Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Hydrogel Synthesis
2.2. The Influence of pH, Adsorbent Weight and Ionic Strength on Adsorption of Ni2+ Ions
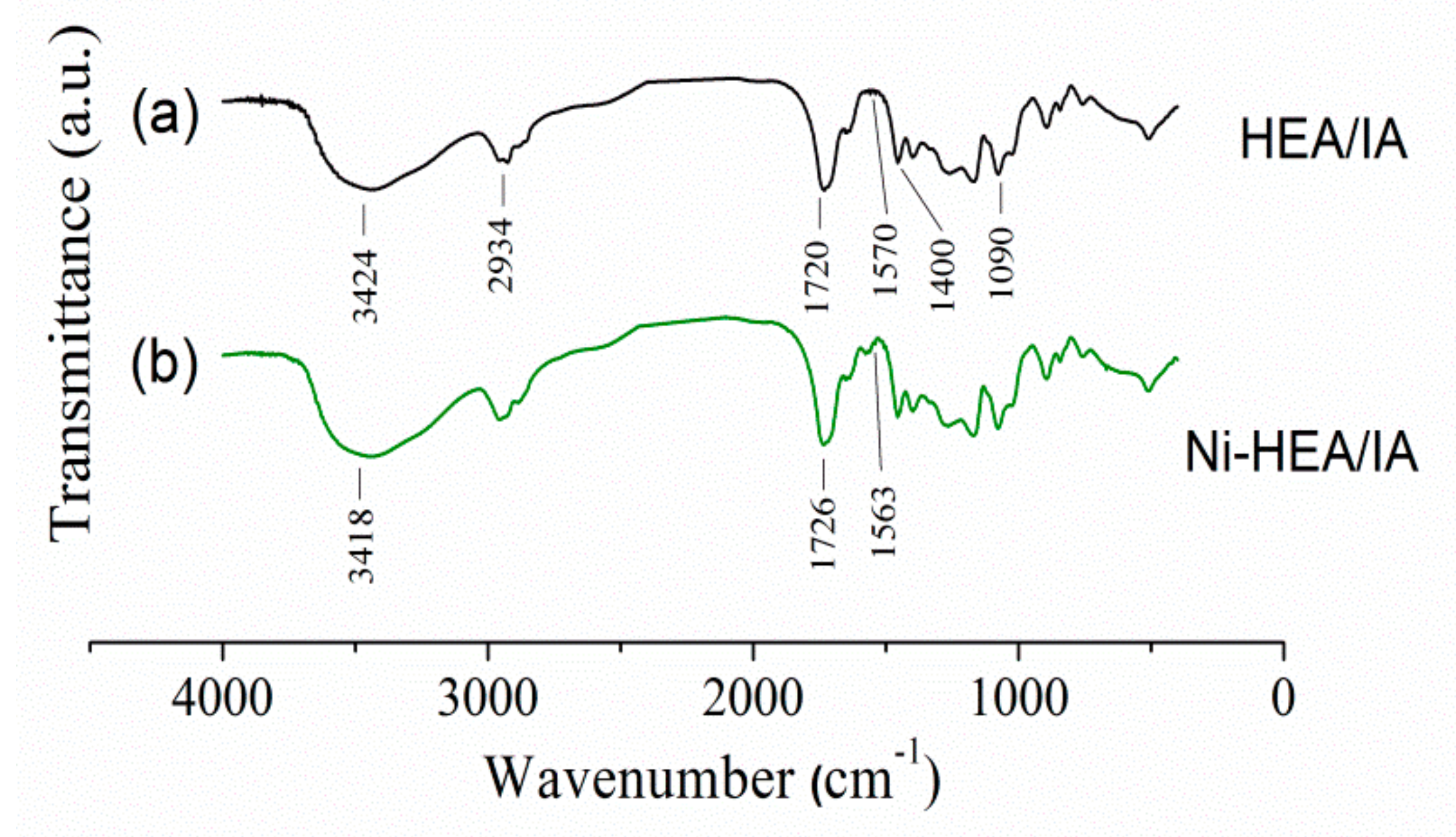
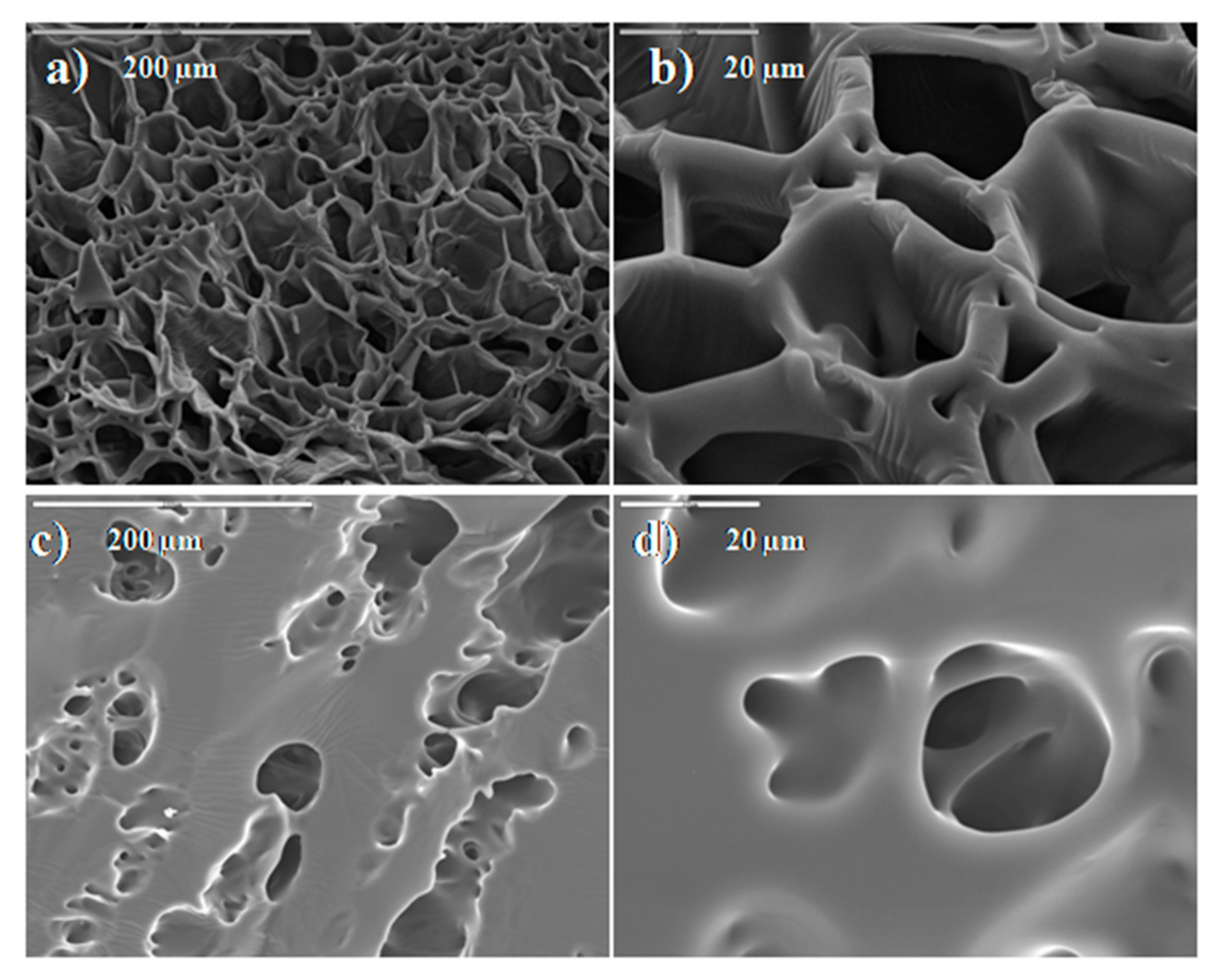
2.3. Characterization of Ni2+ Loaded HEA/10IA Hydrogel
2.4. Adsorption Isotherms
2.5. Adsorption Kinetics and Thermodynamics Studies
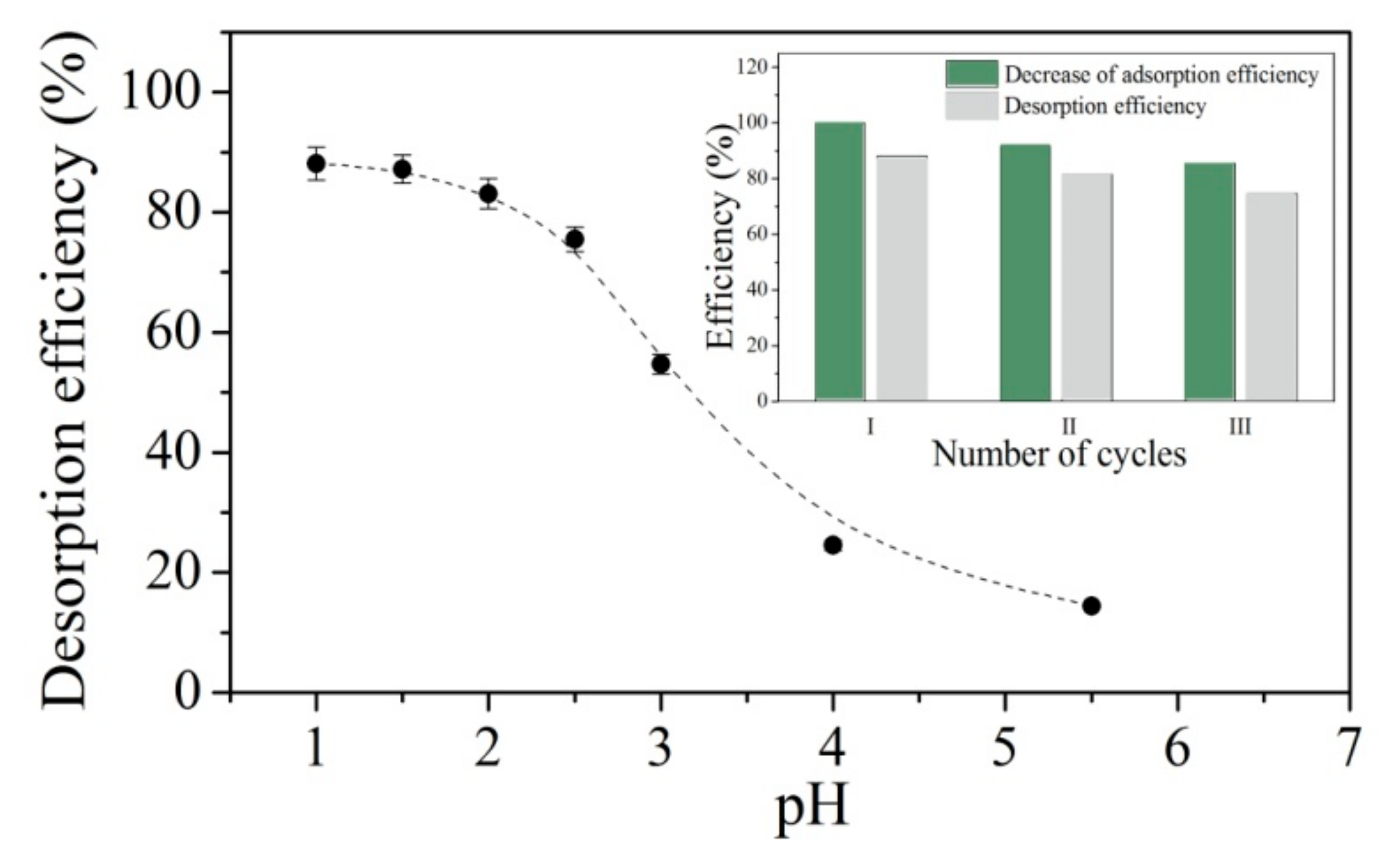
2.6. Desorption and Regeneration Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydrogel Synthesis
4.3. Optimization of Hydrogel Synthesis
4.4. Hydrogels Characterisation
4.5. Adsorption Experiments
4.6. Desorption and Regeneration
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Ur Rashid, T.; Kabir, S.M.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Rehman, T.U.; Shah, L.A.; Khattak, N.S.; Khan, A.; Rehman, N.; Alam, S. Superabsorbent Hydrogels for Heavy Metal Removal. In Trace Metals in the Environment—New Approaches and Recent Advances; IntechOpen: London, UK, 2019. [Google Scholar]
- Patel, A.M.; Patel, R.G.; Patel, M.P. Nickel and copper removal study from aqueous solution using new cationic poly[acrylamide/N,N-DAMB/N,N-DAPB] super absorbent hydrogel. J. Appl. Polym. Sci. 2011, 119, 2485–2493. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from coirpith. Sep. Purif. Technol. 2001, 24, 497–505. [Google Scholar] [CrossRef]
- Zafar, M.N.; Abbas, I.; Nadeem, R.; Sheikh, M.A.; Ghauri, M.A. Removal of Nickel onto Alkali Treated Rice Bran. Water Air Soil Pollut. 2009, 197, 361–370. [Google Scholar] [CrossRef]
- Yu, Q.; Matheickal, J.T.; Yin, P.; Kaewsarn, P. Heavy metal uptake capacities of common marine macro algal biomass. Water Res. 1999, 33, 1534–1537. [Google Scholar] [CrossRef]
- Zhao, M.; Duncan, J.R.; van Hille, R.P. Removal and recovery of zinc from solution and electroplating effluent using Azolla filiculoides. Water Res. 1999, 33, 1516–1522. [Google Scholar] [CrossRef]
