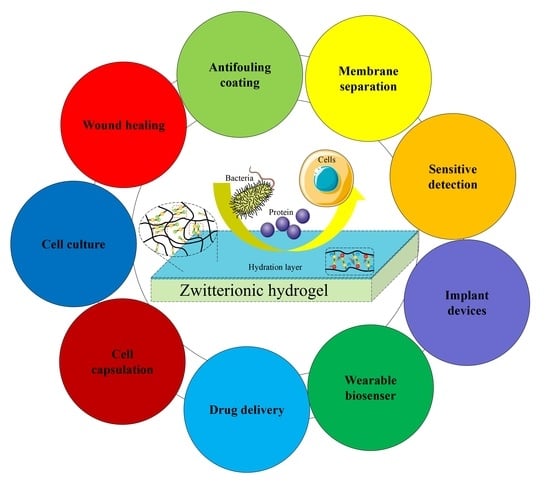
Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application
Abstract
:1. Introduction
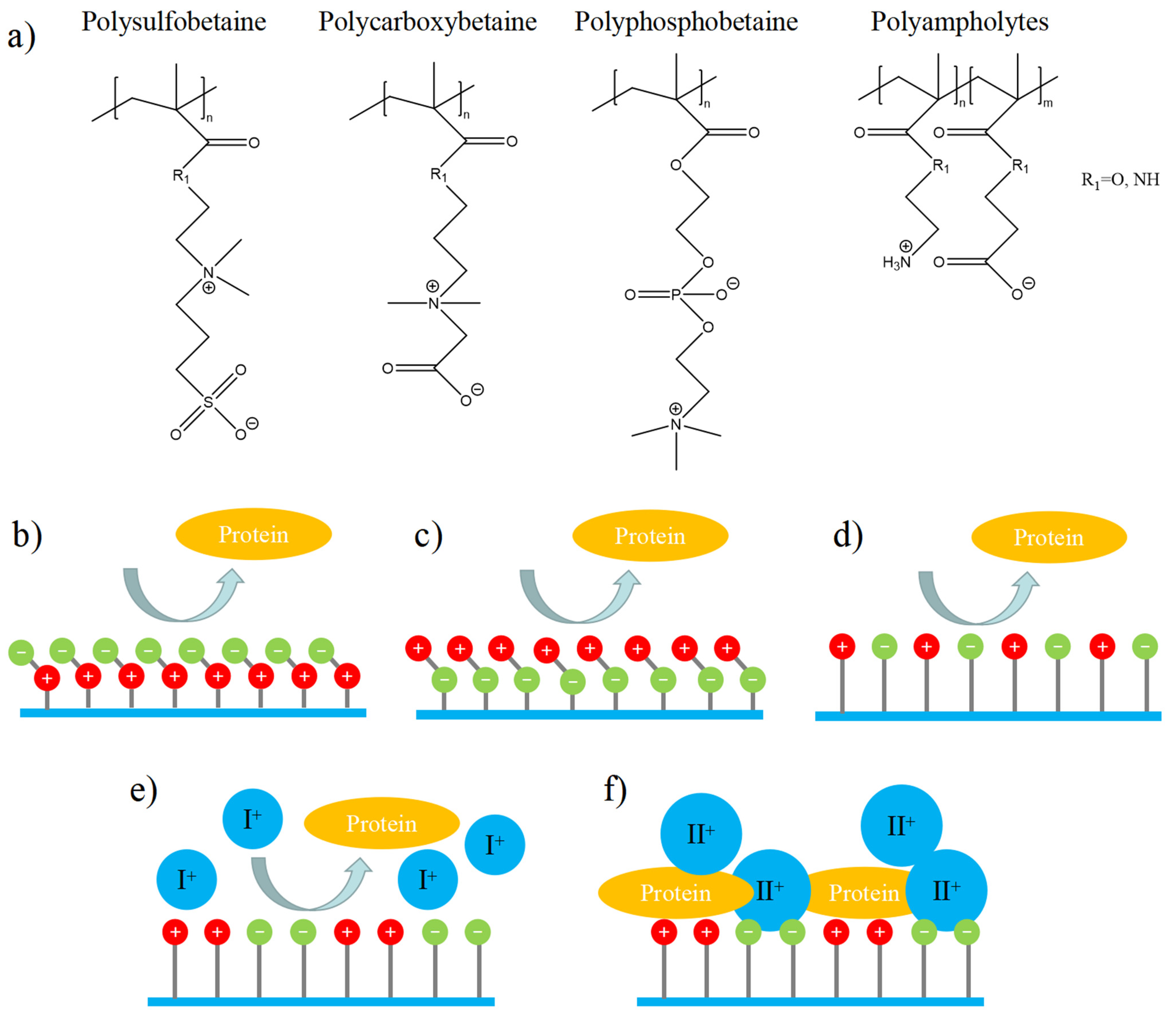
2. Structure of Zwitterionic Materials
3. Zwitterionic Hydrogels
3.1. Preparation of Zwitterionic Hydrogels
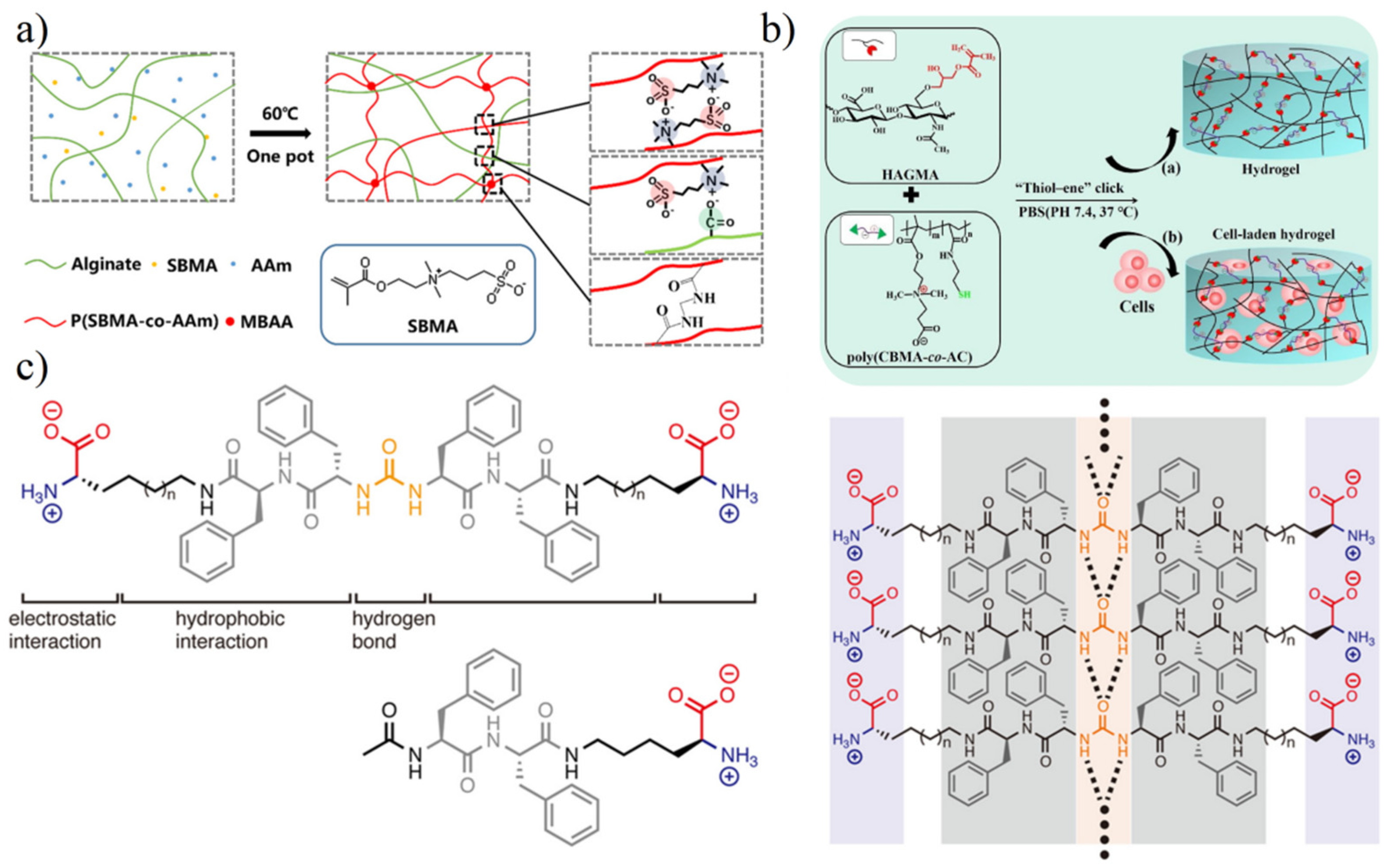
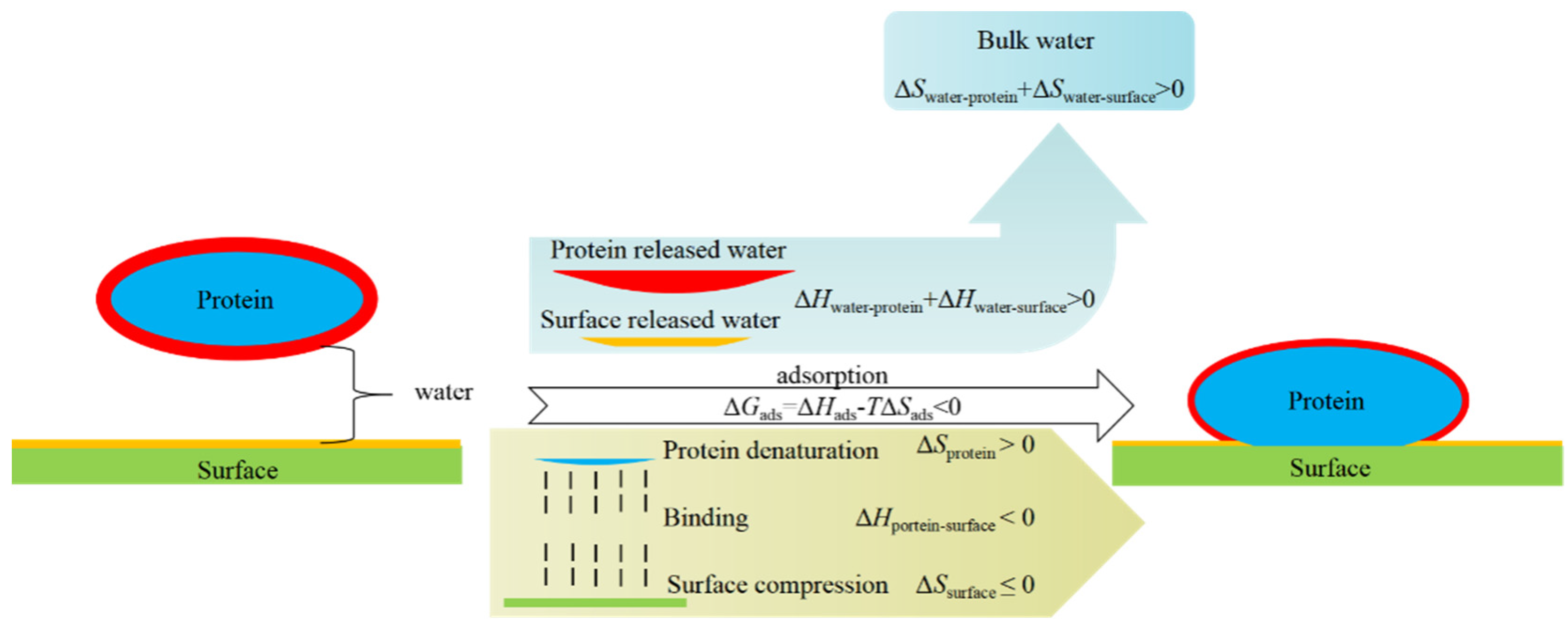
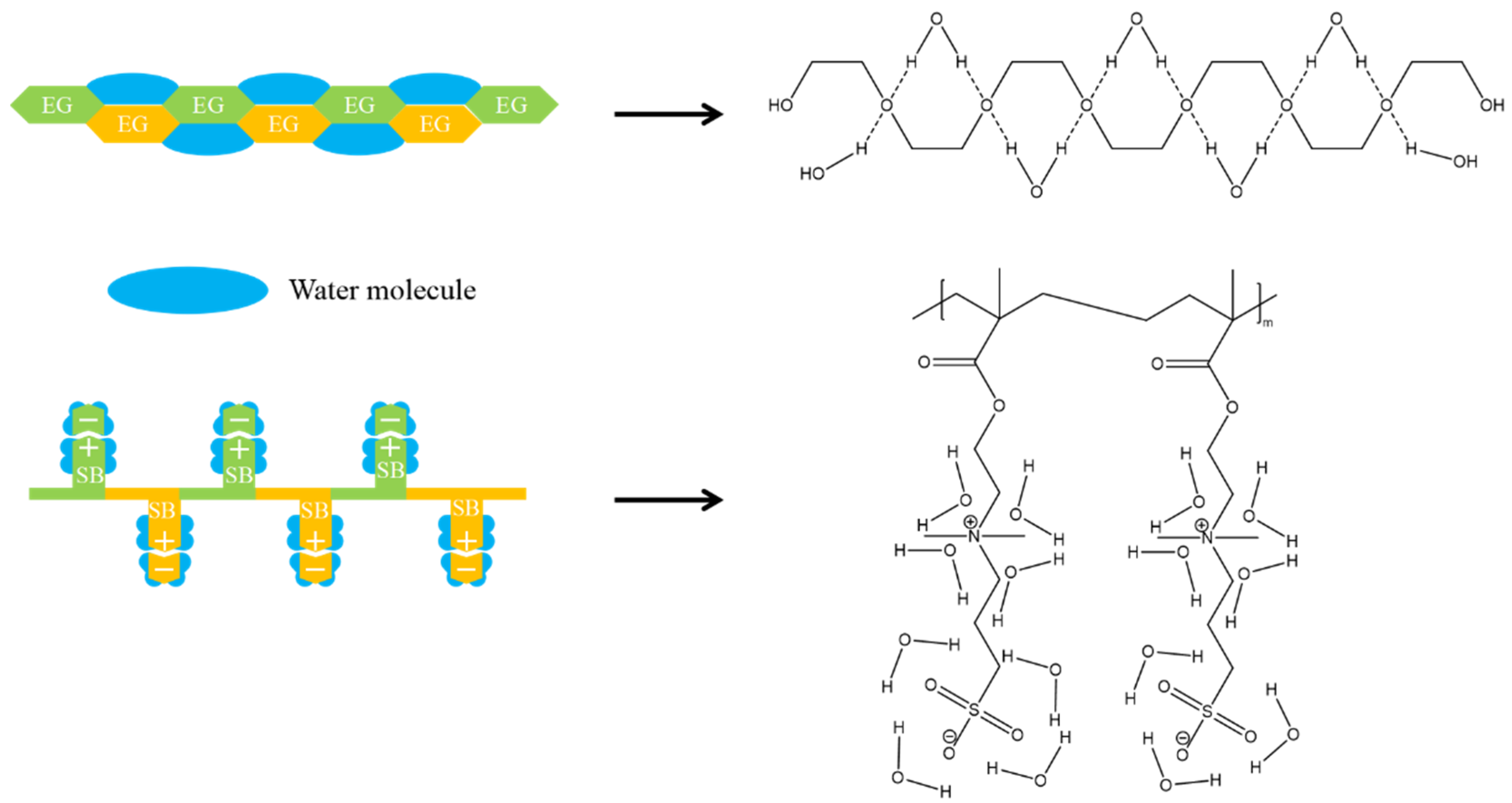
3.2. Properties of Zwitterionic Hydrogels
3.2.1. Swelling Behavior and Mechanical Properties

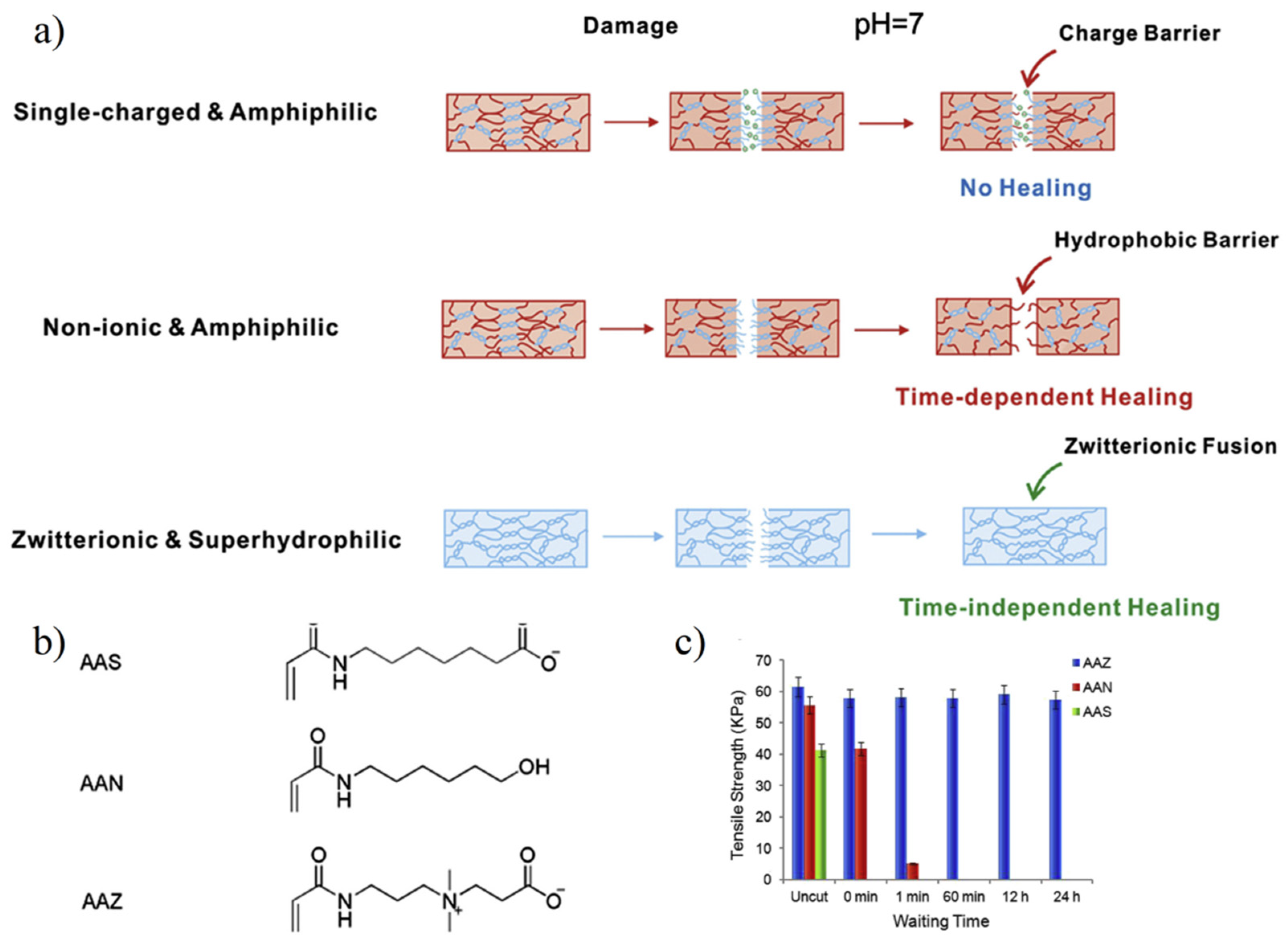
3.2.2. Self-Healing
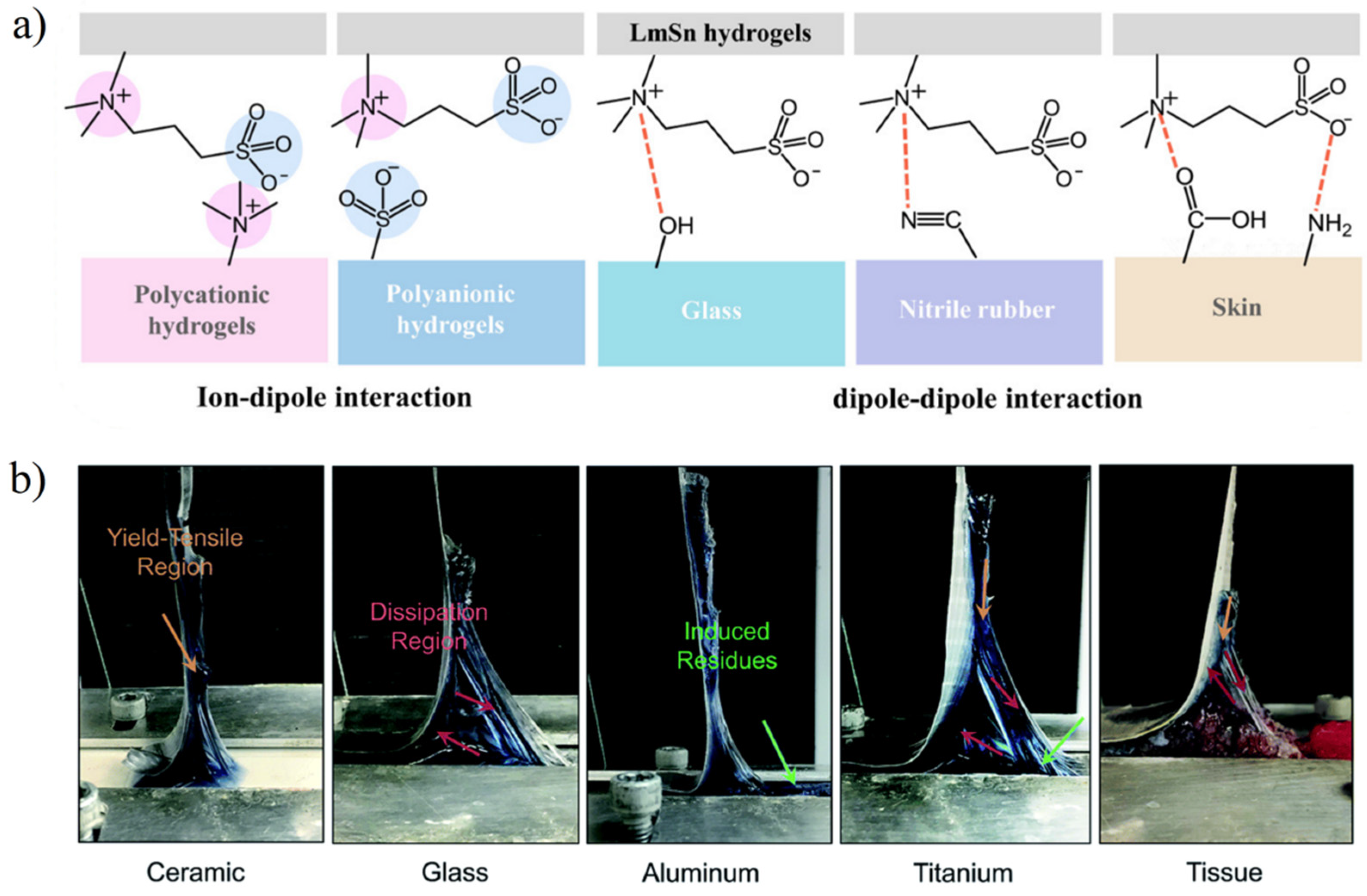
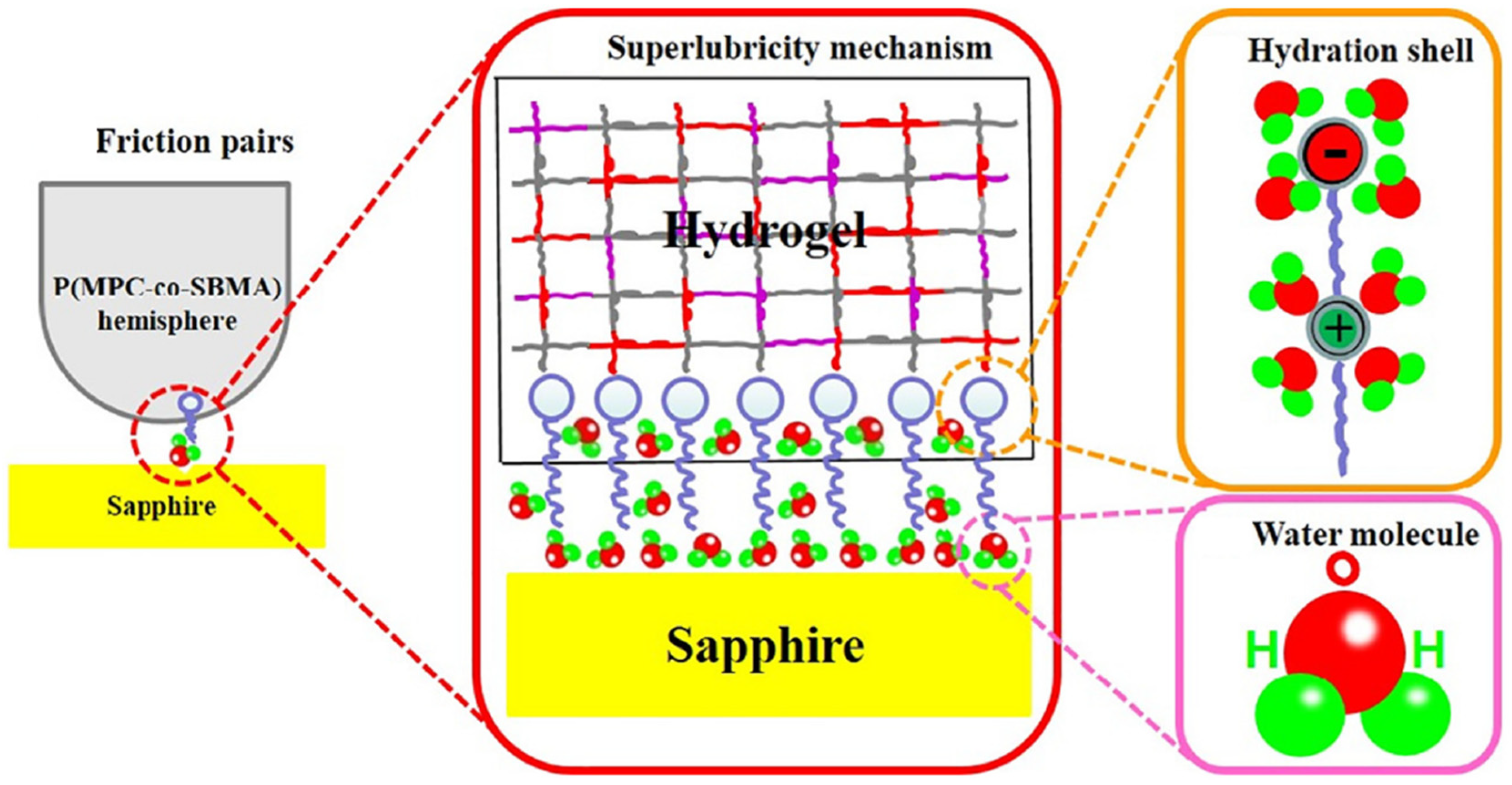
3.2.3. Adhesion and Lubricity
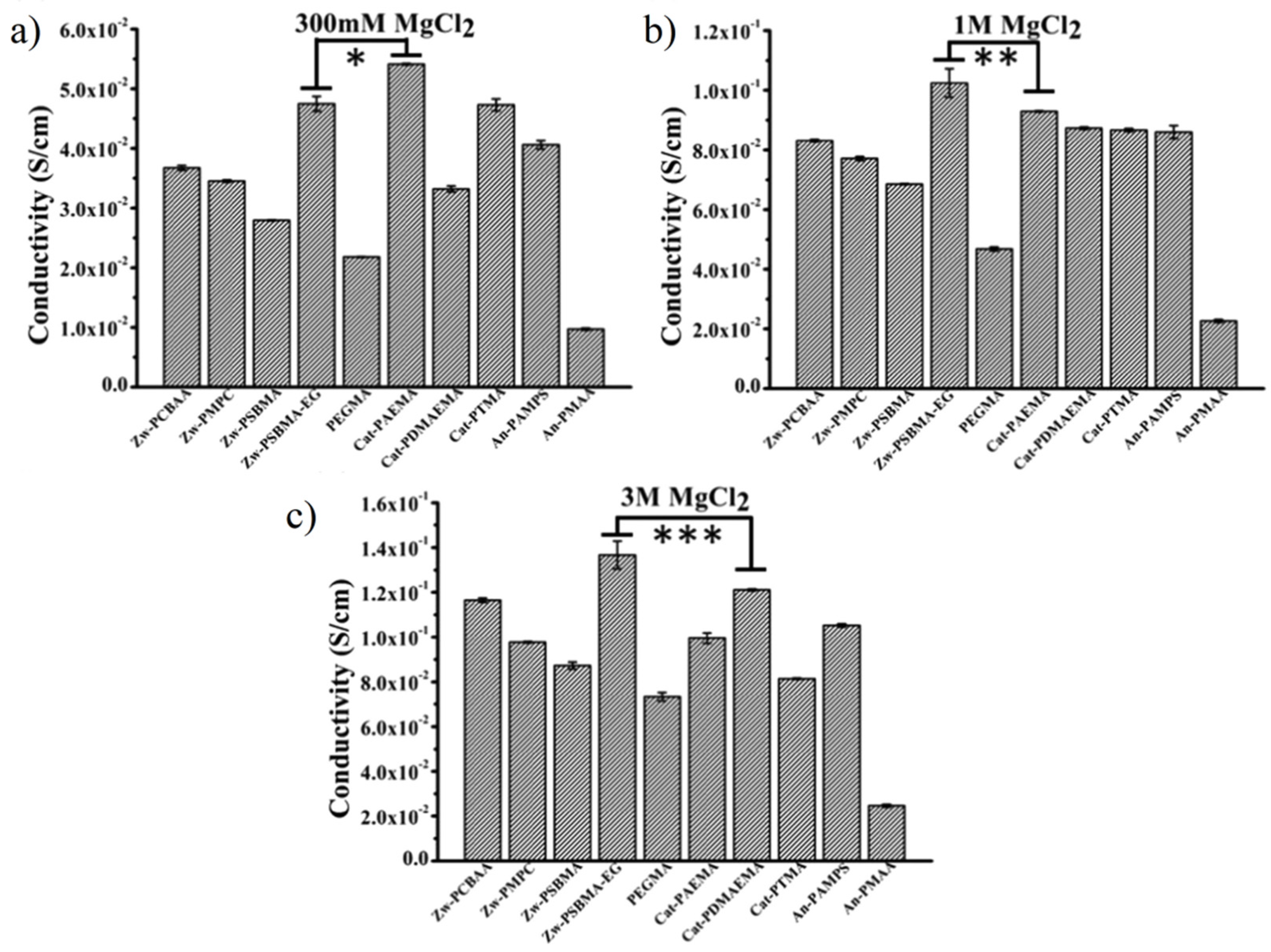
3.2.4. High Ionic Conductivity
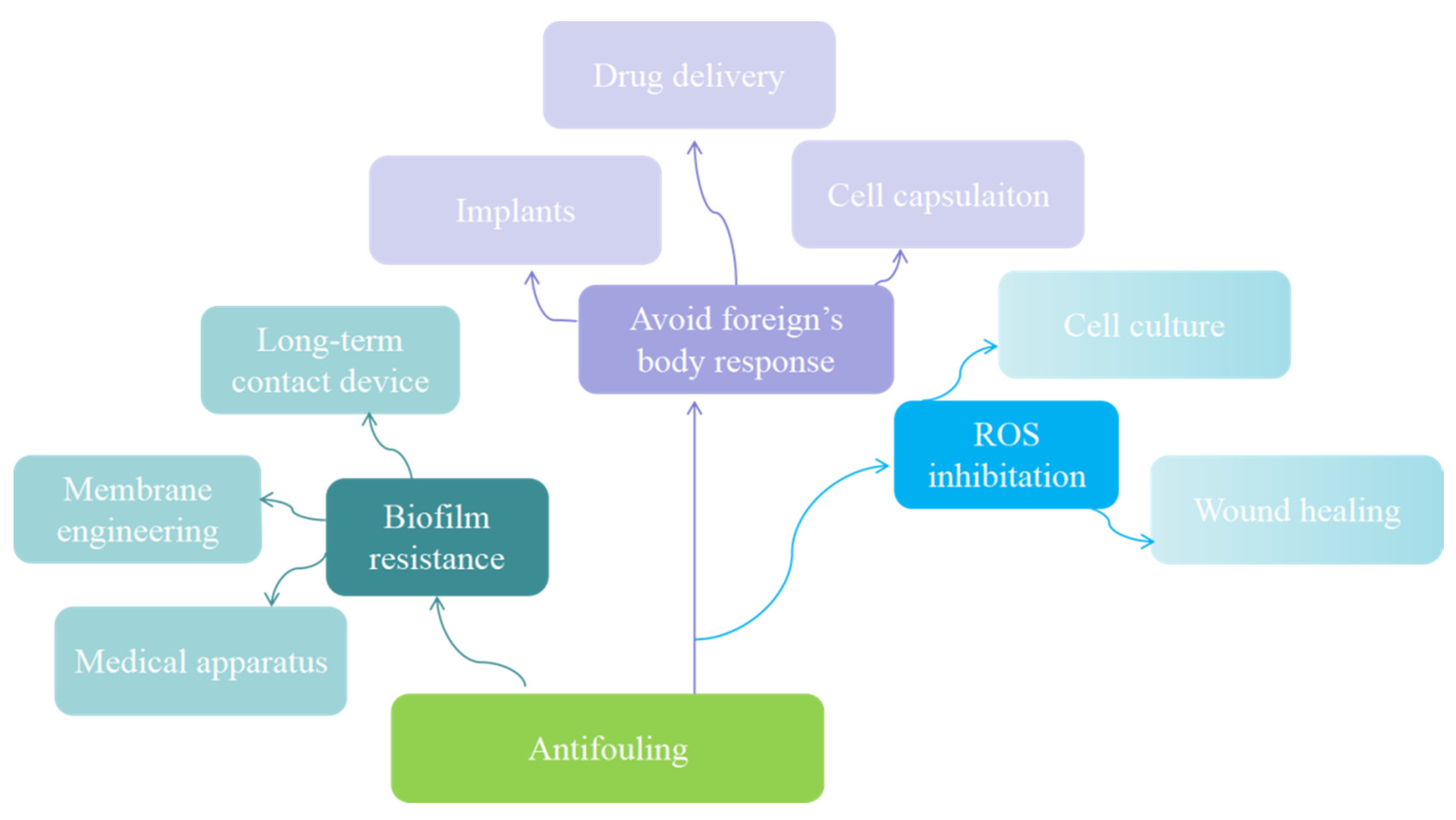
4. Biomedical Applications of Zwitterionic Hydrogels
4.1. Antifouling Coatings for Reusable Medical Apparatus in Physiological Environment
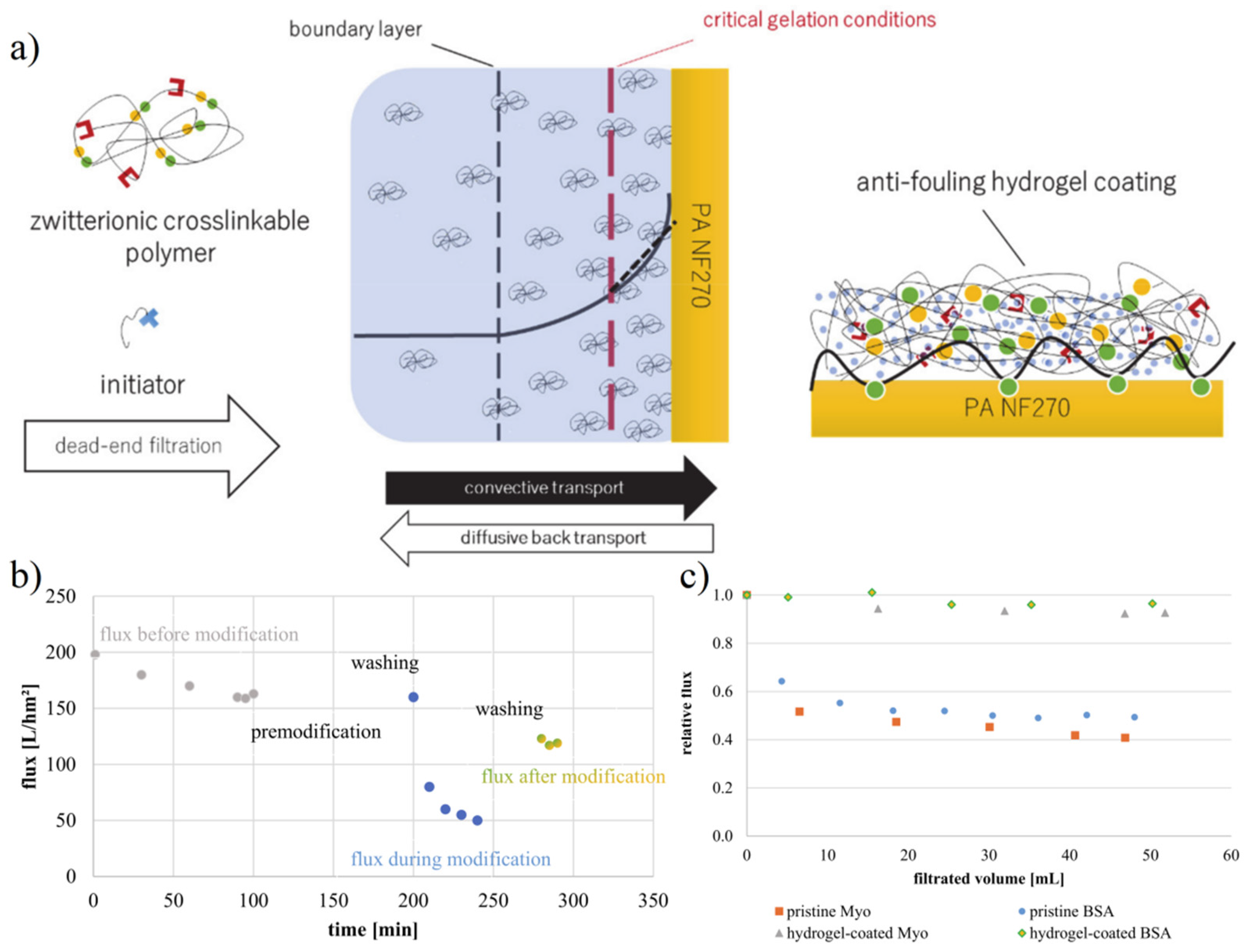
4.2. Membrane Separation Technology
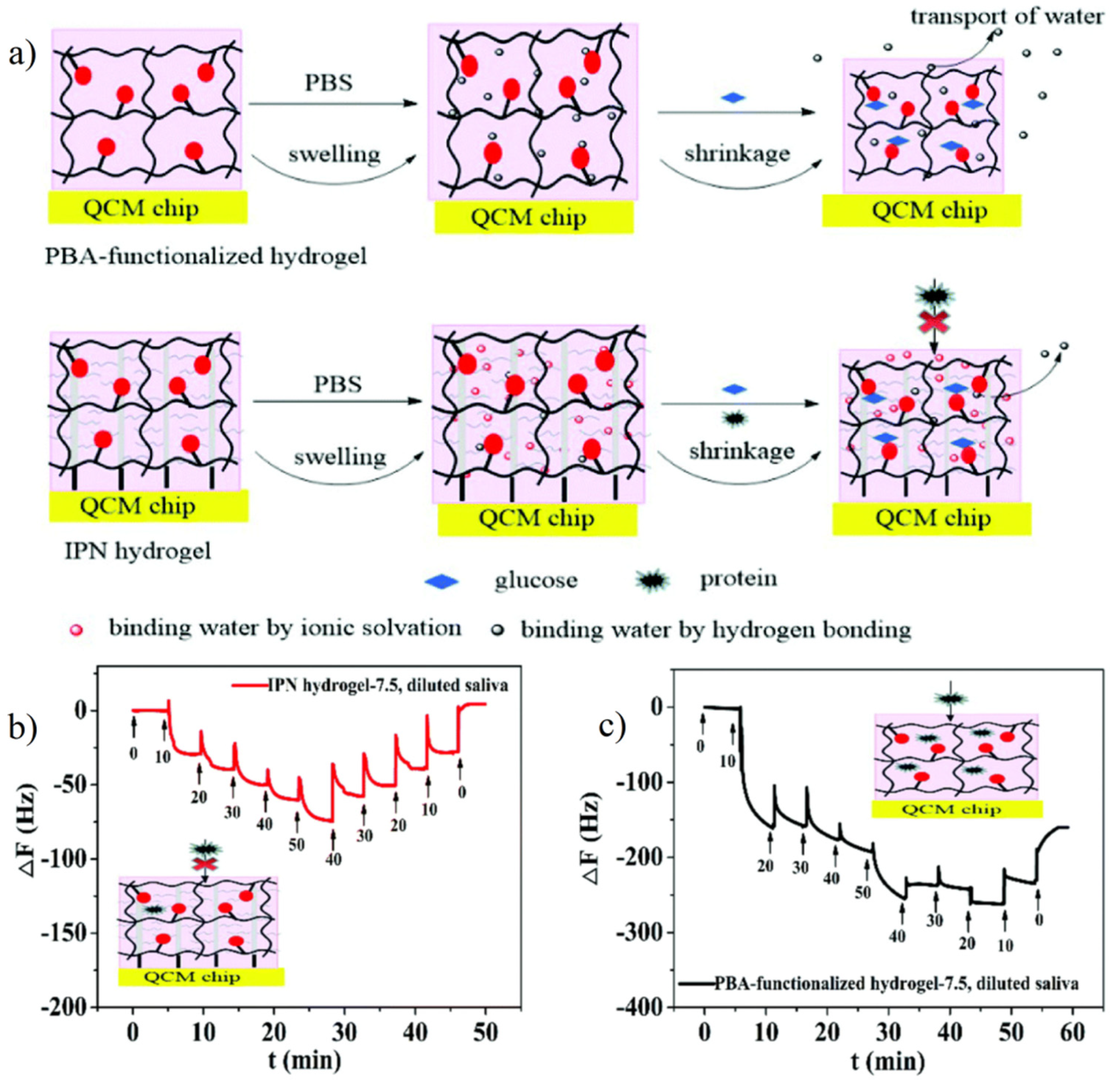
4.3. Antifouling Probe for Sensitive Detection
4.4. Implants
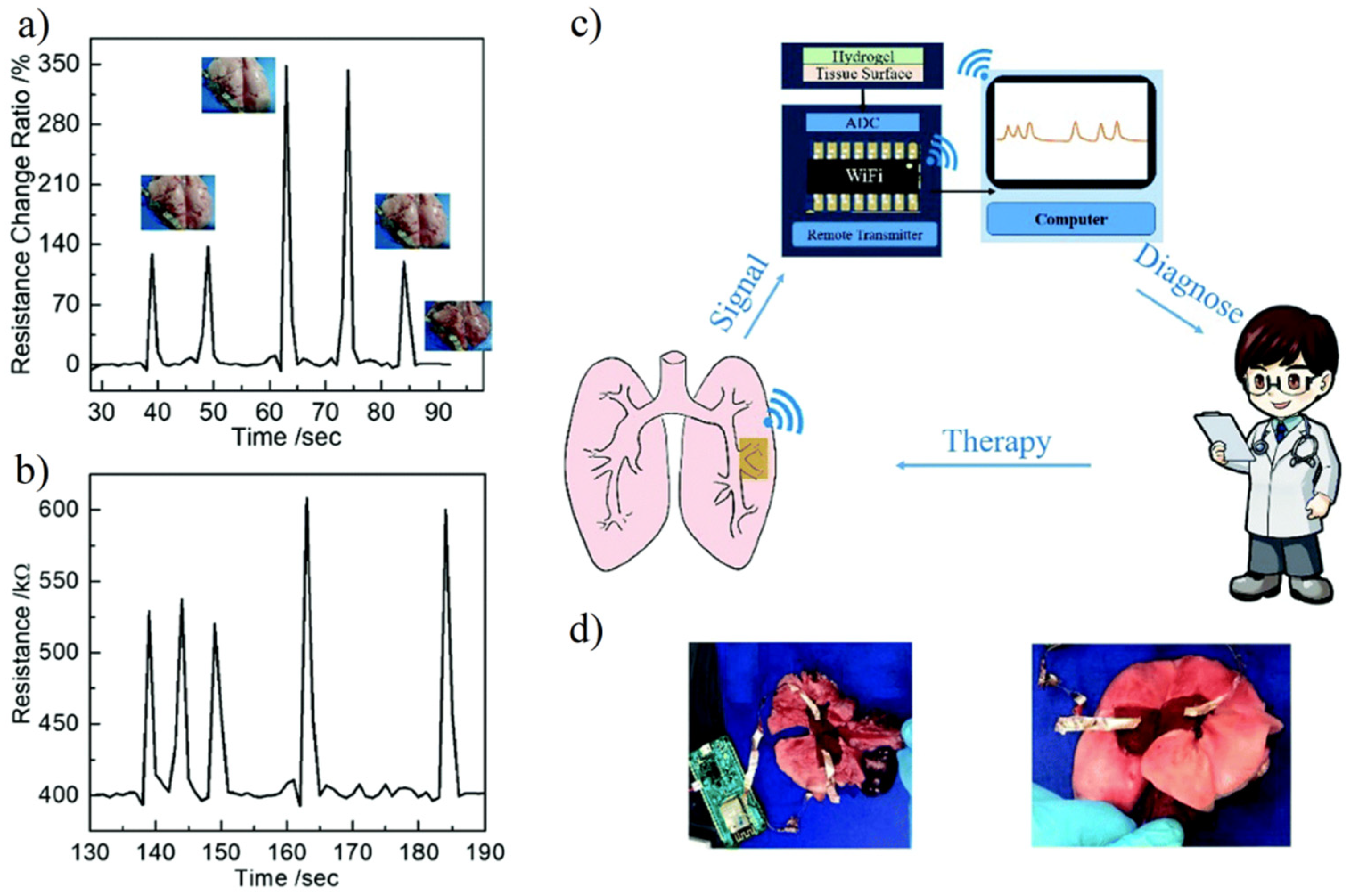
4.5. Biosensor
4.6. Drug Delivery
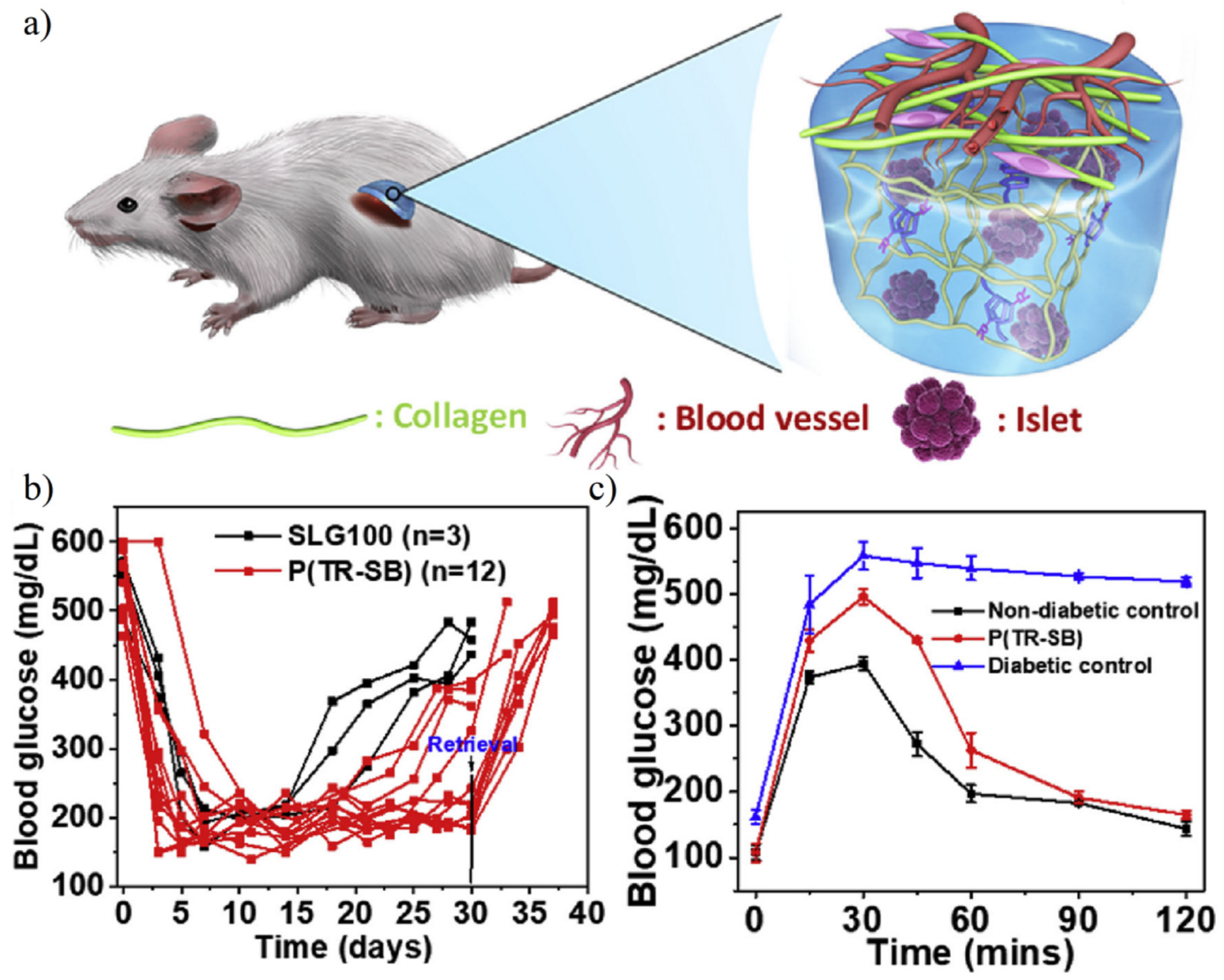
4.7. Cell Capsulation
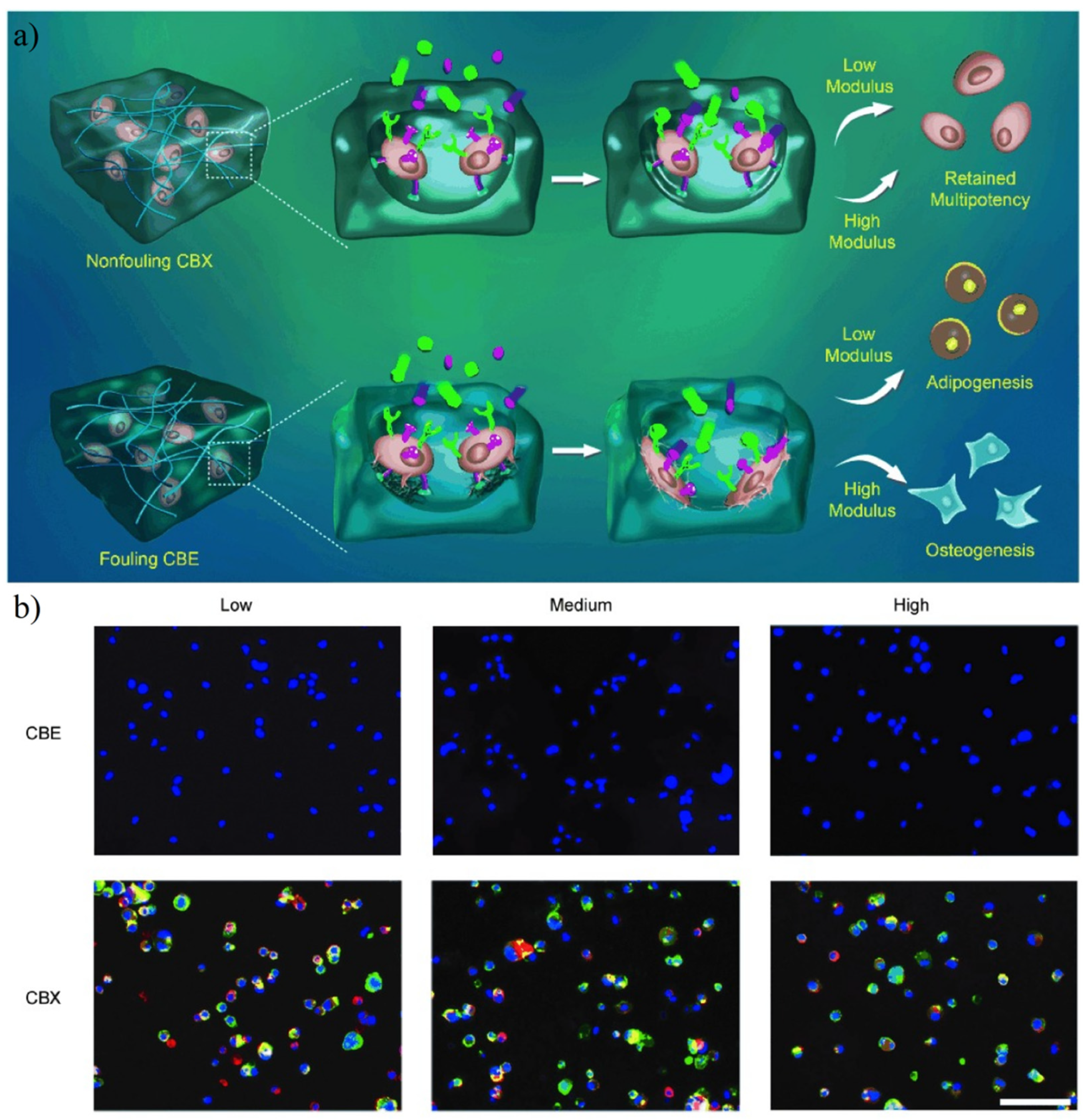
4.8. Cell Culture
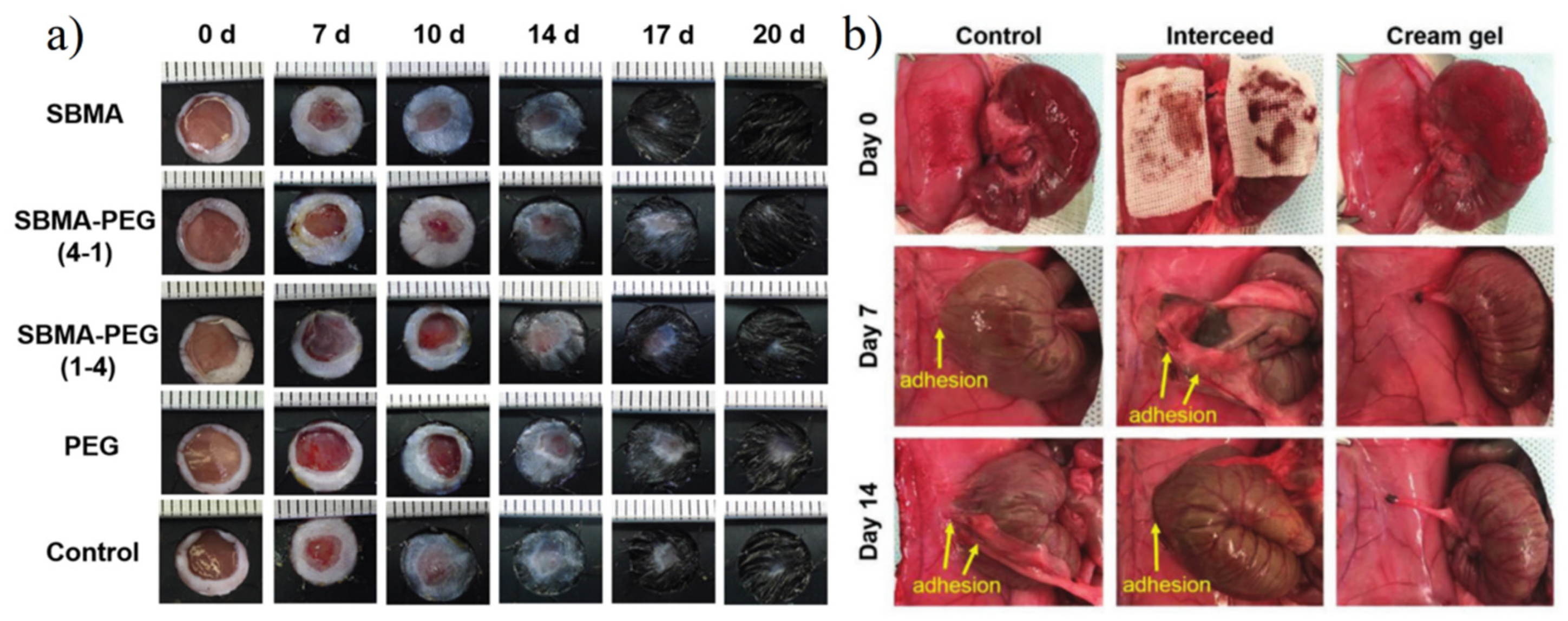
4.9. Wound Healing
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Chan, H.M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Dickinson, G.H.; Teo, S.L.-M.; Rittschof, D. Biomimetic Anchors for Antifouling and Antibacterial Polymer Brushes on Stainless Steel. Langmuir 2011, 27, 7065–7076. [Google Scholar] [CrossRef]
- Rakovsky, A.; Marbach, D.; Lotan, N.; Lanir, Y. Poly(ethylene glycol)-based hydrogels as cartilage substitutes: Synthesis and mechanical characteristics. J. Appl. Polym. Sci. 2009, 112, 390–401. [Google Scholar] [CrossRef]
- Rosso, M.; Nguyen, A.T.; de Jong, E.; Baggerman, J.; Paulusse, J.M.J.; Giesbers, M.; Fokkink, R.G.; Norde, W.; Schroën, K.; Rijn, C.J.M.v.; et al. Protein-Repellent Silicon Nitride Surfaces: UV-Induced Formation of Oligoethylene Oxide Monolayers. Acs. Appl. Mater. Inter. 2011, 3, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Hsieh, P.S.; Dai, L.G.; Huang, C.J. Complete zwitterionic double network hydrogels with great toughness and resistance against foreign body reaction and thrombus. J. Mater. Chem. B 2020, 8, 7390–7402. [Google Scholar] [CrossRef]
- Zhang, Z.; Chao, T.; Chen, S.; Jiang, S. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef]
- Chen, S.F.; Li, L.Y.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhao, C.; Lin, W.; Hu, R.; Wang, Q.; Chen, H.; Li, L.; Chen, S.; Zheng, J. Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Lin, W.; Chen, S. Investigation of the interaction between poly(ethylene glycol) and protein molecules using low field nuclear magnetic resonance. Acta Biomater. 2013, 9, 6414–6420. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.-M.; Erathodiyil, N.; Wu, H.; Lu, H.; Zheng, Y.; Ying, J.Y. Calcium cross-linked zwitterionic hydrogels as antifouling materials. Mater. Today Commun 2020, 23, 100950. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Ladd, J.; Zhang, Z.; Chen, S.; Hower, J.C.; Jiang, S. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 2008, 9, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Zhuang, B.L.; Yu, J. Functional Zwitterionic Polymers on Surface: Structures and Applications. Chem.-Asian J. 2020, 15, 2060–2075. [Google Scholar] [CrossRef]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the hydration of nonfouling material poly(sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, S. Investigation of the hydration of nonfouling material poly(ethylene glycol) by low-field nuclear magnetic resonance. Langmuir 2012, 28, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.F.; Ma, G.L.; Wu, J.; Chen, S.F. Different in vitro and in vivo behaviors between Poly(carboxybetaine methacrylate) and poly(sulfobetaine methacrylate). Colloids Surfaces B 2016, 146, 888–894. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hower, J.; Chen, S.; Bernards, M.T.; Chang, Y.; Jiang, S. Molecular Simulation Studies of Protein Interactions with Zwitterionic Phosphorylcholine Self-Assembled Monolayers in the Presence of Water. Langmuir 2008, 24, 10358–10364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, J.L.; Sun, Y.L.; Wang, Y.; Liang, J.; Luo, J.; Cui, W.G.; Deng, L.F.; Xu, X.Y.; Wang, B.; et al. Gelatin-based composite hydrogels with biomimetic lubrication and sustained drug release. Friction 2021. [Google Scholar] [CrossRef]
- Zhou, L.Q.; Lei, D.Q.; Wang, Q.; Luo, X.L.; Chen, Y.W. Biocompatible Polyphosphorylcholine Hydrogels with Inherent Antibacterial and Nonfouling Behavior Effectively Promote Skin Wound Healing. ACS Appl. Bio. Mater. 2020, 3, 5357–5366. [Google Scholar] [CrossRef]
