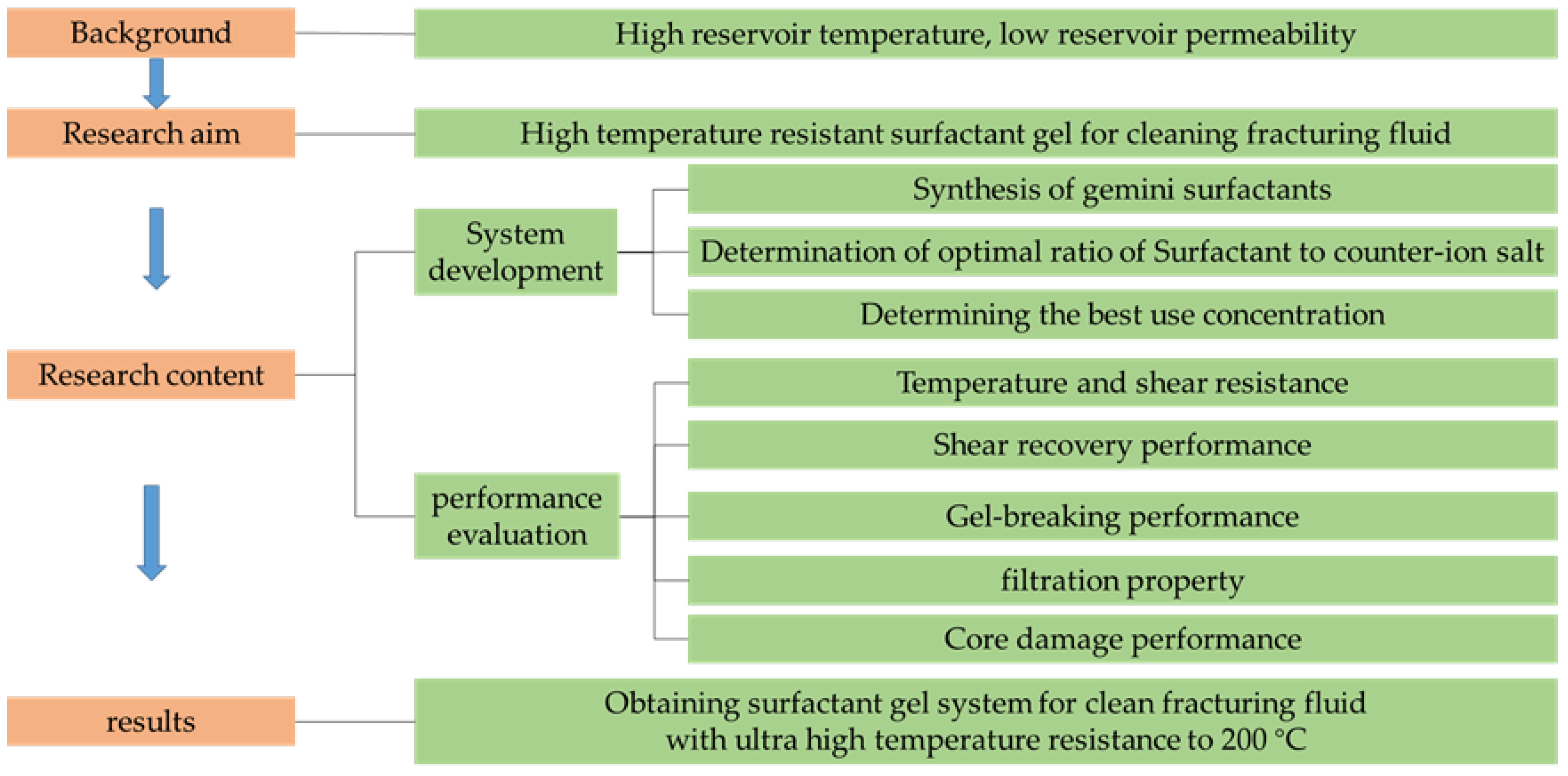
Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C
Abstract
:1. Introduction
2. Results and Discussion
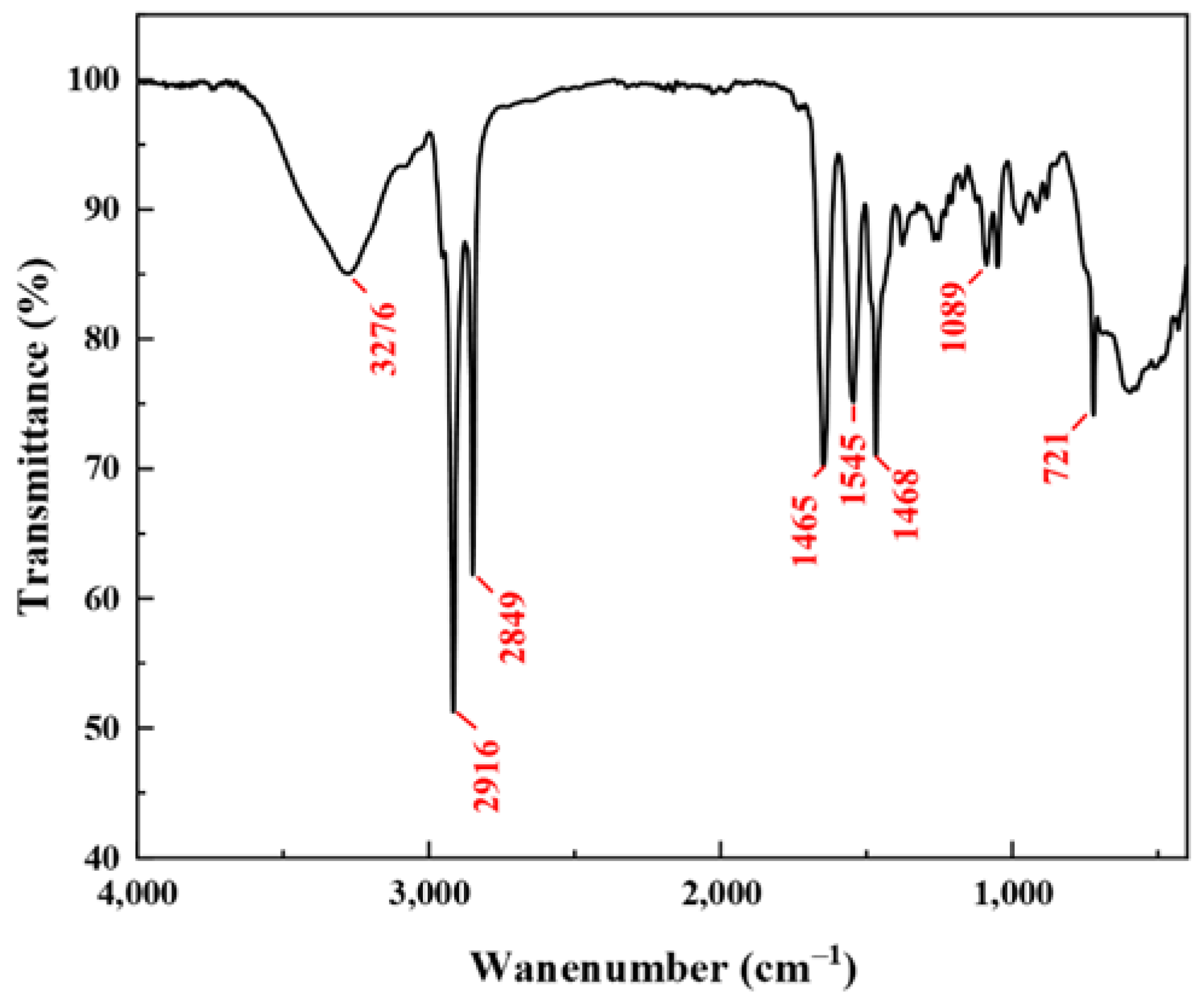
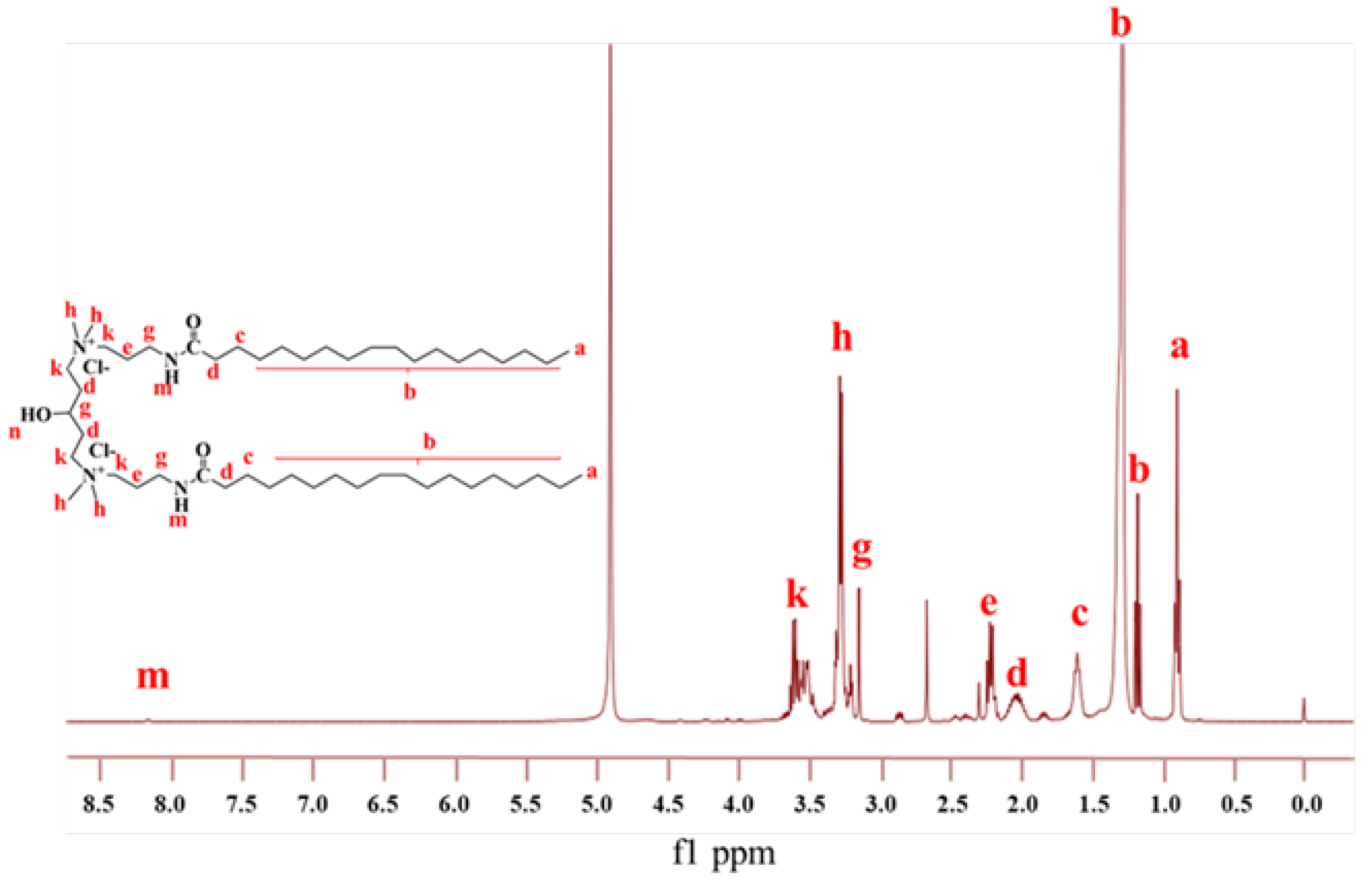
2.1. Structure Characterization
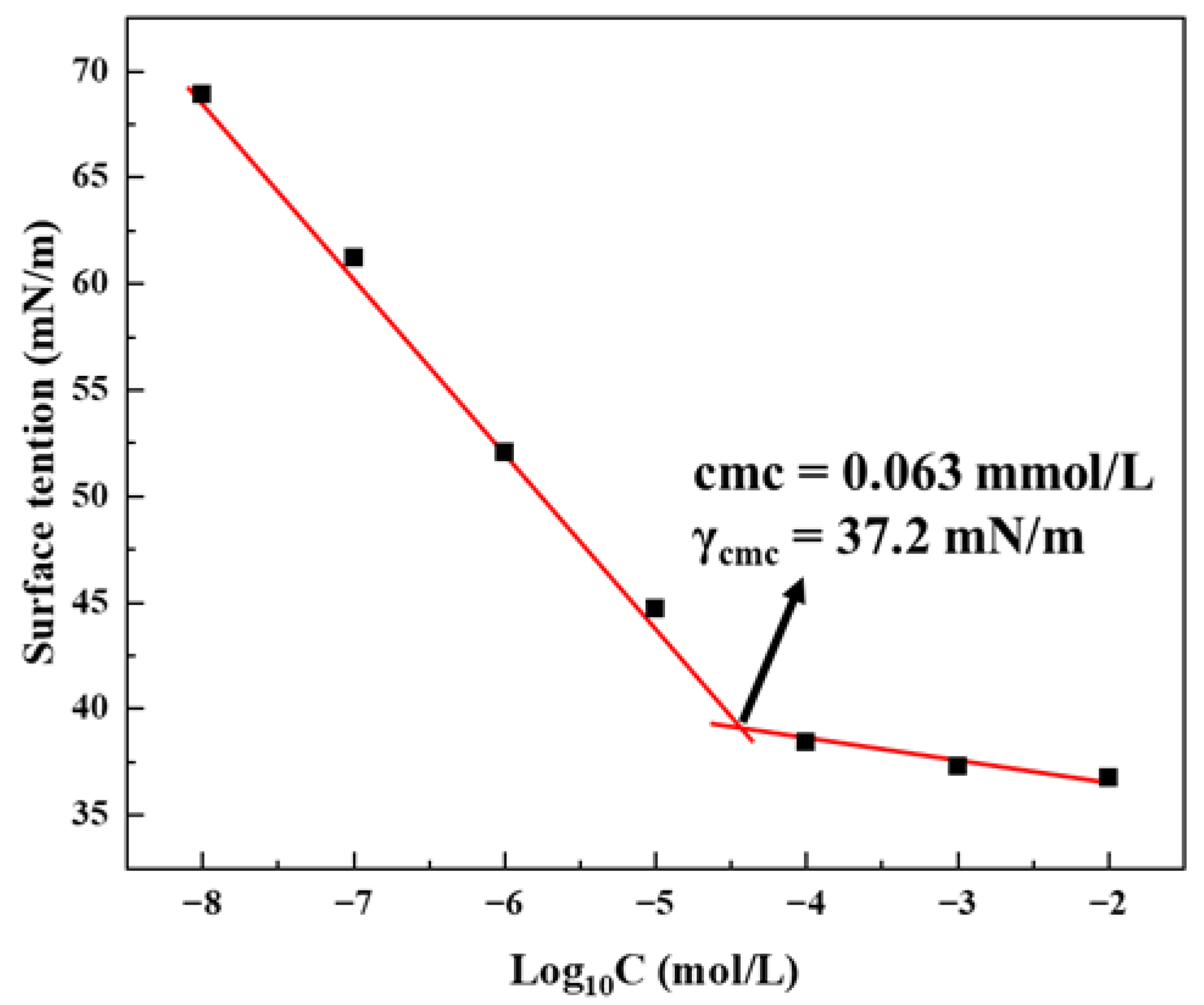
2.2. Surface Activity
2.3. Rheological Properties
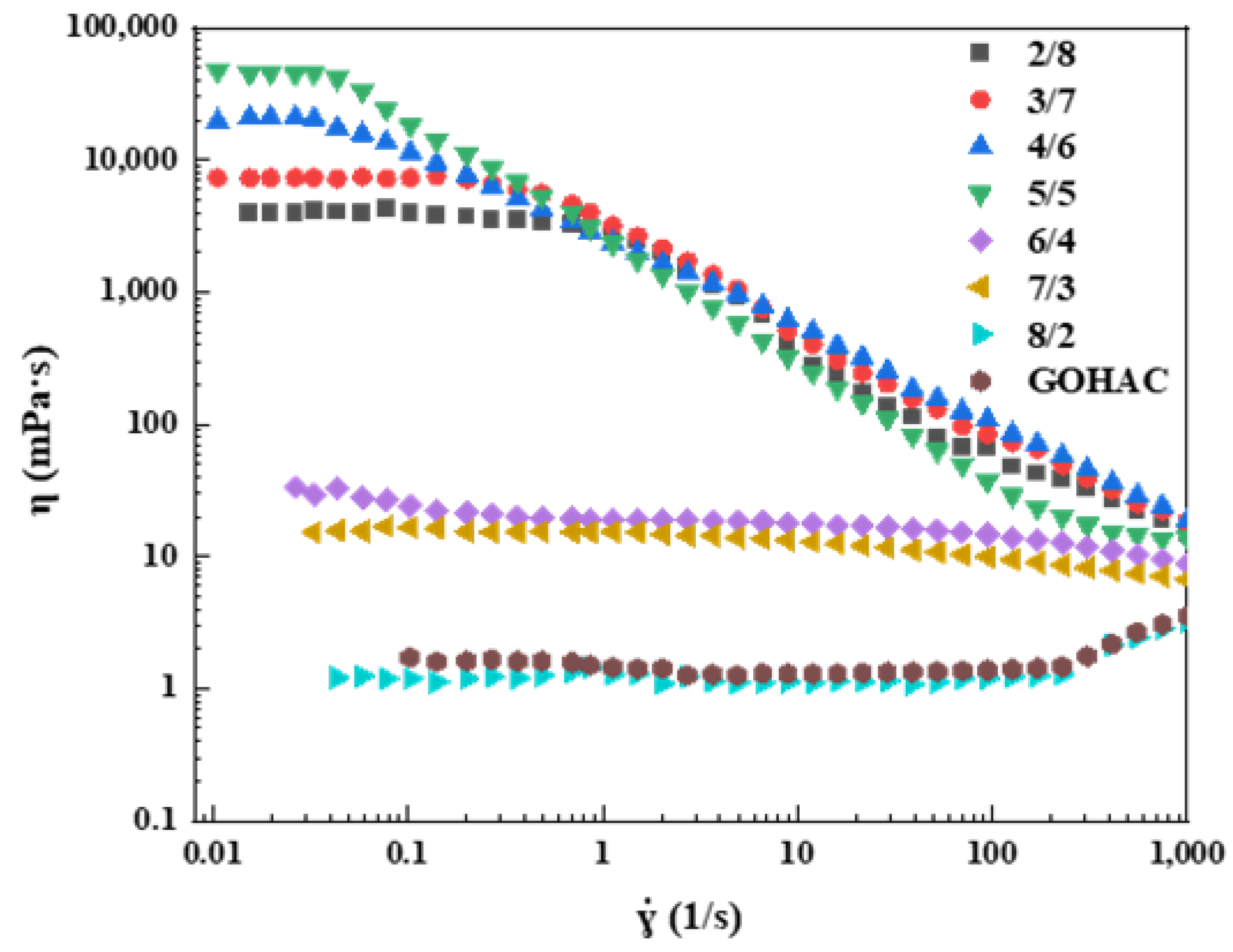
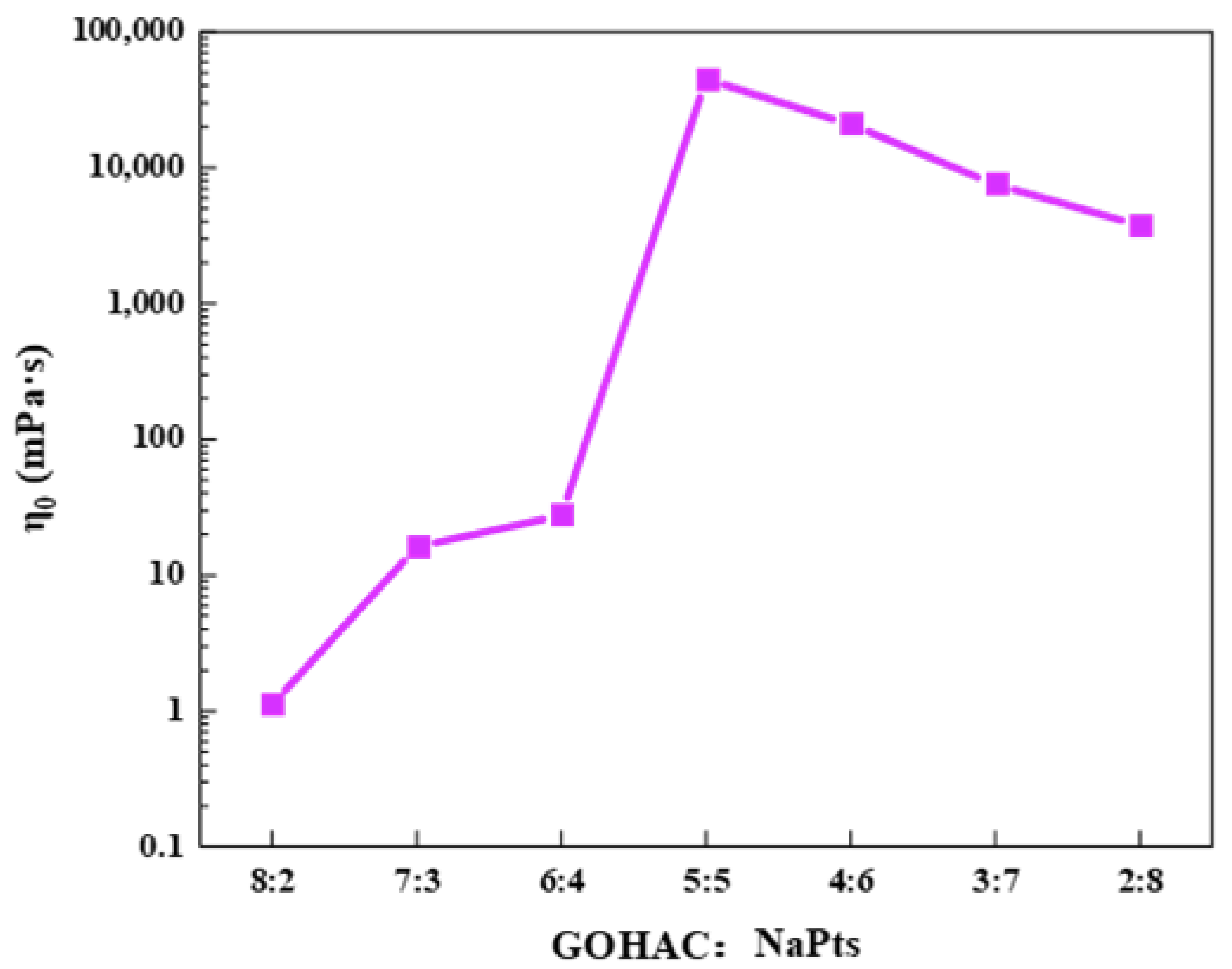
