapy. Appl. Mater. Today 2020, 19, 100608. [Google Scholar] [CrossRef]
- Van Der Burg, S.H.; Arens, R.; Ossendorp, F.; van Hall, T.; Melief, C.J.M. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 2016, 16, 219–233. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [Green Version]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Yang, S.; Palmer, N.; Fox, K.; Kohane, I.S.; Liao, K.P.; Yu, K.-H.; Kou, S.C. Real-world data analyses unveiled the immune-related adverse effects of immune checkpoint inhibitors across cancer types. NPJ Precis. Oncol. 2021, 5, 82. [Google Scholar] [CrossRef]
- Chen, M.; Tan, Y.; Dong, Z.; Lu, J.; Han, X.; Jin, Q.; Zhu, W.; Shen, J.; Cheng, L.; Liu, Z.; et al. Injectable Anti-inflammatory Nanofiber Hydrogel to Achieve Systemic Immunotherapy Post Local Administration. Nano Lett. 2020, 20, 6763–6773. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Fransen, M.F.; van der Maaden, K.; Campos, Y.; García-Couce, J.; Kralisch, D.; Chan, A.; Ossendorp, F.; Cruz, L.J. Thermosensitive hydrogels as sustained drug delivery system for CTLA-4 checkpoint blocking antibodies. J. Control. Release 2020, 323, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Guenancia, C.; Gudjoncik, A.; Hachet, O.; Zeller, M.; Cottin, Y.; Vergely, C. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol. Sci. 2015, 36, 326–348. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S. New HER2-Positive Targeting Agents in Clinical Practice. Curr. Oncol. Rep. 2014, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-W.; Sheu, M.-T.; Chiang, W.-H.; Chiu, Y.-L.; Tu, C.-M.; Wang, W.-Y.; Wu, M.-H.; Wang, Y.-C.; Lu, M.; Ho, H.-O. In situ chemically crosslinked injectable hydrogels for the subcutaneous delivery of trastuzumab to treat breast cancer. Acta Biomater. 2019, 86, 280–290. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tang, Z.; Tian, H.; Chen, X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv. Drug Deliv. Rev. 2016, 98, 64–76. [Google Scholar] [CrossRef]