- Wu, X.X.; He, Y.; Lai, G.C.; Zeng, R.; Tu, M. Biomimetic phosphorylcholine-modified bacterial cellulose membranes with cell fouling resistance. Cellulose 2020, 27, 10061–10075. [Google Scholar] [CrossRef]
- Vales, T.P.; Jee, J.P.; Lee, W.Y.; Cho, S.; Lee, G.M.; Kim, H.J.; Kim, J.S. Development of Poly(2-Methacryloyloxyethyl Phosphorylcholine)-Functionalized Hydrogels for Reducing Protein and Bacterial Adsorption. Materials 2020, 13, 943. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.S.; Guo, H.S.; Yang, J.; Zhao, W.Q.; Zhu, Y.N.; Sui, X.J.; Xu, T.; Zhang, J.M.; Zhang, L. MOF-Based Antibiofouling Hemoadsorbent for Highly Efficient Removal of Protein-Bound Bilirubin. Langmuir 2020, 36, 8753–8763. [Google Scholar] [CrossRef]
- Li, Q.H.; Feng, Z.J.; Song, H.J.; Zhang, J.H.; Dong, A.J.; Kong, D.L.; Wang, W.W.; Huang, P.S. F-19 magnetic resonance imaging enabled real-time, non-invasive and precise localization and quantification of the degradation rate of hydrogel scaffolds in vivo. Biomater. Sci. 2020, 8, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- May, P.; Laghmari, S.; Ulbricht, M. Concentration Polarization Enabled Reactive Coating of Nanofiltration Membranes with Zwitterionic Hydrogel. Membranes 2021, 11, 187. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.L.; Li, M.L.; Fu, F.F.; Zhao, Y.; Yu, J. Zwitterionic Polymer-Grafted Superhydrophilic and Superoleophobic Silk Fabrics for Anti-Oil Applications. Macromol. Rapid Comm. 2020, 41, 2000162. [Google Scholar] [CrossRef]
- Potaufeux, J.E.; Odent, J.; Notta-Cuvier, D.; Lauro, F.; Raquez, J.M. A comprehensive review of the structures and properties of ionic polymeric materials. Polym. Chem. 2020, 11, 5914–5936. [Google Scholar] [CrossRef]
- Li, C.X.; Liu, C.J.; Li, M.L.; Xu, X.; Li, S.Z.; Qi, W.; Su, R.X.; Yu, J. Structures and Antifouling Properties of Self-Assembled Zwitterionic Peptide Monolayers: Effects of Peptide Charge Distributions and Divalent Cations. Biomacromolecules 2020, 21, 2087–2095. [Google Scholar] [CrossRef]
- Yu, Z.L.; Xiao, Z.C.; Shuai, X.T.; Tian, J.W. Local delivery of sunitinib and Ce6viaredox-responsive zwitterionic hydrogels effectively prevents osteosarcoma recurrence. J. Mater. Chem. B 2020, 8, 6418–6428. [Google Scholar] [CrossRef]
- Carr, L.R.; Zhou, Y.B.; Krause, J.E.; Xue, H.; Jiang, S.Y. Uniform zwitterionic polymer hydrogels with a nonfouling and functionalizable crosslinker using photopolymerization. Biomaterials 2011, 32, 6893–6899. [Google Scholar] [CrossRef]
- Cabanach, P.; Pena-Francesch, A.; Sheehan, D.; Bozuyuk, U.; Yasa, O.; Borros, S.; Sitti, M. Zwitterionic 3D-Printed Non-Immunogenic Stealth Microrobots. Adv. Mater. 2020, 32, 2003013. [Google Scholar] [CrossRef]
- Ma, G.L.; Lin, W.F.; Yuan, Z.F.; Wu, J.; Qian, H.F.; Xu, L.B.; Chen, S.F. Development of ionic strength/pH/enzyme triple-responsive zwitterionic hydrogel of the mixed L-glutamic acid and L-lysine polypeptide for site-specific drug delivery. J. Mater. Chem. B 2017, 5, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, L.; Lin, W.; Chen, S. Development of Nonfouling Zwitterionic Copolymerized Peptides Based on Glutamic Acid and Lysine Dimers for Adjustable Enzymatic Degradation. Langmuir 2021, 37, 5776–5782. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, S.Y.; Li, T.Y.; Zhang, L.Q.; Azhar, U.; Ma, J.C.; Zhai, C.C.; Zong, C.Y.; Zhang, S.X. Cytocompatible and non-fouling zwitterionic hyaluronic acid-based hydrogels using thiol-ene “click” chemistry for cell encapsulation. Carbohyd. Polym. 2020, 236, 116021. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, J.Q.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; O’Kelly, M.B.; Nourigat, C.; Jain, P.; Delrow, J.J.; et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Thordarson, P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mater. Chem. B 2020, 8, 863–877. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym. Chem. 2012, 3, 18–33. [Google Scholar] [CrossRef]
- Liyanage, W.; Nilsson, B.L. Substituent Effects on the Self-Assembly/Coassembly and Hydrogelation of Phenylalanine Derivatives. Langmuir 2016, 32, 787–799. [Google Scholar] [CrossRef]
- Abraham, B.L.; Liyanage, W.; Nilsson, B.L. Strategy to Identify Improved N-Terminal Modifications for Supramolecular Phenylalanine-Derived Hydrogelators. Langmuir 2019, 35, 14939–14948. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Tanaka, K.; Chia, J.Y.; Mihara, H. Short self-assembling peptides with a urea bond: A new type of supramolecular peptide hydrogel materials. Peptide Sci. 2021, 113, e24214. [Google Scholar] [CrossRef]
- Carlini, A.S.; Choi, W.; McCallum, N.C.; Gianneschi, N.C. pH-Responsive Charge-Conversion Progelator Peptides. Adv. Funct. Mater. 2021, 31, 2007733. [Google Scholar] [CrossRef]