- Srivastava, V.; Weng, C.H.; Singh, V.K.; Sharma, Y.C. Adsorption of Nickel Ions from Aqueous Solutions by Nano Alumina: Kinetic, Mass Transfer, and Equilibrium Studies. J. Chem. Eng. Data 2011, 56, 1414–1422. [Google Scholar] [CrossRef]
- Zhang, G.; He, Z.; Xu, W. A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chem. Eng. J. 2012, 183, 315–324. [Google Scholar] [CrossRef]
- Yildiz, U.; Kemik, Ö.F.; Hazer, B. The removal of heavy metal ions from aqueous solutions by novel pH-sensitive hydrogels. J. Hazard. Mater. 2010, 183, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Antić, K.M.; Babić, M.M.; Vuković, J.J.J.; Vasiljević-Radović, D.G.; Onjia, A.E.; Filipović, J.M.; Tomić, S.L. Preparation and characterization of novel P(HEA/IA) hydrogels for Cd2+ ion removal from aqueous solution. Appl. Surf. Sci. 2015, 338, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Antić, K.M.; Babić, M.M.; Vuković, J.S.; Onjia, A.E.; Filipović, J.M.; Tomić, S.L. Removal of Pb2+ from aqueous solution by P(HEA/IA) hydrogels. Hem. Ind. 2016, 70, 695–705. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, Y.; Cao, J.; Yilihan, P.; Wen, Y.; Zhou, J. Optimizing adsorption of arsenic(III) by NH2-MCM-41 using response surface methodology. J. Ind. Eng. Chem. 2014, 20, 2792–2800. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veličković, Z.; Kapidžić, A.; Ivanovski, V.; Mitrić, M.; Marinković, A. Efficient multistep arsenate removal onto magnetite modified fly ash. J. Environ. Manag. 2018, 224, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Yang, M.; Yu, F.; Ma, J. Response surface methodological evaluation and optimization for adsorption removal of ciprofloxacin onto graphene hydrogel. J. Mol. Liq. 2019, 284, 124–130. [Google Scholar] [CrossRef]