- Fenton, O.S.; Tibbitt, M.W.; Appel, E.A.; Jhunjhunwala, S.; Webber, M.J.; Langer, R. Injectable Polymer–Nanoparticle Hydrogels for Local Immune Cell Recruitment. Biomacromolecules 2019, 20, 4430–4436. [Google Scholar] [CrossRef]
- Ueda, K.; Akiba, J.; Ogasawara, S.; Todoroki, K.; Nakayama, M.; Sumi, A.; Kusano, H.; Sanada, S.; Suekane, S.; Xu, K.; et al. Growth inhibitory effect of an injectable hyaluronic acid–tyramine hydrogels incorporating human natural interferon-α and sorafenib on renal cell carcinoma cells. Acta Biomater. 2016, 29, 103–111. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Johnson, C.N.; Pattanayek, R.; Potet, F.; Rebbeck, R.T.; Blackwell, D.J.; Nikolaienko, R.; Sequeira, V.; Le Meur, R.; Radwański, P.B.; Davis, J.P.; et al. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of NaV1.5 and RyR2 function. Cell Calcium 2019, 82, 102063. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Meng, J.; Deng, S.; Zhang, L.; Wan, C.; Lu, L.; Huang, J.; Hu, Y.; Zhang, Z.; Li, Y.; et al. Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics 2020, 10, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Qin, Y.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Engineering Dendritic-Cell-Based Vaccines and PD-1 Blockade in Self-Assembled Peptide Nanofibrous Hydrogel to Amplify Antitumor T-Cell Immunity. Nano Lett. 2018, 18, 4377–4385. [Google Scholar] [CrossRef]
- Song, H.; Huang, P.; Niu, J.; Shi, G.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials 2018, 159, 119–129. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Ma, H.; Zhang, J.; Yu, D.; Zhao, R.; Yu, S.; Nie, G.; Wang, H. In Situ Transforming RNA Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy. Nano Lett. 2021, 21, 2224–2231. [Google Scholar] [CrossRef]
- Koh, J.; Kim, S.; Lee, S.N.; Kim, S.-Y.; Kim, J.-E.; Lee, K.Y.; Kim, M.S.; Heo, J.Y.; Park, Y.M.; Ku, B.M.; et al. Therapeutic efficacy of cancer vaccine adjuvanted with nanoemulsion loaded with TLR7/8 agonist in lung cancer model. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102415. [Google Scholar] [CrossRef]