- Jin, X.Q.; Jiang, H.H.; Qiao, F.H.; Huang, W.P.; Bao, X.J.; Wang, Z.K.; Hu, Q.L. Fabrication ofalginate-P(SBMA-co-AAm) hydrogels with ultrastretchability, strain sensitivity, self-adhesiveness, biocompatibility, and self-cleaning function for strain sensors. J. Appl. Polym. Sci. 2021, 138, 49697. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Xiao, S.; Hu, R.; Bhaway, S.M.; Vogt, B.D.; Zhang, M.; Chen, Q.; Ma, J.; Chang, Y.; et al. Salt-responsive polyzwitterionic materials for surface regeneration between switchable fouling and antifouling properties. Acta Biomater. 2016, 40, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Fredrickson, G.H. Theory of polyzwitterion conformations. J. Chem. Phys. 2009, 131, 104901. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.Y.; Tsao, C.; Hung, H.C.; Yao, F.L.; Tang, C.J.; Niu, L.Q.; Ma, J.R.; MacArthur, J.; Sinclair, A.; Wu, K.; et al. High-strength and fibrous capsule-resistant zwitterionic elastomers. Sci. Adv. 2021, 7, eabc5442. [Google Scholar] [CrossRef]
- He, H.; Xuan, X.; Zhang, C.; Song, Y.; Chen, S.; Gong, X.; Ren, B.; Zheng, J.; Wu, J. Simple Thermal Pretreatment Strategy to Tune Mechanical and Antifouling Properties of Zwitterionic Hydrogels. Langmuir 2019, 35, 1828–1836. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.F.; Zhao, C.S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155. [Google Scholar] [CrossRef]
- Liu, S.H.; Ma, J.; Xu, L.B.; Lin, W.F.; Xue, W.L.; Huang, M.; Chen, S.F. An electrospun polyurethane scaffold-reinforced zwitterionic hydrogel as a biocompatible device. J. Mater. Chem. B 2020, 8, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.D.; Chen, L.Q.; Qian, S.X.; Mou, X.Z.; Feng, J. Highly antifouling, biocompatible and tough double network hydrogel based on carboxybetaine-type zwitterionic polymer and alginate. Carbohyd. Polym. 2021, 257, 117627. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Liu, D.; Yuan, Z.; Hung, H.C.; Luozhong, S.; Gu, W.; Wu, K.; Jiang, S. High-Strength and Nonfouling Zwitterionic Triple-Network Hydrogel in Saline Environments. Adv. Mater. 2021, 33, e2102479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, B.P.; Zhang, Y.X.; Liu, Y.L.; Chen, H.; Xiao, S.W.; Chang, Y.; Yang, J.T.; Zheng, J. Micro- and macroscopically structured zwitterionic polymers with ultralow fouling property. J. Colloid. Interf. Sci. 2020, 578, 242–253. [Google Scholar] [CrossRef]
- Xiao, S.W.; He, X.M.; Zhao, Z.Q.; Huang, G.B.; Yan, Z.Z.; He, Z.C.; Zhao, Z.P.; Chen, F.; Yang, J.T. Strong anti-polyelectrolyte zwitterionic hydrogels with superior self-recovery, tunable surface friction, conductivity, and antifreezing properties. Eur. Polym. J. 2021, 148, 110350. [Google Scholar] [CrossRef]
- Xiao, S.W.; He, X.M.; Qian, J.; Wu, X.H.; Huang, G.B.; Jiang, H.J.; He, Z.C.; Yang, J.T. Natural Lipid Inspired Hydrogel-Organogel Bilayer Actuator with a Tough Interface and Multiresponsive, Rapid, and Reversible Behaviors. Ind. Eng. Chem. Res. 2020, 59, 7646–7658. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, Y.; Ren, Y.Y.; Jin, G.Q.; Zhang, C.C.; Chen, W.; Yan, F. Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 2020, 7, 919–927. [Google Scholar] [CrossRef]
- Liu, Q.S.; Chiu, A.; Wang, L.H.; An, D.; Li, W.C.; Chen, E.Y.; Zhang, Y.; Pardo, Y.; McDonough, S.P.; Liu, L.Y.; et al. Developing mechanically robust, triazole-zwitterionic hydrogels to mitigate foreign body response (FBR) for islet encapsulation. Biomaterials 2020, 230, 119640. [Google Scholar] [CrossRef]
- Yang, J.B.; Xu, Z.; Wang, J.J.; Gai, L.G.; Ji, X.X.; Jiang, H.H.; Liu, L.B. Antifreezing Zwitterionic Hydrogel Electrolyte with High Conductivity of 12.6 mS cm−1 at −40 °C through Hydrated Lithium Ion Hopping Migration. Adv. Funct. Mater. 2021, 31, 2009438. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.J.; Zhang, Y.X.; Yang, F.Y.; Liu, Y.L.; Wang, X.Y.; Yang, J.T.; Gong, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Yang, J.B.; Du, Y.X.; Li, X.L.; Qiao, C.D.; Jiang, H.H.; Zheng, J.Y.; Lin, C.G.; Liu, L.B. Fatigue-Resistant, Notch-Insensitive Zwitterionic Polymer Hydrogels with High Self-Healing Ability. Chempluschem 2020, 85, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Lu, S.S.; Li, Q.S.; Ren, Y.W.; Ding, Y.Q.; Wu, H.L.; He, X.H.; Shang, Y.D. High strength zwitterionic nano-micelle hydrogels with superior self-healing, adhesive and ion conductive properties. Eur. Polym. J. 2020, 133, 109761. [Google Scholar] [CrossRef]
- Zheng, A.B.; Wu, D.; Fan, M.; Wang, H.; Liao, Y.G.; Wang, Q.; Yang, Y.J. Injectable zwitterionic thermosensitive hydrogels with low-protein adsorption and combined effect of photothermal-chemotherapy. J. Mater. Chem. B 2020, 8, 10637–10649. [Google Scholar] [CrossRef]