- Arulkumar, M.; Sathishkumar, P.; Palvannan, T. Optimization of Orange G dye adsorption by activated carbon of Thespesia populnea pods using response surface methodology. J. Hazard. Mater. 2011, 186, 827–834. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Optimized waste tea activated carbon for adsorption of Methylene Blue and Acid Blue 29 dyes using response surface methodology. Chem. Eng. J. 2011, 175, 233–243. [Google Scholar] [CrossRef]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Kovacevic, T.; Jovanovic, A.; Cvijetic, I.; Marinkovic, A.D. Kinetics and column adsorption study of diclofenac and heavy-metal ions removal by amino-functionalized lignin microspheres. J. Ind. Eng. Chem. 2021, 93, 302–314. [Google Scholar] [CrossRef]
- Perendija, J.; Veličković, Z.S.; Cvijetić, I.; Lević, S.; Marinković, A.D.; Milošević, M.; Onjia, A. Bio-membrane based on modified cellulose, lignin, and tannic acid for cation and oxyanion removal: Experimental and theoretical study. Process. Saf. Environ. Prot. 2021, 147, 609–625. [Google Scholar] [CrossRef]
- Popović, M.; Stojanović, M.; Veličković, Z.; Kovačević, A.; Miljković, R.; Mirković, N.; Marinković, A. Characterization of potential probiotic strain, L. reuteri B2, and its microencapsulation using alginate-based biopolymers. Int. J. Biol. Macromol. 2021, 183, 423–434. [Google Scholar] [CrossRef]
- Milošević, D.; Lević, S.; Lazarević, S.; Veličković, Z.; Marinković, A.; Petrović, R.; Petrović, P. Hybrid material based on subgleba of mosaic puffball mushroom (Handkea utriformis) as an adsorbent for heavy metal removal from aqueous solutions. J. Environ. Manag. 2021, 297, 113358. [Google Scholar] [CrossRef]
- Nikolić, V.; Tomić, N.; Bugarčić, M.; Sokić, M.; Marinković, A.; Veličković, Z.; Kamberović, Ž. Amino-modified hollow alumina spheres: Effective adsorbent for Cd2+, Pb2+, As(V), and diclofenac removal. Environ. Sci. Pollut. Res. 2021, 28, 27174–27192. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.J.K.; Amr, S.S.A.; Aziz, S.Q.; Aun, N.C.; Sethupathi, S. Wastewater Treatment Processes Optimization Using Response Surface Methodology (RSM) Compared with Conventional Methods: Review and Comparative Study. Middle-East. J. Sci. Res. 2015, 23, 244–252. [Google Scholar] [CrossRef]