- Sun, Z.; Liang, J.; Dong, X.; Wang, C.; Kong, D.; Lv, F. Injectable Hydrogels Coencapsulating Granulocyte-Macrophage Colony-Stimulating Factor and Ovalbumin Nanoparticles to Enhance Antigen Uptake Efficiency. ACS Appl. Mater. Interfaces 2018, 10, 20315–20325. [Google Scholar] [CrossRef]
- Song, H.; Yang, P.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics 2019, 9, 2299–2314. [Google Scholar] [CrossRef]
- Battaglini, E.; Goldstein, D.; Grimison, P.; McCullough, S.; Mendoza-Jones, P.; Park, S.B. Chemotherapy-Induced Peripheral Neurotoxicity in Cancer Survivors: Predictors of Long-Term Patient Outcomes. J. Natl. Compr. Cancer Netw. 2021, 19, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, X.; Yang, X.; Jin, B.; Shao, C.; Kang, W.; Tang, R. Nanomaterial-Based Organelles Protect Normal Cells against Chemotherapy-Induced Cytotoxicity. Adv. Mater. 2018, 30, e1801304. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Cuomo, A.; Spadoni, I.; Magni, E.; Silvola, A.; Conte, A.; Sigismund, S.; Ravenda, P.S.; Bonaldi, T.; Zampino, M.G.; et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat. Med. 2016, 22, 624–631. [Google Scholar] [CrossRef]
- Ren, X.; Wang, N.; Zhou, Y.; Song, A.; Jin, G.; Li, Z.; Luan, Y. An injectable hydrogel using an immunomodulating gelator for amplified tumor immunotherapy by blocking the arginase pathway. Acta Biomater. 2021, 124, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cao, Z.; Zhang, R.; Chen, Y.; Yang, X. Injectable Supramolecular Hydrogel for Locoregional Immune Checkpoint Blockade and Enhanced Cancer Chemo-Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 33874–33884. [Google Scholar] [CrossRef]
- Dong, X.; Yang, A.; Bai, Y.; Kong, D.; Lv, F. Dual fluorescence imaging-guided programmed delivery of doxorubicin and CpG nanoparticles to modulate tumor microenvironment for effective chemo-immunotherapy. Biomaterials 2020, 230, 119659. [Google Scholar] [CrossRef]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.-K.; Mao, Y.; Sun, M.; Sim, S.-P. Mechanism of Action of Camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef]
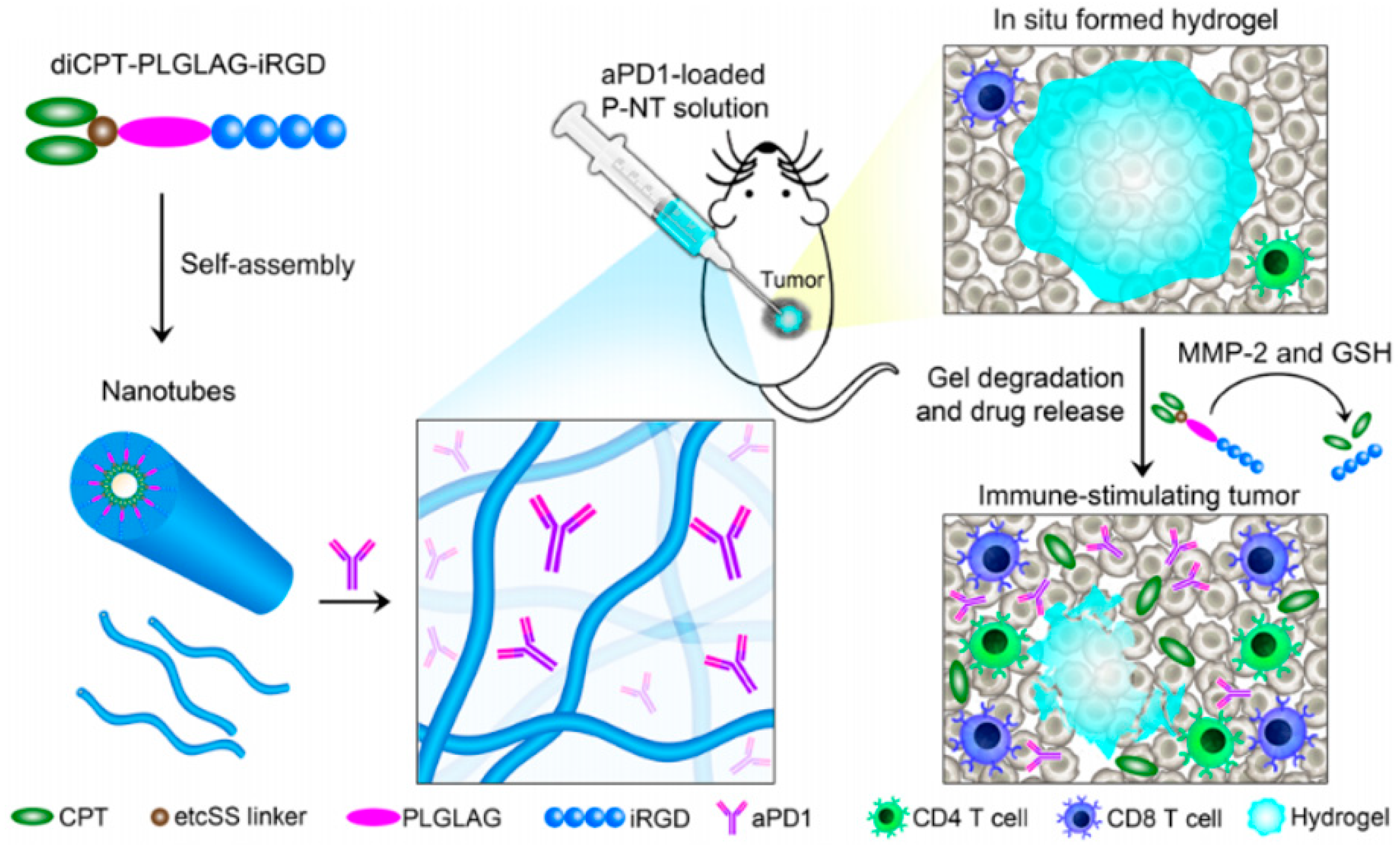