- Pei, X.J.; Zhang, H.; Zhou, Y.; Zhou, L.J.; Fu, J. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater. Horiz. 2020, 7, 1872–1882. [Google Scholar] [CrossRef]
- Sallstrom, N.; Goulas, A.; Martin, S.; Engstrom, D.S. The effect of print speed and material aging on the mechanical properties of a self-healing nanocomposite hydrogel. Addit. Manuf. 2020, 35, 101253. [Google Scholar] [CrossRef]
- Sallstrom, N.; Capel, A.; Lewis, M.P.; Engstrom, D.S.; Martin, S. 3D-printable zwitterionic nano-composite hydrogel system for biomedical applications. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.B.; Zhang, Y.H.; Chen, C.; Yu, F.; Tian, J.; Cai, H.B.; Jiang, X.Z.; Zhang, L.S.; Zhang, W.A. An Antifouling and Antimicrobial Zwitterionic Nanocomposite Hydrogel Dressing for Enhanced Wound Healing. ACS. Biomater. Sci. Eng. 2021, 7, 1621–1630. [Google Scholar] [CrossRef]
- Wang, Z.H.; Scheres, L.; Xia, H.S.; Zuilhof, H. Developments and Challenges in Self-Healing Antifouling Materials. Adv. Funct. Mater. 2020, 30, 1908098. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Liu, S.J.; Sun, F.; Sinclair, A.; Zhang, L.; Shao, Q.; Jiang, S.Y. Zwitterionic fusion in hydrogels and spontaneous and time-independent self-healing under physiological conditions. Biomaterials 2014, 35, 3926–3933. [Google Scholar] [CrossRef]
- Sinclair, A.; O’Kelly, M.B.; Bai, T.; Hung, H.C.; Jain, P.; Jiang, S. Self-Healing Zwitterionic Microgels as a Versatile Platform for Malleable Cell Constructs and Injectable Therapies. Adv. Mater. 2018, 30, e1803087. [Google Scholar] [CrossRef]
- Lin, Y.L.; Zeng, Z.; Li, Y.H.; Sun, S.; Liu, X.T.; He, D.L.; Li, G.J. Self-healing zwitterionic sulfobetaine nanocomposite hydrogels with good mechanical properties. RSC Adv. 2019, 9, 31806–31811. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Mi, L.; Han, X.; Bai, T.; Liu, S.; Li, Y.; Jiang, S. Differences in Cationic and Anionic Charge Densities Dictate Zwitterionic Associations and Stimuli Responses. J. Phys. Chem. B 2014, 118, 6956–6962. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, L.F.; Wu, C.S.; Pei, X.J.; Cong, Y.; Zhang, R.; Fu, J. Antibacterial Zwitterionic Polyelectrolyte Hydrogel Adhesives with Adhesion Strength Mediated by Electrostatic Mismatch. ACS Appl. Mater. Inter. 2020, 12, 46816–46826. [Google Scholar] [CrossRef]
- Wang, L.; Gao, G.; Zhou, Y.; Xu, T.; Chen, J.; Wang, R.; Zhang, R.; Fu, J. Tough, Adhesive, Self-Healable, and Transparent Ionically Conductive Zwitterionic Nanocomposite Hydrogels as Skin Strain Sensors. ACS Appl. Mater. Inter. 2019, 11, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Vegesna, G.K.; Meng, H.; Winter, A.; Lee, B.P. Nitro-Group Functionalization of Dopamine and its Contribution to the Viscoelastic Properties of Catechol-Containing Nanocomposite Hydrogels. Macromol. Chem. Phys. 2015, 216, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Mrlik, M.; Spirek, M.; Al-Khori, J.; Ahmad, A.A.; Mosnacek, J.; AlMaadeed, M.A.; Kasak, P. Mussel-mimicking sulfobetaine-based copolymer with metal tunable gelation, self-healing and antibacterial capability. Arab. J. Chem. 2020, 13, 193–204. [Google Scholar] [CrossRef]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at Physiological Pressures by Polyzwitterionic Brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef] [Green Version]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Wang, Z.N.; Li, J.J.; Liu, Y.H.; Luo, J.B. Macroscale superlubricity achieved between zwitterionic copolymer hydrogel and sapphire in water. Mater. Des. 2020, 188, 108441. [Google Scholar] [CrossRef]
- Wang, Z.N.; Li, J.J.; Liu, Y.H.; Luo, J.B. Synthesis and characterizations of zwitterionic copolymer hydrogels with excellent lubrication behavior. Tribol. Int. 2020, 143, 106026. [Google Scholar] [CrossRef]
- Tiyapiboonchaiya, C.; Pringle, J.M.; Sun, J.; Byrne, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. The zwitterion effect in high-conductivity polyelectrolyte materials. Nat. Mater. 2004, 3, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Wu, H.Y.; Hu, Y.; Young, M.; Wang, H.F.; Lynch, D.; Xu, F.J.; Cong, H.B.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. ACS Appl. Mater. Inter. 2018, 10, 5845–5852. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, H.; Yin, Q.; Wu, J.; Chen, P.; Zhang, G.; Liu, G.; Wu, C.; Xie, Y. A zwitterionic gel electrolyte for efficient solid-state supercapacitors. Nat. Commun. 2016, 7, 11782. [Google Scholar] [CrossRef] [Green Version]
- Kardela, J.H.; Millichamp, I.S.; Ferguson, J.; Parry, A.L.; Reynolds, K.J.; Aldred, N.; Clare, A.S. Nonfreezable Water and Polymer Swelling Control the Marine Antifouling Performance of Polymers with Limited Hydrophilic Content. ACS Appl. Mater. Interfaces 2019, 11, 29477–29489. [Google Scholar] [CrossRef]