- Körbahti, B.K.; Tanyolaç, A. Electrochemical treatment of simulated textile wastewater with industrial components and Levafix Blue CA reactive dye: Optimization through response surface methodology. J. Hazard. Mater. 2008, 151, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Körbahti, B.K.; Aktaş, N.; Tanyolaç, A. Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J. Hazard. Mater. 2007, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.J.K.; Isa, M.H.; Kutty, S.R.M.; Awang, Z.B.; Aziz, H.A.; Mohajeri, S.; Farooqi, I.H. Landfill leachate treatment by electrochemical oxidation. Waste Manag. 2009, 29, 2534–2541. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S.; Bashir, M.J.K. Landfill leachate treatment using powdered activated carbon augmented sequencing batch reactor (SBR) process: Optimization by response surface methodology. J. Hazard. Mater. 2011, 189, 404–413. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S. Optimum Process Parameters for the Treatment of Landfill Leachate Using Powdered Activated Carbon Augmented Sequencing Batch Reactor (SBR) Technology. Sep. Sci. Technol. 2011, 46, 2348–2359. [Google Scholar] [CrossRef]
- Ghafari, S.; Aziz, H.A.; Isa, M.H.; Zinatizadeh, A.A. Application of response surface methodology (RSM) to optimize coagulation-flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J. Hazard. Mater. 2009, 163, 650–656. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Mohamed, A.R.; Abdullah, A.Z.; Mashitah, M.D.; Hasnain Isa, M.; Najafpour, G.D. Process modeling and analysis of palm oil mill effluent treatment in an up-flow anaerobic sludge fixed film bioreactor using response surface methodology (RSM). Water Res. 2006, 40, 3193–3208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-C.; Feng, H.-M.; Lam, M.H.-W.; Lam, P.K.-S.; Ding, Y.-W.; Yu, H.-Q. Removal of Cu(II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. J. Hazard. Mater. 2009, 171, 780–785. [Google Scholar] [CrossRef]