2.3.1. Steady Shear Properties
2.3.2. Dynamic Viscoelasticity
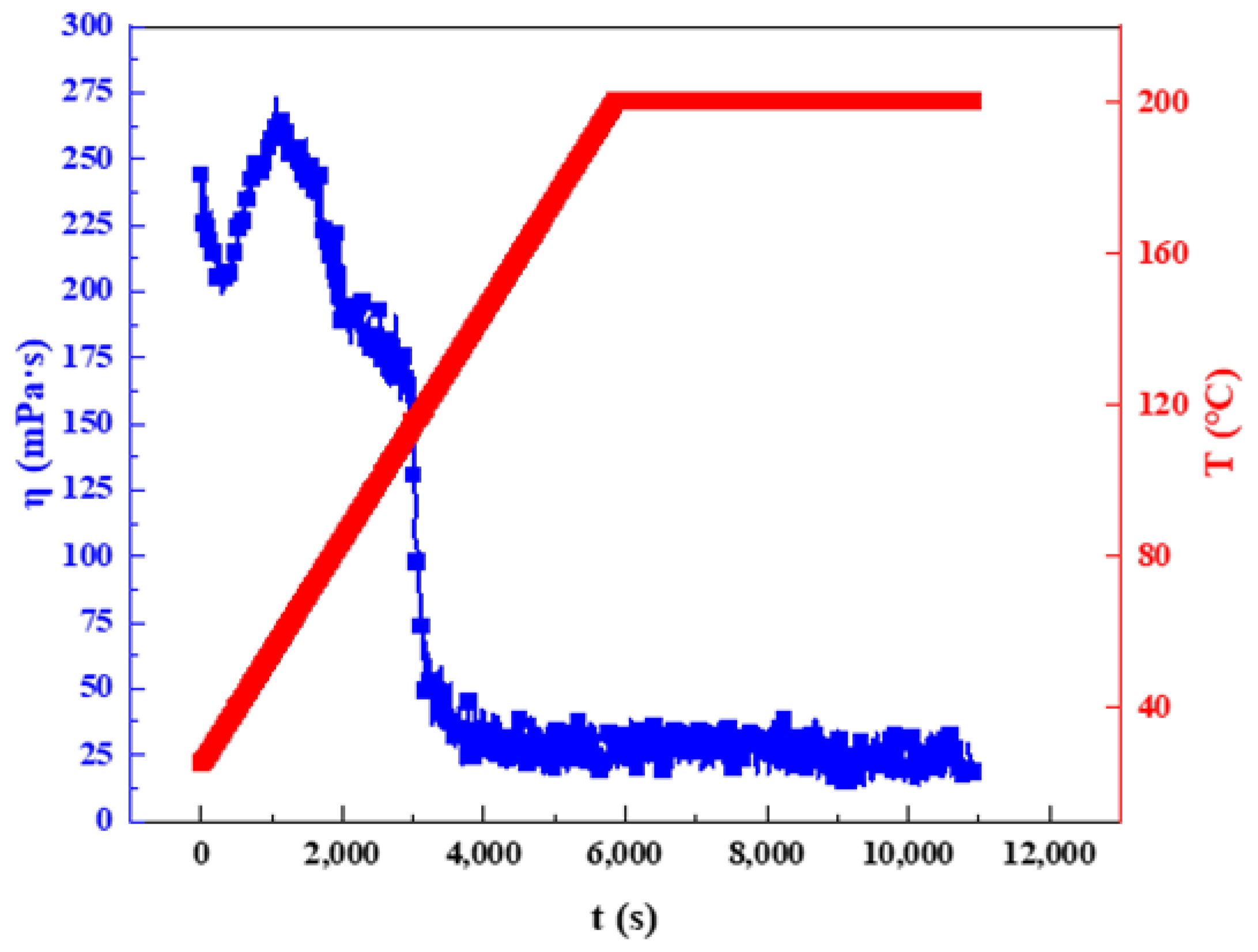
2.3.3. High-Temperature Thermal Stability
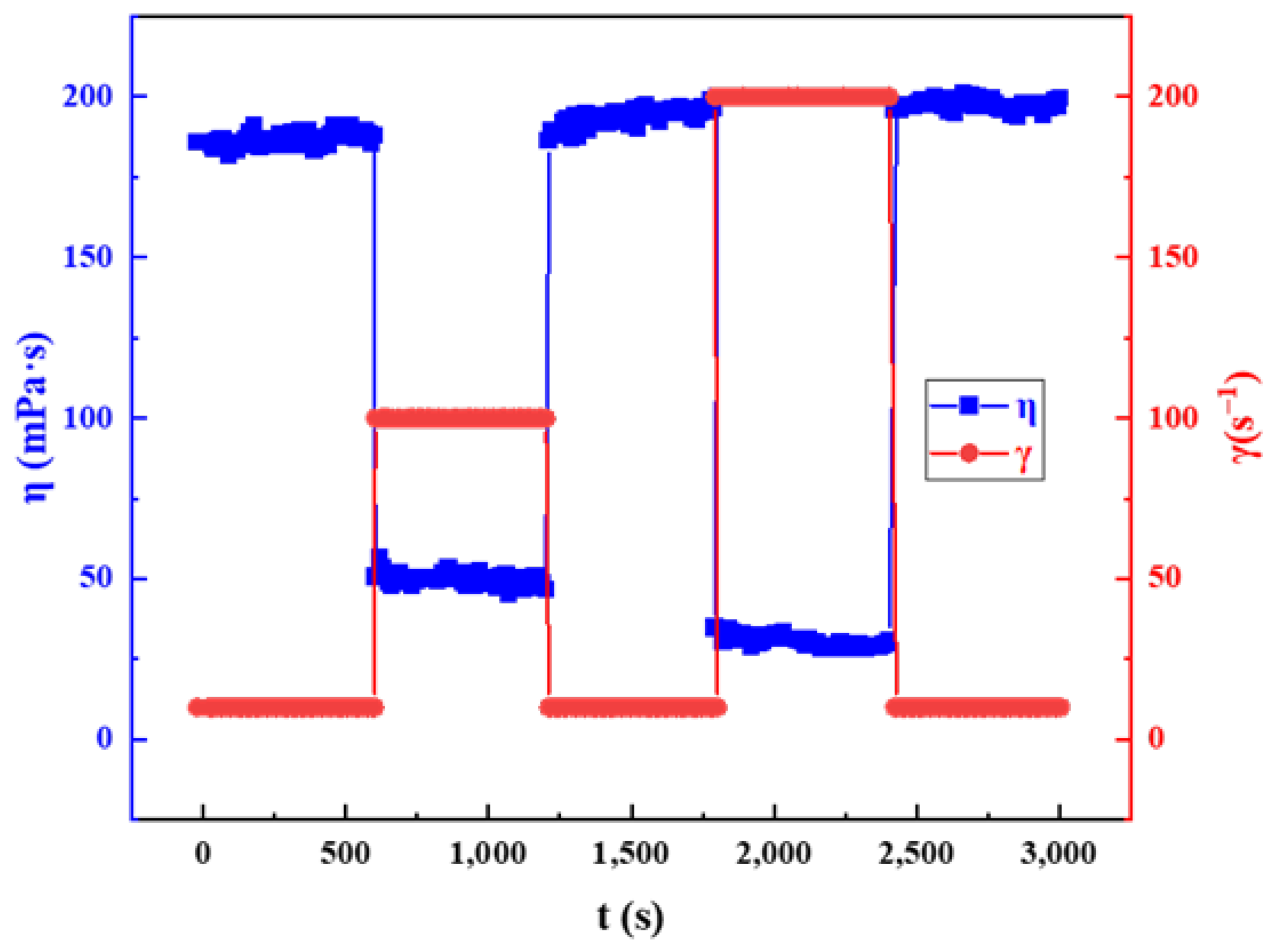
2.3.4. Shear Recovery
2.4. Gel-Breaking Performance
2.5. Filtration Evaluation
2.6. Core Permeability Damage
3. Conclusions
4. Materials and Methods
4.1. Material
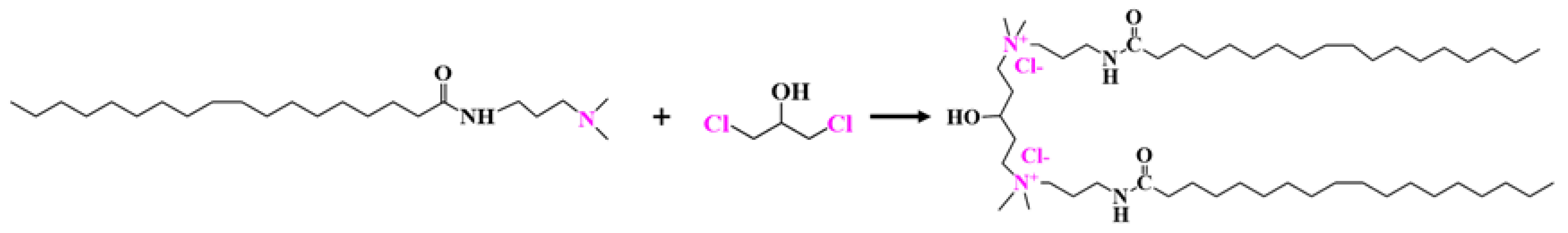