- Horbund, H.M.; Freiberger, A. Slime films and their role in marine fouling: A review. Ocean Eng. 1970, 1, 631–634. [Google Scholar] [CrossRef]
- Whalan, S.; Webster, N.S. Sponge larval settlement cues: The role of microbial biofilms in a warming ocean. Sci. Rep. 2014, 4, 4072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchetti De Gregoris, T.; Khandeparker, L.; Anil, A.C.; Mesbahi, E.; Burgess, J.G.; Clare, A.S. Characterisation of the bacteria associated with barnacle, Balanus amphitrite, shell and their role in gregarious settlement of cypris larvae. J. Exp. Mar. Biol. Ecol. 2012, 413, 7–12. [Google Scholar] [CrossRef]
- Zhao, C.; Yuan, X.; Bai, S.; Sun, P.; Zhao, Y.; Zhu, K.; Ren, L.; Li, X. Antifogging and antibacterial properties of amphiphilic coatings based on zwitterionic copolymers. Sci. China Technol. Sci. 2020, 64, 817–826. [Google Scholar] [CrossRef]
- Koc, J.; Schonemann, E.; Arnuthalingam, A.; Clarke, J.; Finlay, J.A.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A. Low-Fouling Thin Hydrogel Coatings Made of Photo-Cross-Linked Polyzwitterions. Langmuir 2019, 35, 1552–1562. [Google Scholar] [CrossRef]
- Jensen, M.J.; Peel, A.; Horne, R.; Chamberlain, J.; Xu, L.J.; Hansen, M.R.; Guymon, C.A. Antifouling and Mechanical Properties of Photografted Zwitterionic Hydrogel Thin-Film Coatings Depend on the Cross-Link Density. ACS Biomater. Sci. Eng. 2021, 7, 4494–4502. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Chen, S.; Horbett, T.A.; Ratner, B.D.; Jiang, S. Blood compatibility of surfaces with superlow protein adsorption. Biomaterials 2008, 29, 4285–4291. [Google Scholar] [CrossRef]
- Liu, G.Y.; Li, K.J.; Wang, H.B.; Ma, L.; Yu, L.; Nie, Y. Stable Fabrication of Zwitterionic Coating Based on Copper-Phenolic Networks on Contact Lens with Improved Surface Wettability and Broad-Spectrum Antimicrobial Activity. ACS Appl. Mater. Inter. 2020, 12, 16125–16136. [Google Scholar] [CrossRef]
- Wu, S.J.; Yuk, H.; Wu, J.; Nabzdyk, C.S.; Zhao, X. A Multifunctional Origami Patch for Minimally Invasive Tissue Sealing. Adv. Mater. 2021, 33, e2007667. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Mi, L.; Mendiola, J.; Ella-Menye, J.R.; Zhang, L.; Xue, H.; Jiang, S.Y. Reversibly Switching the Function of a Surface between Attacking and Defending against Bacteria. Angew. Chem. Int. Edit. 2012, 51, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Tang, Q.; Li, L.L.; Humble, J.; Wu, H.Y.; Liu, L.Y.; Cheng, G. Switchable Antimicrobial and Antifouling Hydrogels with Enhanced Mechanical Properties. Adv. Healthc. Mater. 2013, 2, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Ishihara, K.; Huang, C.J. Polyelectrolyte and Antipolyelectrolyte Effects for Dual Salt-Responsive Interpenetrating Network Hydrogels. Biomacromolecules 2019, 20, 3524–3534. [Google Scholar] [CrossRef]
- Lin, W.F.; Zhang, J.; Wang, Z.; Chen, S.F. Development of robust biocompatible silicone with high resistance to protein adsorption and bacterial adhesion. Acta. Biomater. 2011, 7, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.Q.; Lin, W.F.; Wang, Z.; Wang, L.G.; Zhang, J.; Ma, G.L.; Chen, S.F. Development of Nonstick and Drug-Loaded Wound Dressing Based on the Hydrolytic Hydrophobic Poly(carboxybetaine) Ester Analogue. ACS Appl. Mater. Inter. 2013, 5, 10489–10494. [Google Scholar] [CrossRef]
- Wang, G.Z.; Wang, L.G.; Lin, W.F.; Wang, Z.; Zhang, J.; Ji, F.Q.; Ma, G.L.; Yuan, Z.F.; Chen, S.F. Development of Robust and Recoverable Ultralow-Fouling Coatings Based on Poly(carboxybetaine) Ester Analogue. ACS Appl. Mater. Inter. 2015, 7, 16938–16945. [Google Scholar] [CrossRef]
- Ma, J.; Lin, W.F.; Xu, L.B.; Liu, S.H.; Xue, W.L.; Chen, S.F. Resistance to Long-Term Bacterial Biofilm Formation Based on Hydrolysis-Induced Zwitterion Material with Biodegradable and Self-Healing Properties. Langmuir 2020, 36, 3251–3259. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zong, Y.; Yang, N.; Zhang, N.; Jiang, B.; Zhang, L.H.; Xiao, X.M. Surface hydrophilic modification of PVDF membranes based on tannin and zwitterionic substance towards effective oil-in-water emulsion separation. Sep. Purif. Technol. 2020, 234, 116015. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Quilitzsch, M.; Osmond, R.; Krug, M.; Heijnen, M.; Ulbricht, M. Macro-initiator mediated surface selective functionalization of ultrafiltration membranes with anti-fouling hydrogel layers applicable to ready-to-use capillary membrane modules. J. Membr. Sci. 2016, 518, 328–337. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Tran, T.; Pan, S.W.; Chen, X.Y.; Lin, X.C.; Blevins, A.K.; Ding, Y.F.; Lin, H.Q. Zwitterionic Hydrogel-Impregnated Membranes with Polyamide Skin Achieving Superior Water/Salt Separation Properties. ACS Appl. Mater. Inter. 2020, 12, 49192–49199. [Google Scholar] [CrossRef]