- Wang, F.; Xu, D.; Su, H.; Zhang, W.; Sun, X.; Monroe, M.K.; Chakroun, R.W.; Wang, Z.; Dai, W.; Oh, R.; et al. Supramolecular prodrug hydrogelator as an immune booster for checkpoint blocker–based immunotherapy. Sci. Adv. 2020, 6, eaaz8985. [Google Scholar] [CrossRef]
- Wang, F.; Su, H.; Xu, D.; Dai, W.; Zhang, W.; Wang, Z.; Anderson, C.F.; Zheng, M.; Oh, R.; Wan, F.; et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat. Biomed. Eng. 2020, 4, 1090–1101. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zhang, X.; Yu, S.; Wen, D.; Hu, Q.; Ye, Y.; Bomba, H.; Hu, X.; Liu, Z.; et al. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018, 10, eaan3682. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.; Hu, Q.; Wen, D.; Chen, Q.; Chen, G.; Lu, Y.; Wang, J.; Cheng, H.; Lu, W.; Gu, Z. A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Adv. Mater. 2019, 31, e1806957. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.I.; Ho, A.Y.; McArthur, H.L. Combined Radiation Therapy and Immune Checkpoint Blockade Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 153–164. [Google Scholar] [CrossRef]
- Morris, Z.S.; Guy, E.I.; Francis, D.M.; Gressett, M.M.; Werner, L.; Carmichael, L.L.; Yang, R.; Armstrong, E.A.; Huang, S.; Navid, F.; et al. In Situ Tumor Vaccination by Combining Local Radiation and Tumor-Specific Antibody or Immunocytokine Treatments. Cancer Res. 2016, 76, 3929–3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Zhang, D.; Qian, H.; Chu, Y.; Yang, Y.; Shao, J.; Xu, Q.; Liu, B. Superior Antitumor Efficacy of IFN-α2b-Incorporated Photo-Cross-Linked Hydrogels Combined with T Cell Transfer and Low-Dose Irradiation Against Gastric Cancer. Int. J. Nanomed. 2020, 15, 3669–3680. [Google Scholar] [CrossRef]
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, Z.; Lin, M.; Peng, Y.; Wang, R.; Du, Y.; Zhou, Y.; Li, J.; Qi, X. Phototherapy and anti-GITR antibody-based therapy synergistically reinvigorate immunogenic cell death and reject established cancers. Biomaterials 2021, 269, 120648. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Lan, Q.-H.; Jiang, X.; Du, C.-C.; Zhai, Y.-Y.; Shen, X.; Xu, H.-L.; Xiao, J.; Kou, L.; Zhao, Y.-Z. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics 2020, 10, 11719–11736. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, L.; Wu, H.; Zheng, W.; Kan, D.; Cheng, R.; Yan, J.; Yu, C.; Sun, S.-K. Biocompatible Iodine–Starch–Alginate Hydrogel for Tumor Photothermal Therapy. ACS Biomater. Sci. Eng. 2019, 5, 3654–3662. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.W.; Li, J.; Pu, K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. [Google Scholar] [CrossRef]
- Song, C.; Phuengkham, H.; Kim, Y.S.; Dinh, V.V.; Lee, I.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Um, S.H.; Lee, H.; et al. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat. Commun. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, Y.; Guo, M.; Du, S.; Han, N. Recent strategies for nano-based PTT combined with immunotherapy: From a biomaterial point of view. Theranostics 2021, 11, 7546–7569. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Shi, K.; Yang, F.; Liao, J.F.; Han, R.X.; Yuan, L.P.; Hao, Y.; Pan, M.; Xiao, Y.; Qian, Z.Y.; et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/adfm.202001059 (accessed on 27 September 2021).
- Wu, Y.; Li, Q.; Shim, G.; Oh, Y.-K. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J. Control. Release 2021, 330, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Y.; Du, Y.; Zhang, Y.; Wang, X.; Ding, Y.; Yang, X.; Meng, F.; Tu, J.; Luo, L.; et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019, 10, 487. [Google Scholar] [CrossRef]
- Hou, X.-L.; Dai, X.; Yang, J.; Zhang, B.; Zhao, D.-H.; Li, C.-Q.; Yin, Z.-Y.; Zhao, Y.-D.; Liu, B. Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J. Mater. Chem. B 2020, 8, 8623–8633. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Fan, Q.; Dai, H.; Zhou, X.; Xu, J.; Ma, Q.; Maruyama, A.; Wang, C. Physiologically triggered injectable red blood cell-based gel for tumor photoablation and enhanced cancer immunotherapy. Biomaterials 2021, 271, 120724. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zheng, H.; Chan, M.T.V.; Wu, W.K.K. Immune consequences induced by photodynamic therapy in non-melanoma skin cancers: A review. Environ. Sci. Pollut. Res. 2018, 25, 20569–20574. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