4.2. Synthesis of GOHAC
4.3. Structure Characterization
4.4. Viscoelastic Surfactant Gel Sample Preparation
4.5. Surface Tension Measurement
4.6. Rheological Measurement
4.7. High-Temperature Thermal Stability Evaluation Method
4.8. Gel-Breaking Performance Test
4.9. Filtration Test
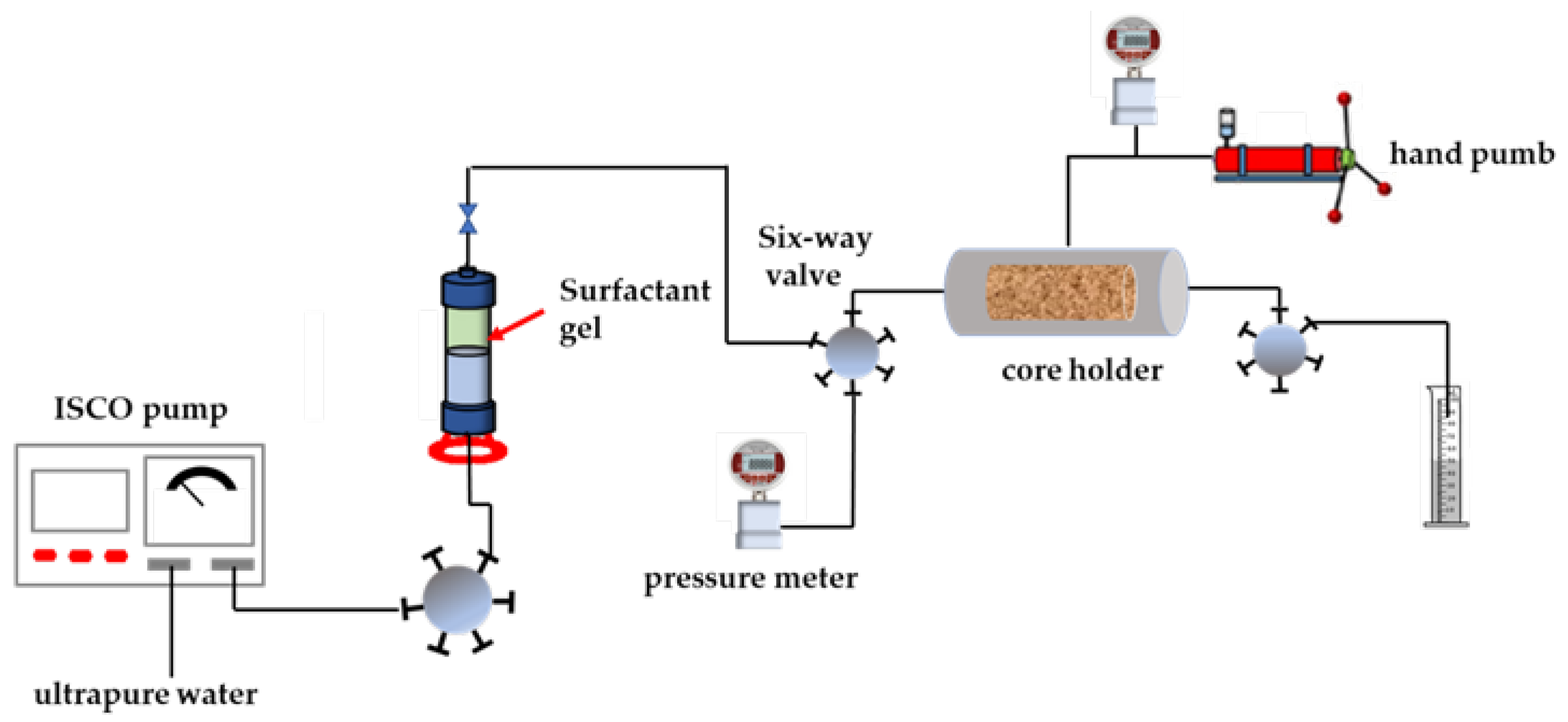
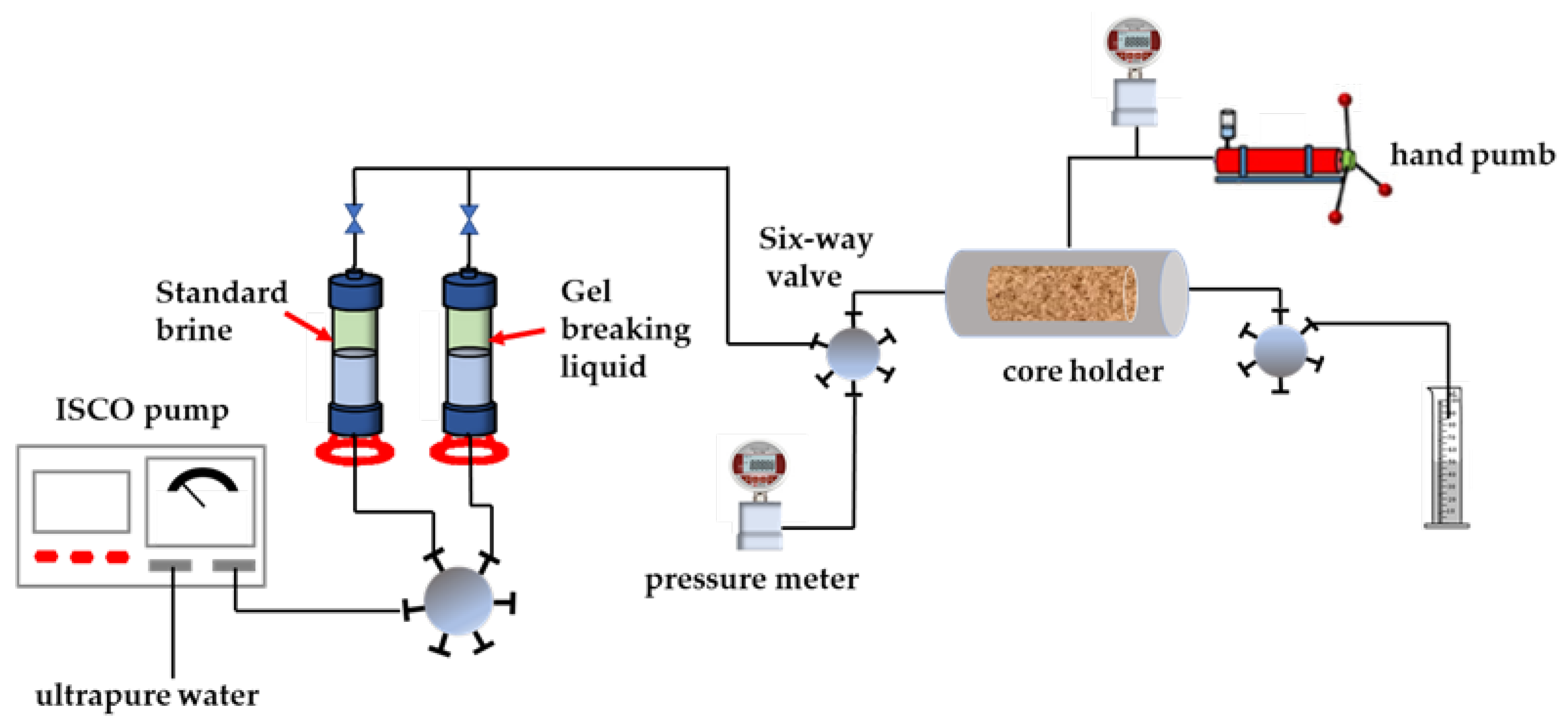
4.10. Core Permeability Damage Experiment
- (1)
- The core was treated to a preset size, cleaned with a KQ-300DE ultrasonic cleaner for 10 min, and dried in a 95 °C oven for 24 h. After fully drying, the core was taken out and cooled to room temperature.
- (2)
- The standard brine with the concentration of 2.0 wt% KCl, 5.5 wt% NaCl, 0.45 wt% MgCl2 and 0.55 wt% CaCl2 was prepared. In addition, the core was put into the standard brine for vacuum saturation for 24 h.
- (3)
- Preparation of gel breaking liquid: the surfactant gel is fully mixed with 1% kerosene and is stationary for 24 h, and the clear liquid is taken for use.
- (4)
- Determination of initial permeability: a confining pressure of 2 MPa was applied around the core holder and the standard brine was displaced into the core by the ISCO pump at a flow rate of 0.5 mL/min. The stable pressure value was recorded, and the initial permeability was calculated according to formula (4).
- (5)
- Simulating the damage process: the glue-breaking liquid was injected from the other end of the core at the same injection rate, with an injection volume of 1.5 PV. After the injection was completed, the valves at both ends of the core holder were closed so that the gel-breaking liquid stayed in the core for 2 h to simulate the soaking well process.