- Laghmari, S.; May, P.; Ulbricht, M. Polyzwitterionic hydrogel coating for reverse osmosis membranes by concentration polarization-enhanced in situ ?click? reaction that is applicable in modules. J. Membr. Sci. 2021, 629, 119274. [Google Scholar] [CrossRef]
- Ma, W.; Rajabzadeh, S.; Matsuyama, H. Preparation of antifouling poly(vinylidene fluoride) membranes via different coating methods using a zwitterionic copolymer. Appl. Surf. Sci. 2015, 357, 1388–1395. [Google Scholar] [CrossRef]
- Bernstein, R.; Belfer, S.; Freger, V. Improving performance of spiral wound RO elements by in situ concentration polarization-enhanced radical graft polymerization. J. Membr. Sci. 2012, 405–406, 79–84. [Google Scholar] [CrossRef]
- Venault, A.; Ye, C.-C.; Lin, Y.-C.; Tsai, C.-W.; Jhong, J.-F.; Ruaan, R.-C.; Higuchi, A.; Chinnathambi, A.; Ho, H.-T.; Chang, Y. Zwitterionic fibrous polypropylene assembled with amphiphatic carboxybetaine copolymers for hemocompatible blood filtration. Acta. Biomater. 2016, 40, 130–141. [Google Scholar] [CrossRef]
- Yang, T.; He, X.; Zhou, X.; Lei, Z.; Wang, Y.; Yang, J.; Cai, D.; Chen, S.-L.; Wang, X. [f54INVITED] Surface plasmon cavities on optical fiber end-facets for biomolecule and ultrasound detection. Opt. Laser Technol. 2018, 101, 468–478. [Google Scholar] [CrossRef]
- Yang, T.; Chen, S.; He, X.; Guo, H.; Sun, X. How to convincingly measure low concentration samples with optical label-free biosensors. Sens. Actuators B Chem. 2020, 306, 127568. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Dou, Q.; Wang, S.W.; Hu, D.B.; Yang, B.; Zhao, Z.P.; Liu, H.L.; Dai, Q. The development of an antifouling interpenetrating polymer network hydrogel film for salivary glucose monitoring. Nanoscale 2020, 12, 22787–22797. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Lu, T.; Xie, Y.; Zhao, W.F.; Sun, S.D.; Zhao, C.S. Zwitterionic polymer functionalization of polysulfone membrane with improved antifouling property and blood compatibility by combination of ATRP and click chemistry. Acta Biomater. 2016, 40, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Sabate Del Rio, J.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef]
- Roh, Y.H.; Seo, J.; Kim, J.Y.; Kim, H.U.; Mun, S.J.; Seo, J.H.; Bong, K.W. Phosphorylcholine-based encoded hydrogel microparticles with enhanced fouling resistance for multiplex immunoassays. Analyst 2020, 145, 5482–5490. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Chapin, S.C.; Doyle, P.S. Hydrogel microparticles from lithographic processes: Novel materials for fundamental and applied colloid science. Curr. Opin. Colloid. Interface Sci. 2011, 16, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Parada, G.; Yu, Y.; Riley, W.; Lojovich, S.; Tshikudi, D.; Ling, Q.; Zhang, Y.; Wang, J.; Ling, L.; Yang, Y.; et al. Ultrathin and Robust Hydrogel Coatings on Cardiovascular Medical Devices to Mitigate Thromboembolic and Infectious Complications. Adv. Healthc. Mater. 2020, 9, e2001116. [Google Scholar] [CrossRef]
- Cutiongco, M.F.; Kukumberg, M.; Peneyra, J.L.; Yeo, M.S.; Yao, J.Y.; Rufaihah, A.J.; Le Visage, C.; Ho, J.P.; Yim, E.K. Submillimeter Diameter Poly(Vinyl Alcohol) Vascular Graft Patency in Rabbit Model. Front. Bioeng. Biotechnol. 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanEpps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Wallace, A.; Albadawi, H.; Patel, N.; Khademhosseini, A.; Zhang, Y.S.; Naidu, S.; Knuttinen, G.; Oklu, R.J.C.D. Anti-fouling strategies for central venous catheters. Cardiovasc. Diagn. Ther. 2017, 7, S246–S257. [Google Scholar] [CrossRef]
- Koc, J.; Schonemann, E.; Wanka, R.; Aldred, N.; Clare, A.S.; Gardner, H.; Swain, G.W.; Hunsucker, K.; Laschewsky, A.; Rosenhahn, A. Effects of crosslink density in zwitterionic hydrogel coatings on their antifouling performance and susceptibility to silt uptake. Biofouling 2020, 36, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, P.; Wang, J.; Wang, X.; He, Y.; Song, P.; Wang, R. Rational Design of Polycationic Hydrogel with Excellent Combination Functions for Flexible Wearable Electronic Devices. Macromol. Mater. Eng. 2021, 2100593. [Google Scholar] [CrossRef]
- Sheng, G.; Ni, J.; Xing, K.; Fan, L.; Dai, T.; Yu, J.; Dai, X.; Chen, R.; Wu, J.; Li, N.; et al. Infection microenvironment-responsive multifunctional peptide coated gold nanorods for bimodal antibacterial applications. J. Colloid. Interface Sci. 2021, 41, 100379. [Google Scholar] [CrossRef]
