Cellulose-g-poly(2-(dimethylamino)ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application
Abstract
1. Introduction
2. Results and Discussion
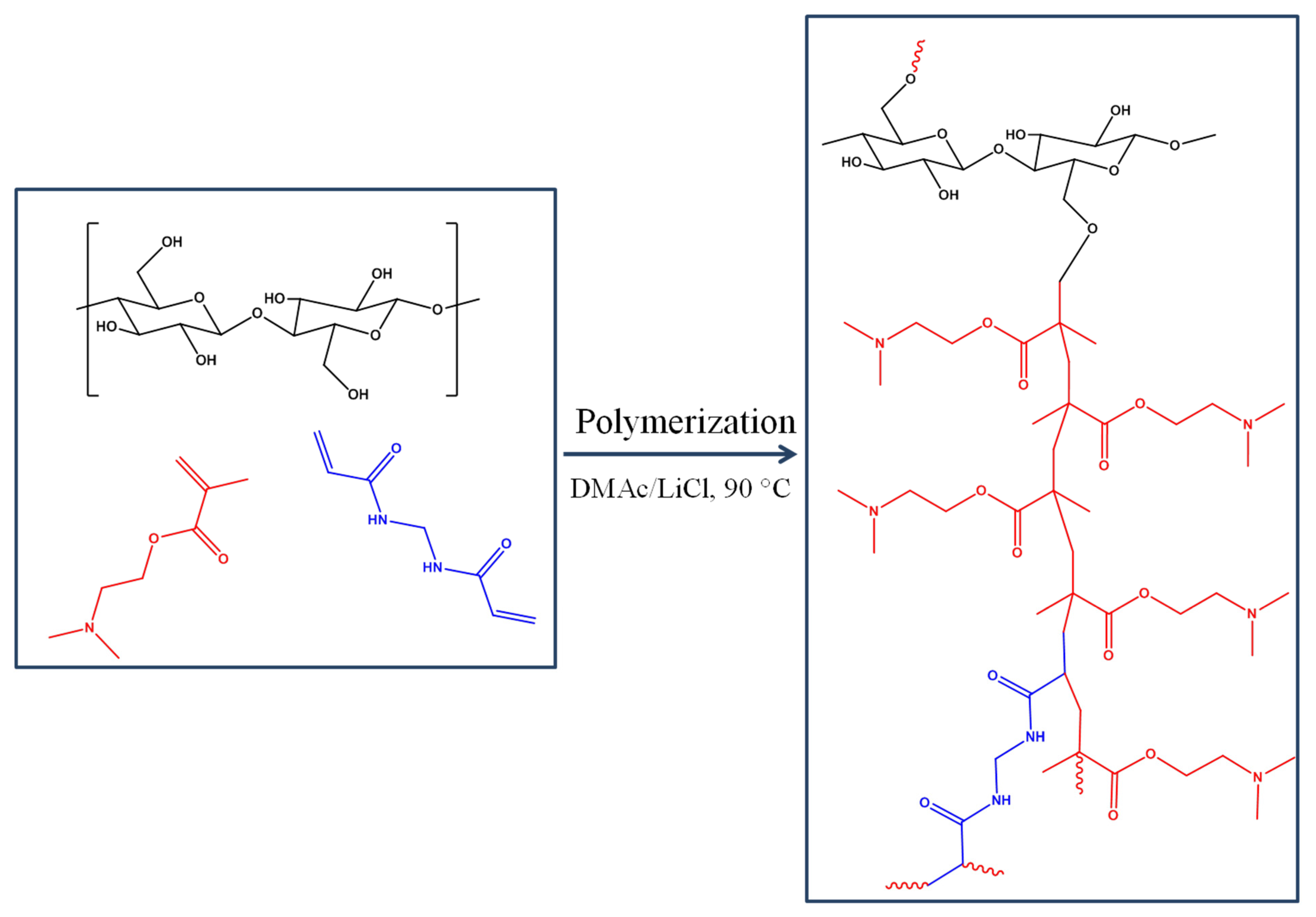
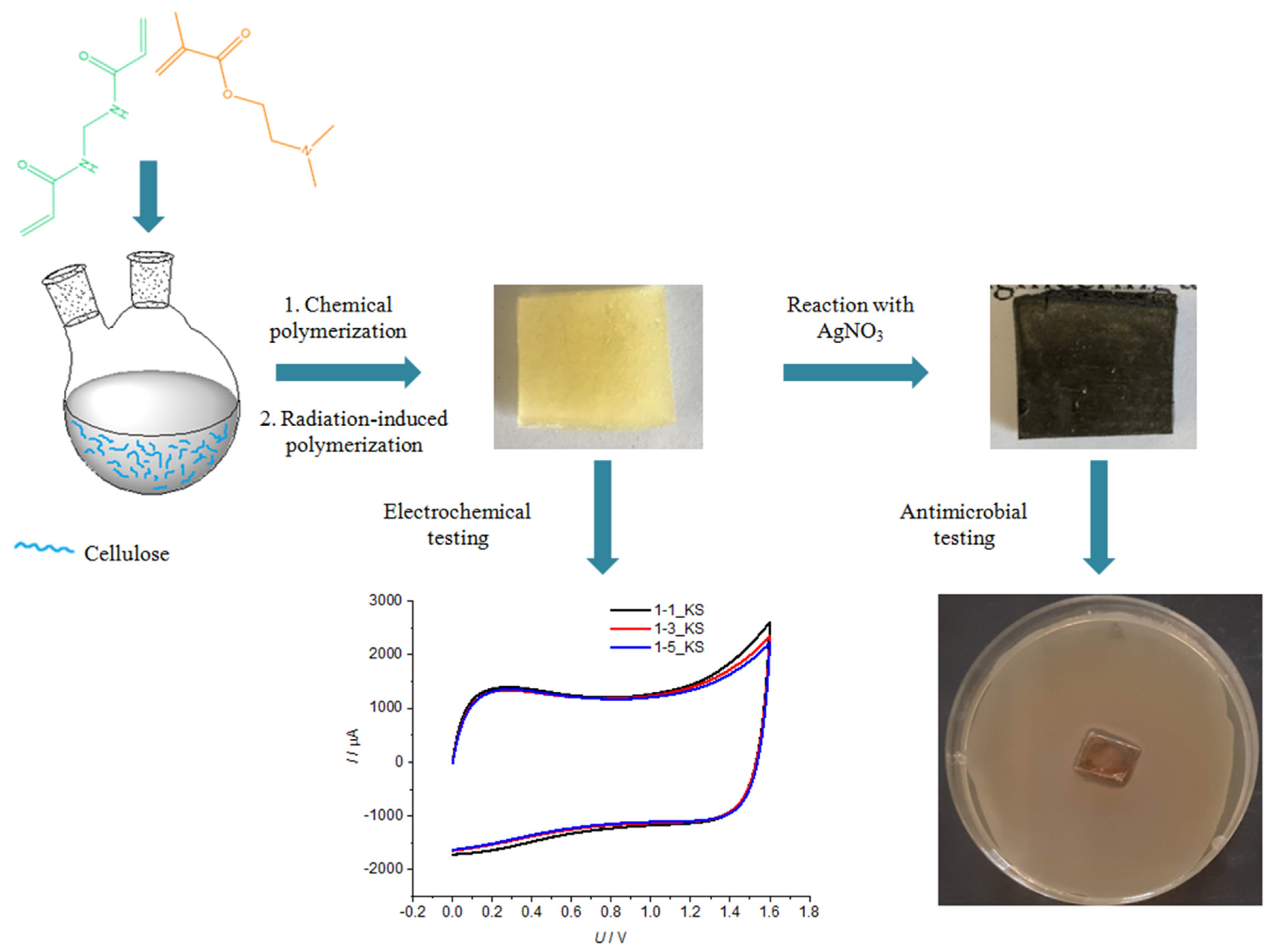