- (6)
- Determination of permeability after damaging: the test steps were the same as Step 4.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sander, R.; Pan, Z.; Connell, L.D. Laboratory measurement of low permeability unconventional gas reservoir rocks: A review of experimental methods. J. Nat. Gas Sci. Eng. 2017, 37, 248–279. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. The control of asphaltene precipitation in oil wells. Pet. Sci. Technol. 2018, 36, 443–449. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. Experimental and modeling analysis of asphaltene precipitation in the near wellbore region of oil wells. Pet. Sci. Technol. 2018, 36, 1030–1036. [Google Scholar] [CrossRef]
- Chieng, Z.H.; Mohyaldinn, M.E.; Hassan, A.M.; Bruining, H. Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid. Polymers 2020, 12, 1470. [Google Scholar] [CrossRef]
- Yang, M.; Lu, Y.; Ge, Z.; Zhou, Z.; Chai, C.; Wang, H.; Zhang, L.; Bo, T. Viscoelastic surfactant fracturing fluids for use in coal seams: Effects of surfactant composition and formulation. Chem. Eng. Sci. 2020, 215, 115370. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Li, W.; Zeng, P.; Zheng, Z.; Feng, Y.; Yin, H. Comparative studies on hydraulic fracturing fluids for high-temperature and high-salt oil reservoirs: Synthetic polymer versus guar gum. ACS Omega 2021, 6, 25421–25429. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Guo, J.; Lai, J.; Wang, D.; He, L.; Qin, Y. A study of relation between suspension behavior and microstructure and viscoelastic property of guar gum fracturing fluid. J. Pet. Sci. Eng. 2014, 124, 432–435. [Google Scholar] [CrossRef]
- Gaillard, N.; Thomas, A.; Favero, C. Novel Associative Acrylamide-based Polymers for Proppant Transport in Hydraulic Fracturing Fluids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013; p. SPE-164072-MS. [Google Scholar]
- Ma, K.; Jiang, H.; Li, J.; Zhao, L. Experimental study on the micro alkali sensitivity damage mechanism in low-permeability reservoirs using QEMSCAN. J. Nat. Gas Sci. Eng. 2016, 36, 1004–1017. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Wang, C.; Adenutsi, C.D. Experimental study on microscopic formation damage of low permeability reservoir caused by HPG fracturing fluid. J. Nat. Gas Sci. Eng. 2016, 36, 486–495. [Google Scholar] [CrossRef]
- Hu, R.; Tang, S.; Mpelwa, M.; Jiang, Z.; Feng, S. Research progress of viscoelastic surfactants for enhanced oil recovery. Energy Explor. Exploit. 2021, 39, 1324–1348. [Google Scholar] [CrossRef]
- Kang, W.; Mushi, S.J.; Yang, H.; Wang, P.; Hou, X. Development of smart viscoelastic surfactants and its applications in fracturing fluid: A review. J. Pet. Sci. Eng. 2020, 190, 107107. [Google Scholar] [CrossRef]
- Philippova, O.E.; Molchanov, V.S. Enhanced rheological properties and performance of viscoelastic surfactant fluids with embedded nanoparticles. Curr. Opin. Colloid Interface Sci. 2019, 43, 52–62. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Zheng, L. Salt-induced wormlike micelles formed by N-alkyl-N-methylpyrrolidinium bromide in aqueous solution. Colloid Surf. A Physicochem. Eng. Asp. 2012, 396, 16–21. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Chen, Z.; Chen, Y.; Zhao, J. Clean fracturing fluids for tight reservoirs: Opportunities with viscoelastic surfactant. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1446–1459. [Google Scholar] [CrossRef]
- Gomaa, A.M.M.; Gupta, D.V.S.V.S.; Carman, P. Viscoelastic Behavior and Proppant Transport Properties of a New High-Temperature Viscoelastic Surfactant-Based Fracturing Fluid. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015; p. SPE-173745-MS. [Google Scholar]
- Yan, Z.; Dai, C.; Zhao, M.; Sun, Y.; Zhao, G. Development, formation mechanism and performance evaluation of a reusable viscoelastic surfactant fracturing fluid. J. Ind. Eng. Chem. 2016, 37, 115–122. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhao, J.; Tian, J.; Lin, C.; Mao, J. Development of a sulfonic gemini zwitterionic viscoelastic surfactant with high salt tolerance for seawater-based clean fracturing fluid. Chem. Eng. Sci. 2019, 207, 688–701. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Zhou, Z.; Abass, H.; Li, X.; Bearinger, D.; Frank, W. Mechanisms of imbibition during hydraulic fracturing in shale formations. J. Pet. Sci. Eng. 2016, 141, 125–132. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, F.; Wang, S.; Fan, H.; Wen, D.; Gao, S.; Tian, Y.; Yang, X.; He, H.; Zhang, X. Mechanisms of Spontaneous Imbibition and Wettability Reversal of Sandstone Cores by a Novel Imbibition Agent. Energy Fuels 2022, 36, 1316–1325. [Google Scholar] [CrossRef]
- Dai, C.; Wang, K.; Liu, Y.; Li, H.; Wei, Z.; Zhao, M. Reutilization of Fracturing Flowback Fluids in Surfactant Flooding for Enhanced Oil Recovery. Energy Fuels 2015, 29, 2304–2311. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Aleshina, A.L.; Arkharova, N.A.; Orekhov, A.S.; Kuklin, A.I.; Philippova, O.E. Disruption of cationic/anionic viscoelastic surfactant micellar networks by hydrocarbon as a basis of enhanced fracturing fluids clean-up. Nanomaterials 2020, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Mao, J.; Mao, J.; Jiang, Q.; Fu, M.; Lin, C.; Chen, A.; Cun, M.; Du, A.; Xiao, S. The role of KCl in cationic Gemini viscoelastic surfactant based clean fracturing fluids. Colloid Surf. A Physicochem. Eng. Asp. 2020, 606, 125510. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H.; Zhang, W. High performance clean fracturing fluid using a new tri-cationic surfactant. Polymers 2018, 10, 535. [Google Scholar] [CrossRef]
- Mpelwa, M.; Tang, S.; Jin, L.; Hu, R.; Wang, C.; Hu, Y. The study on the properties of the newly extended Gemini surfactants and their application potentials in the petroleum industry. J. Pet. Sci. Eng. 2020, 186, 106799. [Google Scholar] [CrossRef]
- Hou, B.; Jia, R.; Fu, M.; Wang, Y.; Ma, C.; Jiang, C.; Yang, B. A novel high temperature tolerant and high salinity resistant gemini surfactant for enhanced oil recovery. J. Mol. Liq. 2019, 296, 112114. [Google Scholar] [CrossRef]