- Xu, L.C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goor, O.J.G.M.; Brouns, J.E.P.; Dankers, P.Y.W. Introduction of anti-fouling coatings at the surface of supramolecular elastomeric materials via post-modification of reactive supramolecular additives. Polym. Chem. 2017, 8, 5228–5238. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhou, S.; Wu, L. Self-repairing nonfouling polyurethane coatings via 3D-grafting of PEG-b-PHEMA-b-PMPC copolymer. RSC Adv. 2015, 5, 104907–104914. [Google Scholar] [CrossRef]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Bonyadi, S.Z.; Demott, C.J.; Grunlan, M.A.; Dunn, A.C. Cartilage-like tribological performance of charged double network hydrogels. J. Mech. Behav. Biomed. 2021, 114, 104202. [Google Scholar] [CrossRef]
- Milner, P.E.; Parkes, M.; Puetzer, J.L.; Chapman, R.; Stevens, M.M.; Cann, P.; Jeffers, J.R.T. A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018, 65, 102–111. [Google Scholar] [CrossRef]
- Ren, K.; Ke, X.; Chen, Z.; Zhao, Y.; He, L.; Yu, P.; Xing, J.; Luo, J.; Xie, J.; Li, J. Zwitterionic polymer modified xanthan gum with collagen II-binding capability for lubrication improvement and ROS scavenging. Carbohydr. Polym. 2021, 274, 118672. [Google Scholar] [CrossRef]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Nur, R.; Matsuhisa, N.; Jiang, Z.; Nayeem, M.O.G.; Yokota, T.; Someya, T. A Highly Sensitive Capacitive-type Strain Sensor Using Wrinkled Ultrathin Gold Films. Nano Lett. 2018, 18, 5610–5617. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Park, J.; Wang, S.; Li, M.; Ahn, C.; Hyun, J.K.; Kim, D.S.; Kim, D.K.; Rogers, J.A.; Huang, Y.; Jeon, S. Three-dimensional nanonetworks for giant stretchability in dielectrics and conductors. Nat. Commun. 2012, 3, 916. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, N.; Lv, T.; Yao, Y.; Peng, H.; Shi, J.; Cao, S.; Chen, T. Ag-Doped PEDOT:PSS/CNT composites for thin-film all-solid-state supercapacitors with a stretchability of 480%. J. Mater. Chem. A 2018, 6, 941–947. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Ryplida, B.; Lee, K.D.; In, I.; Park, S.Y. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar] [CrossRef]
- Erfani, A.; Flynn, N.H.; Aichele, C.P.; Ramsey, J.D. Encapsulation and delivery of protein from within poly(sulfobetaine) hydrogel beads. J. Appl. Polym. Sci. 2020, 137, e49550. [Google Scholar] [CrossRef]
- Xue, W.L.; Trital, A.; Shen, J.; Wang, L.G.; Chen, S.F. Zwitterionic Polypeptide-Based Nanodrug Augments pH-Triggered Tumor Targeting via Prolonging Circulation Time and Accelerating Cellular Internalization. ACS Appl. Mater. Inter. 2020, 12, 46639–46652. [Google Scholar] [CrossRef]
- Perry, J.L.; Reuter, K.G.; Kai, M.P.; Herlihy, K.P.; Jones, S.W.; Luft, J.C.; Napier, M.; Bear, J.E.; DeSimone, J.M. PEGylated PRINT nanoparticles: The impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012, 12, 5304–5310. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Jia, X.R.; Du, J.; Ying, X.; Lu, W.L.; Lou, J.N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef]
- Shen, W.; Chang, Y.; Liu, G.; Wang, H.; Cao, A.; An, Z. Biocompatible, Antifouling, and Thermosensitive Core−Shell Nanogels Synthesized by RAFT Aqueous Dispersion Polymerization. Macromolecules 2011, 44, 2524–2530. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chen, C.-H.; Chen, B.-M.; Su, Y.-C.; Chen, Y.-T.; Hershfield, M.S.; Lee, M.-T.M.; Cheng, T.-L.; Chen, Y.-T.; Roffler, S.R.; et al. A genome-wide association study identifies a novel susceptibility locus for the immunogenicity of polyethylene glycol. Nat. Commun. 2017, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, E.J.; Tsao, C.; Kasten, S.A.; Boeri, M.V.; Dao, T.L.; DeBus, S.J.; Cadleux, C.L.; Baker, C.A.; Otto, T.C.; et al. Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents. Sci. Transl. Med. 2019, 11, eaau7091. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.C.; Li, B.Q.; Liu, N.; Wu, G.L.; Gao, H.; Ma, J.B. A hydrazone crosslinked zwitterionic polypeptide nanogel as a platform for controlled drug delivery. RSC Adv. 2014, 4, 50301–50311. [Google Scholar] [CrossRef]
- Men, Y.; Peng, S.; Yang, P.; Jiang, Q.; Zhang, Y.; Shen, B.; Dong, P.; Pang, Z.; Yang, W. Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 23509–23521. [Google Scholar] [CrossRef] [PubMed]
- She, D.; Huang, H.; Li, J.; Peng, S.; Wang, H.; Yu, X. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem. Eng. J. 2021, 408, 127359. [Google Scholar] [CrossRef]
- Ekkelenkamp, A.E.; Jansman, M.M.T.; Roelofs, K.; Engbersen, J.F.J.; Paulusse, J.M.J. Surfactant-free preparation of highly stable zwitterionic poly(amido amine) nanogels with minimal cytotoxicity. Acta Biomater. 2016, 30, 126–134. [Google Scholar] [CrossRef]