- Takahashi, A.; Nagasawa, M. Excluded volume of polyelectrolyte in salt solutions. J. Am. Chem. Soc. 1964, 86, 5543–5548. [Google Scholar] [CrossRef]
- Ceylan, D.; Okay, O. Macroporous Polyisobutylene Gels: A Novel Tough Organogel with Superfast Responsivity. Macromolecules 2007, 40, 8742–8749. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, W.; Kan, X.; Dong, L.; Jiang, Z.; Li, H.; Yang, H.; Cheng, R. Sorption of methylene blue by carboxymethyl cellulose and reuse process in a secondary sorption. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 143–151. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z. Synthesis and characterization of poly(HEA/MALA) hydrogel and its application in removal of heavy metal ions from water. Chem. Eng. J. 2013, 215–216, 894–902. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 2009, 153, 70–79. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Fu, S.; Zheng, L.; Zhan, H. Adsorption behavior of Cd2+, Pb2+ and Ni2+ from aqueous solutions on cellulose-based hydrogels. Bioresources 2012, 7, 2752–2765. [Google Scholar]
- Zhou, J.; Zhang, L.; Deng, Q.; Wu, X. Synthesis and characterization of cellulose derivates prepared in NaOH/urea aqueous solutions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5911–5920. [Google Scholar] [CrossRef]
- Miretzky, P.; Muñoz, C. Enhanced metal removal from aqueous solution by Fenton activated macrophyte biomass. Desalination 2011, 271, 20–28. [Google Scholar] [CrossRef]
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Hua, S.; Wang, A. Adsorption behavior of Cu2+ from aqueous solutions onto starch-g-poly(acrylic acid)/sodium humate hydrogels. Desalination 2010, 263, 170–175. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]




| MODEL | Equation | Parameter | HEA/10IA |
|---|---|---|---|
| Langmuir | KL (L/g) | 5.45 | |
| qm (mg/g) | 269.54 | ||
| R2 | 0.9988 | ||
| χ2 | 5.0713 | ||
| Freundlich | KF (mg g−1 (L mg−1)1/n) | 2.18 | |
| N | 1.25 | ||
| R2 | 0.9944 | ||
| χ2 | 29.5 | ||