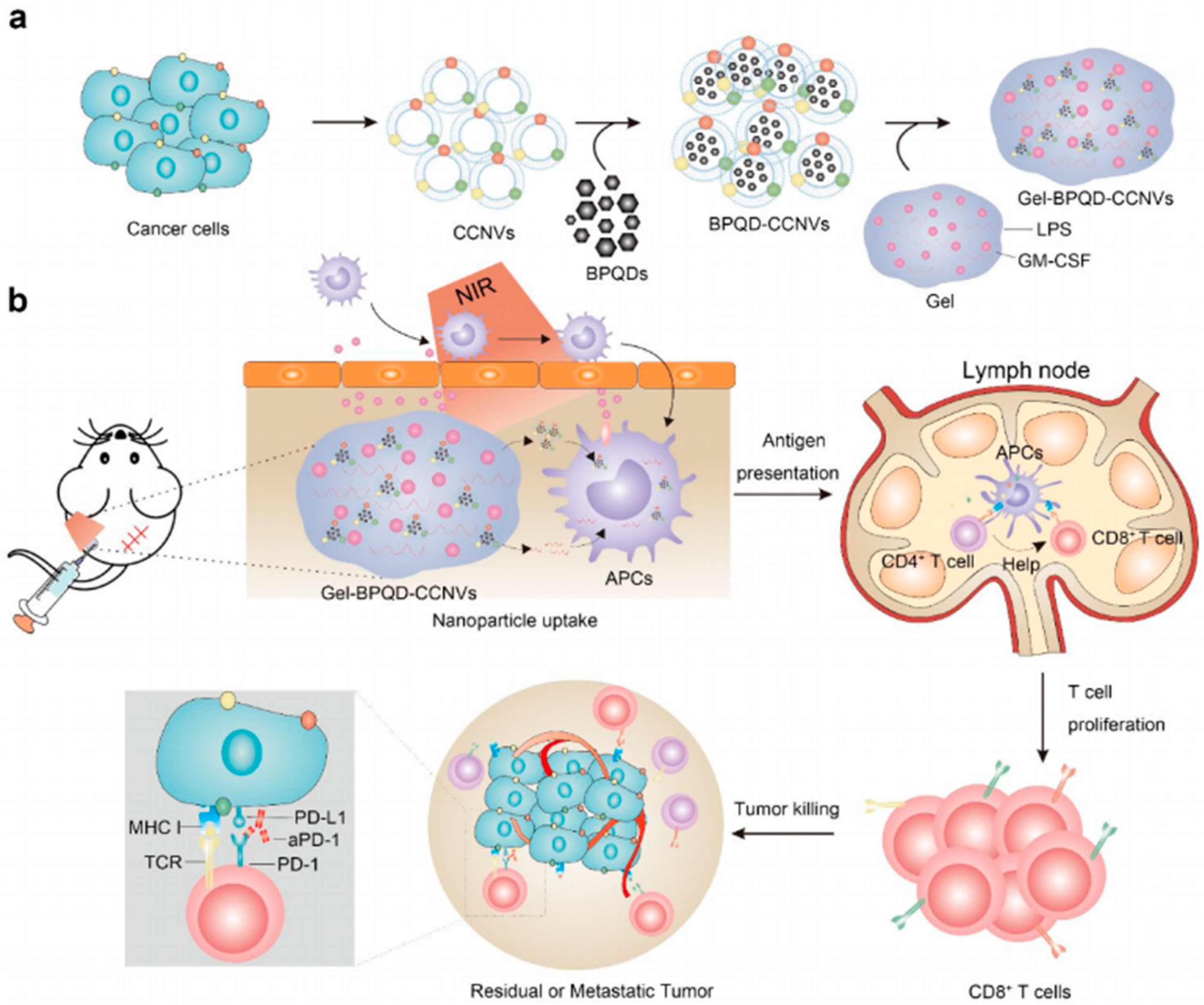
- Meng, Z.; Zhou, X.; Xu, J.; Han, X.; Dong, Z.; Wang, H.; Zhang, Y.; She, J.; Xu, L.; Wang, C.; et al. Light-Triggered In Situ Gelation to Enable Robust Photodynamic-Immunotherapy by Repeated Stimulations. Adv. Mater. 2019, 31, e1900927. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Zhu, W.; Jiang, Y.; Li, X.; Pan, J.; Zhang, X.; Zhang, X.; Sun, S. Persistent Luminescence Immune Hydrogel for Photodynamic-Immunotherapy of Tumors In Vivo. Adv. Funct. Mater. 2021, 31, 2104472. [Google Scholar] [CrossRef]
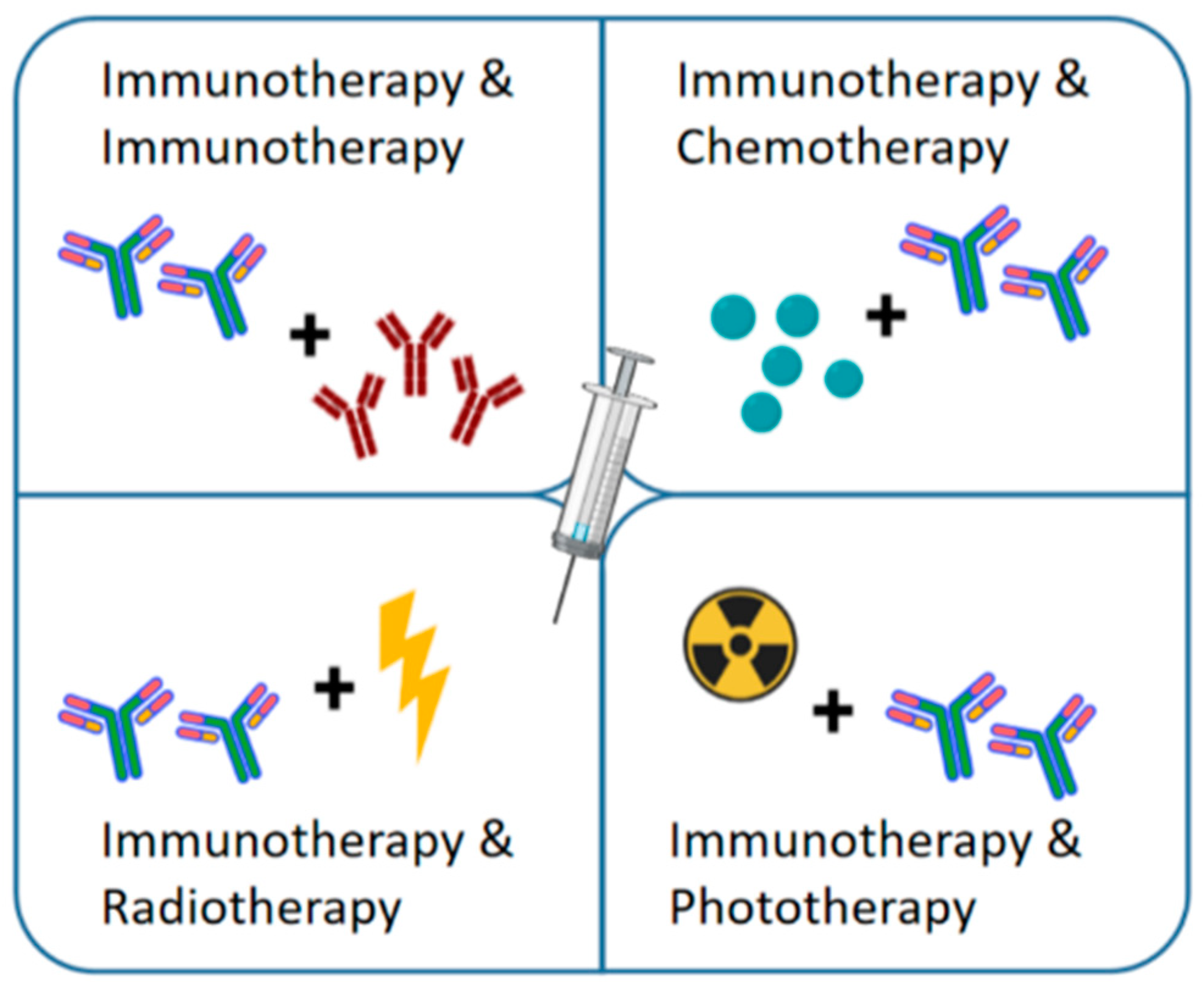


| Therapeutic Strategy | Advantage | Disadvantage | Phase | Reference |
|---|---|---|---|---|
| Immunotherapy | Simple hydrogel composition and synthesis process | Limited therapeutic effect | Preclinical step | [35,42,43,46,48] [15,49,51,53,54] [36,37,56,59,87] |
| Chemoimmunotherapy | High immune response, better treatment effect | Hydrogels are usually complex in composition and their long-term biosafety in vivo needs to be confirmed | Preclinical step | [63,64,65,66,68] [69,70,71] |
| Radioimmunotherapy | High immune response, better antitumor effect at the distal end | Safety of radioactive elements | Preclinical step | [33,75] |
| Photoimmunotherapy | High immune response, good therapeutic effect, and great time and space control | The limited penetration of near infrared light to tissues and the toxicity of photosensitizers | Preclinical step | [65,83,84,85,86] [89,93,94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Guo, X.; Wu, Y.; Gao, J. Locally Injectable Hydrogels for Tumor Immunotherapy. Gels 2021, 7, 224. https://doi.org/10.3390/gels7040224
Zhang X, Guo X, Wu Y, Gao J. Locally Injectable Hydrogels for Tumor Immunotherapy. Gels. 2021; 7(4):224. https://doi.org/10.3390/gels7040224
Chicago/Turabian StyleZhang, Xinyi, Xiaonan Guo, Yan Wu, and Jie Gao. 2021. "Locally Injectable Hydrogels for Tumor Immunotherapy" Gels 7, no. 4: 224. https://doi.org/10.3390/gels7040224