- Tayanloo-Beik, A.; Rabbani, Z.; Soveyzi, F.; Alavi-Moghadam, S.; Rezaei-Tavirani, M.; Goodarzi, P.; Arjmand, B.; Larijani, B. Cellular therapy for treatment of spinal cord injury in Zebrafish model. Mol. Biol. Rep. 2021, 48, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Strand, B.L.; Coron, A.E.; Skjak-Braek, G. Current and Future Perspectives on Alginate Encapsulated Pancreatic Islet. Stem. Cells Transl. Med. 2017, 6, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Iwata, H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv. Drug Deliv. Rev. 2010, 62, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.L.; Liu, B.; Jones, P.M.; Pickup, J.C. Polysaccharide multilayer nanoencapsulation of insulin-producing beta-cells grown as pseudoislets for potential cellular delivery of insulin. Biomacromolecules 2010, 11, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Chiu, A.; Wang, L.H.; An, D.; Zhong, M.; Smink, A.M.; de Haan, B.J.; de Vos, P.; Keane, K.; Vegge, A.; et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat. Commun. 2019, 10, 5262. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Cuchiara, M.L.; Coskun, S.; Banda, O.A.; Horter, K.L.; Hirschi, K.K.; West, J.L. Bioactive poly(ethylene glycol) hydrogels to recapitulate the HSC niche and facilitate HSC expansion in culture. Biotechnol. Bioeng. 2016, 113, 870–881. [Google Scholar] [CrossRef]
- Bai, T.; Sun, F.; Zhang, L.; Sinclair, A.; Liu, S.J.; Ella-Menye, J.R.; Zheng, Y.; Jiang, S.Y. Restraint of the Differentiation of Mesenchymal Stem Cells by a Nonfouling Zwitterionic Hydrogel. Angew. Chem. Int. Ed. 2014, 53, 12729–12734. [Google Scholar] [CrossRef]
- Csaszar, E.; Kirouac, D.C.; Yu, M.; Wang, W.; Qiao, W.; Cooke, M.P.; Boitano, A.E.; Ito, C.; Zandstra, P.W. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell 2012, 10, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Kilian, K.A.; Mrksich, M. Directing Stem Cell Fate by Controlling the Affinity and Density of Ligand–Receptor Interactions at the Biomaterials Interface. Angew. Chem. 2012, 124, 4975–4979. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Sinclair, A.; Sun, F.; Jain, P.; Hung, H.C.; Zhang, P.; Ella-Menye, J.R.; Liu, W.G.; Jiang, S.Y. Harnessing isomerization-mediated manipulation of nonspecific cell/matrix interactions to reversibly trigger and suspend stem cell differentiation. Chem. Sci. 2016, 7, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Cankova, Z.; Iwanaszko, M.; Lichtor, S.; Mrksich, M.; Ameer, G.A. Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, 6816–6821. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Z.; Chen, A.; He, H.; He, C.; Shuai, X.; Li, X.; Chen, S.; Zhang, Y.; Ren, B.; et al. Sulfated zwitterionic poly(sulfobetaine methacrylate) hydrogels promote complete skin regeneration. Acta Biomater. 2018, 71, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Q.; An, Y.; Huang, W.; Xuan, T.X.; Zhang, Q.W.; Ye, M.Q.; Luo, S.; Xuan, X.; He, H.C.; Zheng, J.; et al. Highly Water-Preserving Zwitterionic Betaine-Incorporated Collagen Sponges With Anti-oxidation and Anti-inflammation for Wound Regeneration. Front. Cell Dev. Biol. 2020, 8, 491. [Google Scholar] [CrossRef]
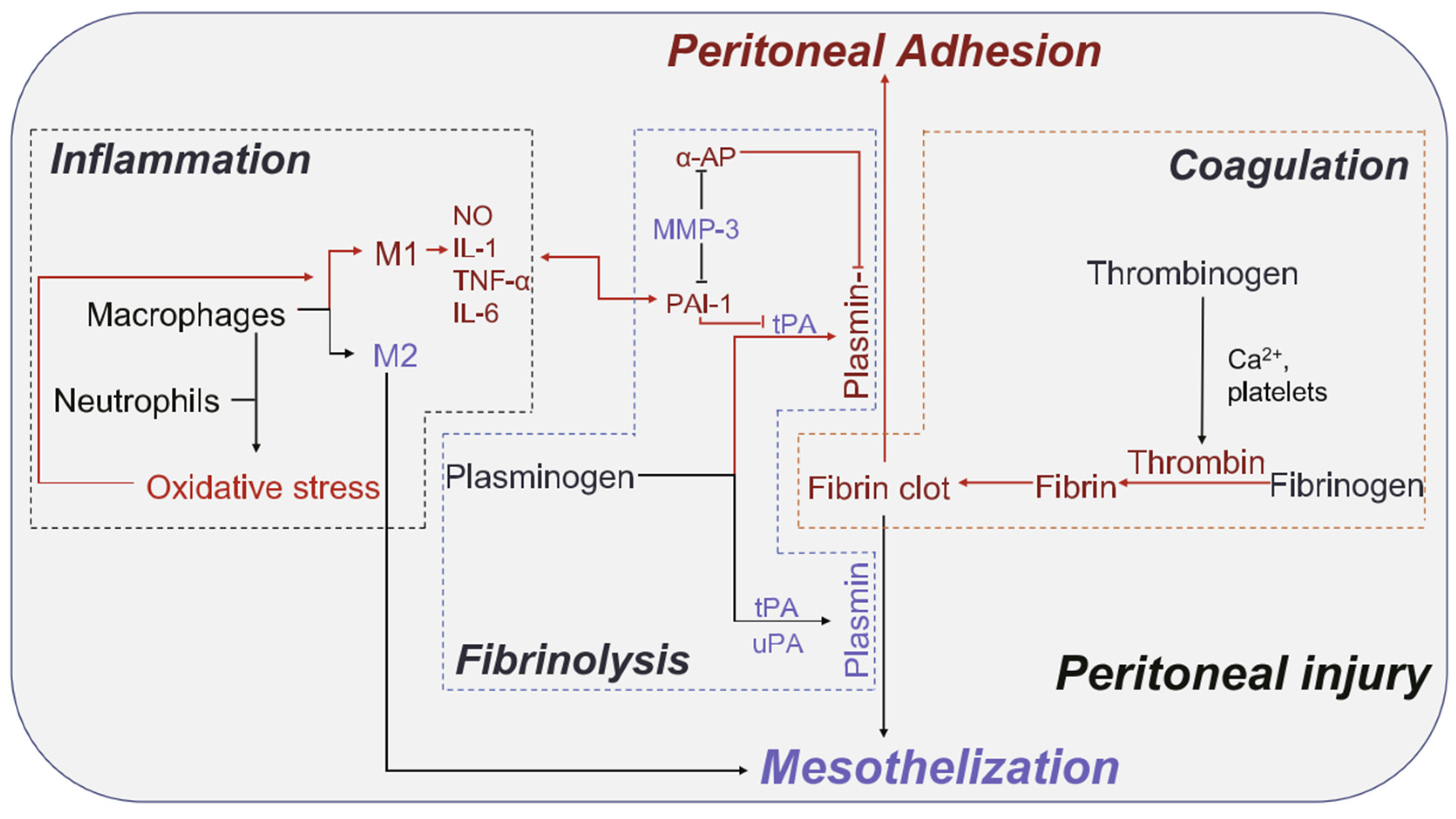
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020, 116, 84–104. [Google Scholar] [CrossRef]
- Zhang, E.S.; Yang, J.H.; Wang, K.; Song, B.Y.; Zhu, H.; Han, X.F.; Shi, Y.J.; Yang, C.B.; Zeng, Z.P.; Cao, Z.Q. Biodegradable Zwitterionic Cream Gel for Effective Prevention of Postoperative Adhesion. Adv. Funct. Mater. 2021, 31, 2009431. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, J.M.; Cao, L.L.; Jiao, Q.; Zhou, J.H.; Yang, L.J.; Zhang, H.; Wei, Y.P. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Inter. 2021, 13, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Fang, Y.L.; Hsieh, P.S.; Li, C.C.; Dai, N.T.; Huang, C.J. Non-sticky and antimicrobial zwitterionic nanocomposite dressings for infected chronic wounds. Biomater. Sci. 2017, 5, 1072–1081. [Google Scholar] [CrossRef]
- Xiao, Z.C.; Zheng, X.Y.; An, Y.; Wang, K.N.; Zhang, J.W.; He, H.C.; Wu, J. Zwitterionic hydrogel for sustained release of growth factors to enhance wound healing. Biomater. Sci. 2021, 9, 882–891. [Google Scholar] [CrossRef] [PubMed]




| Sample | Srequibrium, Water | SRequibrium, NaCl | SRequibrium, NaCl/SRequibrium, Water |
|---|---|---|---|
| PNaSS | 18.68 | 6.21 | 0.33 |
| PSBMA1NaSS0.5 | 18.22 | 7.51 | 0.41 |
| PSBMA1NaSS1 | 33.27 | 10.22 | 0.30 |
| PSBMA1NaSS2 | 71.94 | 16.39 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. https://doi.org/10.3390/gels8010046
Liu S, Tang J, Ji F, Lin W, Chen S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels. 2022; 8(1):46. https://doi.org/10.3390/gels8010046
Chicago/Turabian StyleLiu, Sihang, Jingyi Tang, Fangqin Ji, Weifeng Lin, and Shengfu Chen. 2022. "Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application" Gels 8, no. 1: 46. https://doi.org/10.3390/gels8010046
APA StyleLiu, S., Tang, J., Ji, F., Lin, W., & Chen, S. (2022). Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels, 8(1), 46. https://doi.org/10.3390/gels8010046