- Chu, Z.L.; Feng, Y.J. Thermo-switchable surfactant gel. Chem. Commun. 2011, 47, 7191–7193. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Zhu, Z.X.; Dai, L.Y.; Liu, L.Y.; Shi, W. Introducing Hydroxyl into Cationic Surfactants as Viscoelastic Surfactant Fracturing Fluid with High Temperature Resistance. Russ. J. Appl. Chem. 2016, 89, 2016–2026. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, X.; Wu, Y.; Dai, C.; Gao, M.; Yan, R.; Cheng, Y.; Li, Y.; Song, X.; Wang, X.; et al. Development, performance evaluation and enhanced oil recovery regulations of a zwitterionic viscoelastic surfactant fracturing-flooding system. Colloid Surf. A Physicochem. Eng. Asp. 2021, 630, 127568. [Google Scholar] [CrossRef]
- Mao, J.C.; Yang, X.J.; Wang, D.L.; Li, Y.M.; Zhao, J.Z. A novel gemini viscoelastic surfactant (VES) for fracturing fluids with good temperature stability. RSC Adv. 2016, 6, 88426–88432. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.C.; Zhao, J.Z.; Zhang, W.L.; Liao, Z.J.; Xu, T.; Du, A.Q.; Zhang, Z.Y.; Yang, X.J.; Ni, Y.H. Preparation of a novel sulfonic Gemini zwitterionic viscoelastic surfactant with superior heat and salt resistance using a rigid-soft combined strategy. J. Mol. Liq. 2020, 318, 114057. [Google Scholar] [CrossRef]
- Cun, M.; Mao, J.C.; Sun, H.L.; Wei, G.; Tang, F.; Zhang, W.L.; Tian, J.Z.; Yang, X.J.; Lin, C.; Huang, Z.G. Development of a novel temperature-resistant and salt-resistant double-cationic surfactant with “super thick hydration layer” for clean fracturing fluid. Colloid Surf. A Physicochem. Eng. Asp. 2021, 617, 126306. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, Y.; Yang, W.; Wang, J.; Fan, Y.; Lu, J. Experimental study of sulfonate gemini surfactants as thickeners for clean fracturing fluids. Energies 2018, 11, 3182. [Google Scholar] [CrossRef]
- Mata, J.; Varade, D.; Bahadur, P. Aggregation behavior of quaternary salt based cationic surfactants. Thermochim. Acta 2005, 428, 147–155. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Zhao, M.; Zou, C.; Feng, Y.; Wu, Y.; Dai, C. The effect and enhancement mechanism of hydrophobic interaction and electrostatic interaction on zwitterionic wormlike micelles. Colloid Surf. A Physicochem. Eng. Asp. 2022, 648, 129424. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Sang, Q.; Han, C.; Lv, D.; Zhang, Q.; Liu, M.; Wei, X. Comparative study of the viscoelastic micellar solutions of R 16 HTAC and CTAC in the presence of sodium salicylate. J. Mol. Liq. 2017, 234, 149–156. [Google Scholar] [CrossRef]
- Wei, X.; Geng, P.; Han, C.; Guo, Y.; Chen, X.; Zhang, J.; Zhang, Y.; Sun, D.; Zhou, S. Rheological Properties of Viscoelastic Solutions in a Cationic Surfactant–Organic Salts–Water System. Ind. Eng. Chem. Res. 2016, 55, 5556–5564. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Kaler, E.W. Highly viscoelastic wormlike micellar solutions formed by cationic surfactants with long unsaturated tails. Langmuir 2001, 17, 300–306. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Fritz, G.; Kaler, E.W. Wormlike micelles formed by synergistic self-assembly in mixtures of anionic and cationic surfactants. Langmuir 2002, 18, 3797–3803. [Google Scholar] [CrossRef]
- Kumar, R.; Kalur, G.C.; Ziserman, L.; Danino, D.; Raghavan, S.R. Wormlike micelles of a C22-tailed zwitterionic betaine surfactant: From viscoelastic solutions to elastic gels. Langmuir 2007, 23, 12849–12856. [Google Scholar] [CrossRef]
- Shulevich, Y.V.; Ilyin, S.; Dukhanina, E.; Ozerin, A.; Tutaev, D.; Navrotskii, A.; Kulichikhin, V.; Novakov, I. Rheological properties of associates of ionic monomers with micelles of oppositely charged surfactants. Russ. Chem. Bull. 2016, 65, 1161–1166. [Google Scholar] [CrossRef]
- Reinicke, A.; Rybacki, E.; Stanchits, S.; Huenges, E.; Dresen, G. Hydraulic fracturing stimulation techniques and formation damage mechanisms—Implications from laboratory testing of tight sandstone–proppant systems. Geochemistry 2010, 70, 107–117. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhang, Z.; Yang, B.; Zhang, Y.; Zhao, J. Study of a novel gemini viscoelastic surfactant with high performance in clean fracturing fluid application. Polymers 2018, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, X.; Gao, Z.; Wu, X.; Li, L.; Guo, X.; Yang, Y. Preparation and performance evaluation of nanoparticle modified clean fracturing fluid. Colloid Surf. A Physicochem. Eng. Asp. 2022, 636, 128117. [Google Scholar] [CrossRef]
- Mao, J.; Yang, X.; Chen, Y.; Zhang, Z.; Zhang, C.; Yang, B.; Zhao, J. Viscosity reduction mechanism in high temperature of a Gemini viscoelastic surfactant (VES) fracturing fluid and effect of counter-ion salt (KCl) on its heat resistance. J. Pet. Sci. Eng. 2018, 164, 189–195. [Google Scholar] [CrossRef]
- Parris, M.; Mirakyan, A.; Abad, C.; Chen, Y.; Mueller, F. A New Shear-Tolerant High-Temperature Fracturing Fluid. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009; p. SPE-121755-MS. [Google Scholar]
- Commission, N. Recommended Practices on Measuring the Properties of Waterbased Fracturing Fluid; Chinese Oil and Gas Industry Standards: Beijing, China, 2008. [Google Scholar]
- Almubarak, T.; Ng, J.H.; Sokhanvarian, K.; Khaldi, M.; Nasr-El-Din, H.A. Development of a Mixed Polymer Hydraulic Fracturing Fluid for High Temperature Applications. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Houston, TX, USA, 23–25 July 2018; p. URTEC-2896329-MS. [Google Scholar]




| Q (mL·min−1) | A (cm2) | k (m2) | L (cm) | φ | C (m·s−1/2) |
|---|---|---|---|---|---|
| 0.54 | 4.91 | 1.21 × 10−15 | 4.97 | 19.35% | 2.90 × 10−4 |
| Core No. | Fracturing Fluid Type | Length (cm) | Diameter (cm) | Initial Permeability (mD) | Damage Permeability (mD) | Damage Rate (%) |
|---|---|---|---|---|---|---|
| 1 | Surfactant gel | 5.01 | 2.50 | 3.85 | 3.61 | 6.23% |
| 2 | guanidine gum | 4.98 | 2.50 | 3.79 | 2.63 | 30.61% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Dai, C.; Gao, M.; Wang, X.; Liu, S.; Jin, X.; Li, T.; Zhao, M. Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C. Gels 2022, 8, 600. https://doi.org/10.3390/gels8100600
Liu P, Dai C, Gao M, Wang X, Liu S, Jin X, Li T, Zhao M. Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C. Gels. 2022; 8(10):600. https://doi.org/10.3390/gels8100600
Chicago/Turabian StyleLiu, Peng, Caili Dai, Mingwei Gao, Xiangyu Wang, Shichun Liu, Xiao Jin, Teng Li, and Mingwei Zhao. 2022. "Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C" Gels 8, no. 10: 600. https://doi.org/10.3390/gels8100600
APA StyleLiu, P., Dai, C., Gao, M., Wang, X., Liu, S., Jin, X., Li, T., & Zhao, M. (2022). Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 °C. Gels, 8(10), 600. https://doi.org/10.3390/gels8100600