| Redlich–Peterson | KR (L/g) | 1.69 | |
| Β | 0.53 | ||
| aR (Lmol) | 0.074 | ||
| R2 | 0.9951 | ||
| χ2 | 2.2826 | ||
| Temkin | KT (L/g) | 0.10 | |
| B | 53.92 | ||
| b (J/mol) | 45.95 | ||
| R2 | 0.8498 | ||
| χ2 | 39.3581 | ||
| Dubinin–Radushkevich | qm (mg g−1) × 10−6 | 0.49 | |
| E (kJ/mol) | 0.64 | ||
| β × 106 | 1.23 | ||
| R2 | 0.7726 | ||
| χ2 | 259.6563 |
| C0 (mg/L) | HEA/10IA |
|---|---|
| 10.00 | 0.9483 |
| 53.13 | 0.7810 |
| 98.72 | 0.6539 |
| 202.24 | 0.4832 |
| 307.53 | 0.3687 |
| 427.11 | 0.2932 |
| 518.06 | 0.2578 |
| Hydrogel | qm (mg/g) | Reference |
|---|---|---|
| HEA/MALA | 58.2 | [37] |
| Modified silica | 12.4 | [38] |
| C-g-AA | 380.1 | [39] |
| Chitosan(chitin)/cellulose composite biosorbents | 13.2 | [40] |
| Magnetic p(AMPS) hydrogels | 114.9 | [41] |
| HEA/10IA | 225.4 | Present study |
| qe,exp (mg/g) | Pseudo-First Order Model | Pseudo-Second Order Model | |||||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe,cal (mg/g) | R2 | k2 (g mg−1 h−1) | qe,cal (mg/g) | R2 | ||
| HEA/10IA | 7.81 ± 0.24 | 0.123 | 5.663 | 0.9299 | 1.922 | 8.753 | 0.9904 |
| Temperature | qe,exp | ∆Go | ∆Ho | ∆So | |
|---|---|---|---|---|---|
| (°C) | (mg/g) | (kJ/mol) | (kJ/mol) | (J/mol/K) | |
| Ni2+ | 10 | 7.96 ± 0.19 | 0.208 | −4.084 | −15.13 |
| 25 | 7.81 ± 0.24 | 0.449 | |||
| 50 | 7.44 ± 0.16 | 0.814 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antić, K.; Onjia, A.; Vasiljević-Radović, D.; Veličković, Z.; Tomić, S.L. Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology. Gels 2021, 7, 225. https://doi.org/10.3390/gels7040225
Antić K, Onjia A, Vasiljević-Radović D, Veličković Z, Tomić SL. Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology. Gels. 2021; 7(4):225. https://doi.org/10.3390/gels7040225
Chicago/Turabian StyleAntić, Katarina, Antonije Onjia, Dana Vasiljević-Radović, Zlate Veličković, and Simonida Lj. Tomić. 2021. "Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology" Gels 7, no. 4: 225. https://doi.org/10.3390/gels7040225