2.1. Polymerization Reaction
2.2. Structural Characterization of Synthesized Cel-g-PDMAEMA Hydrogels
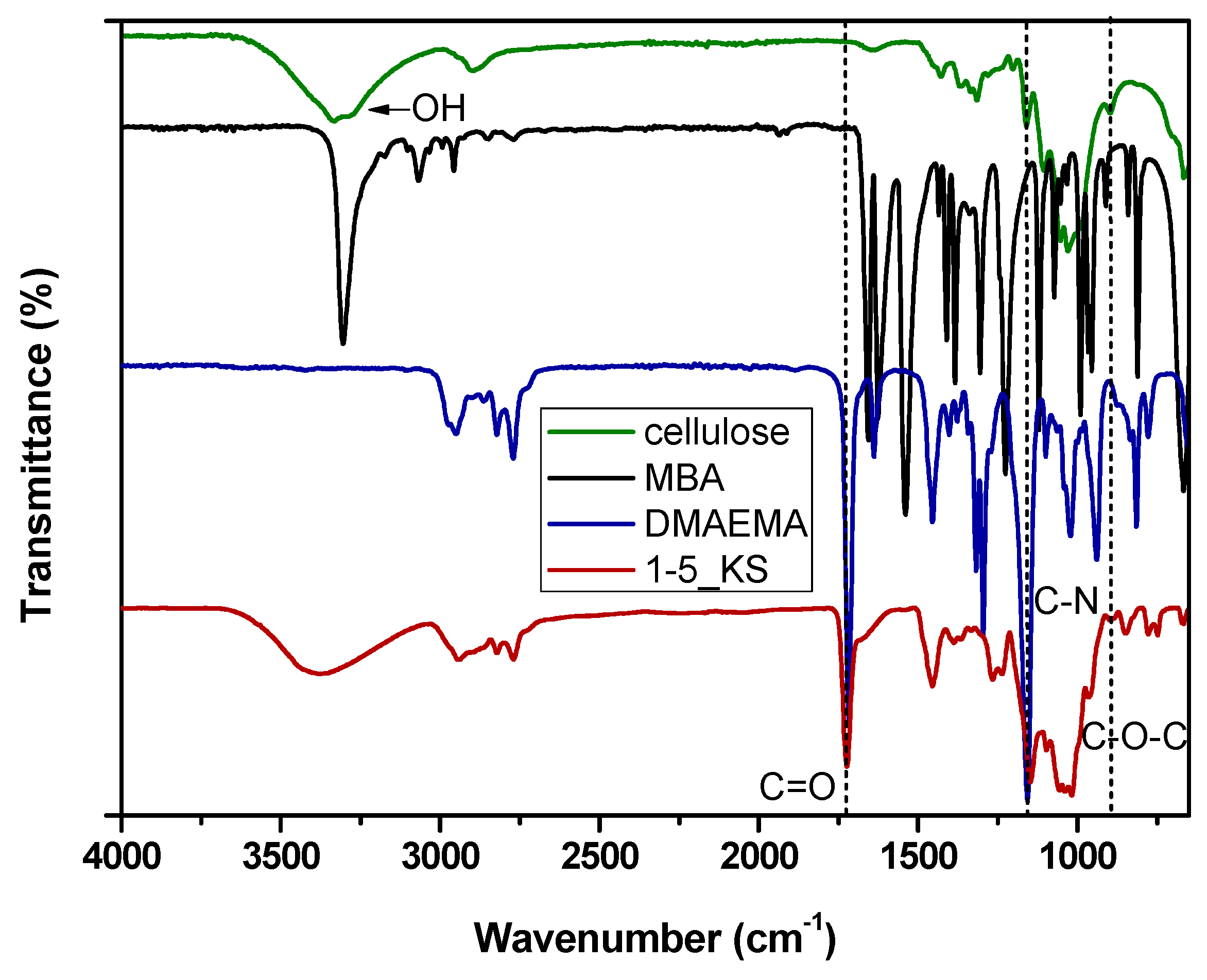
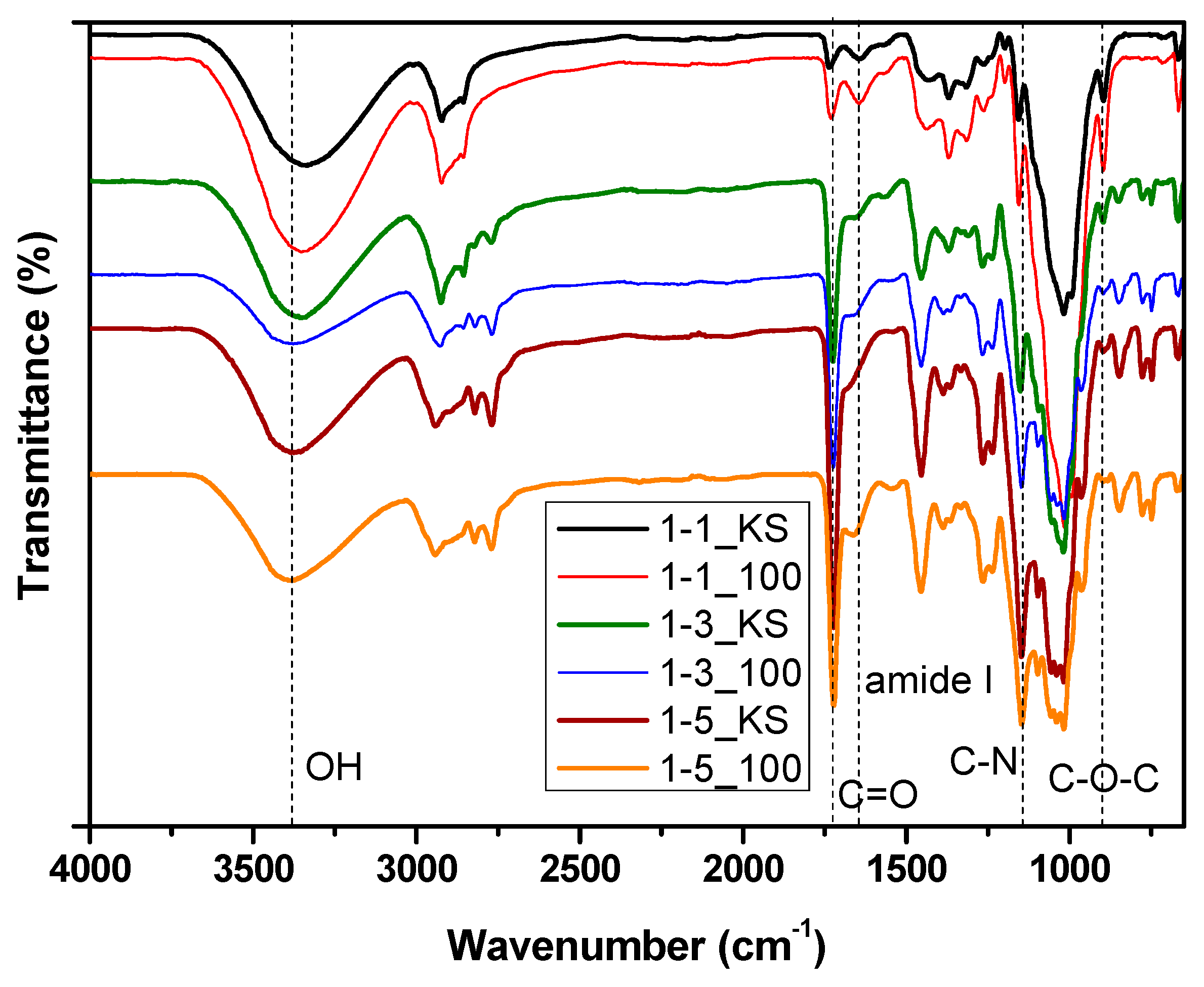
2.2.1. FTIR Spectroscopy Analysis
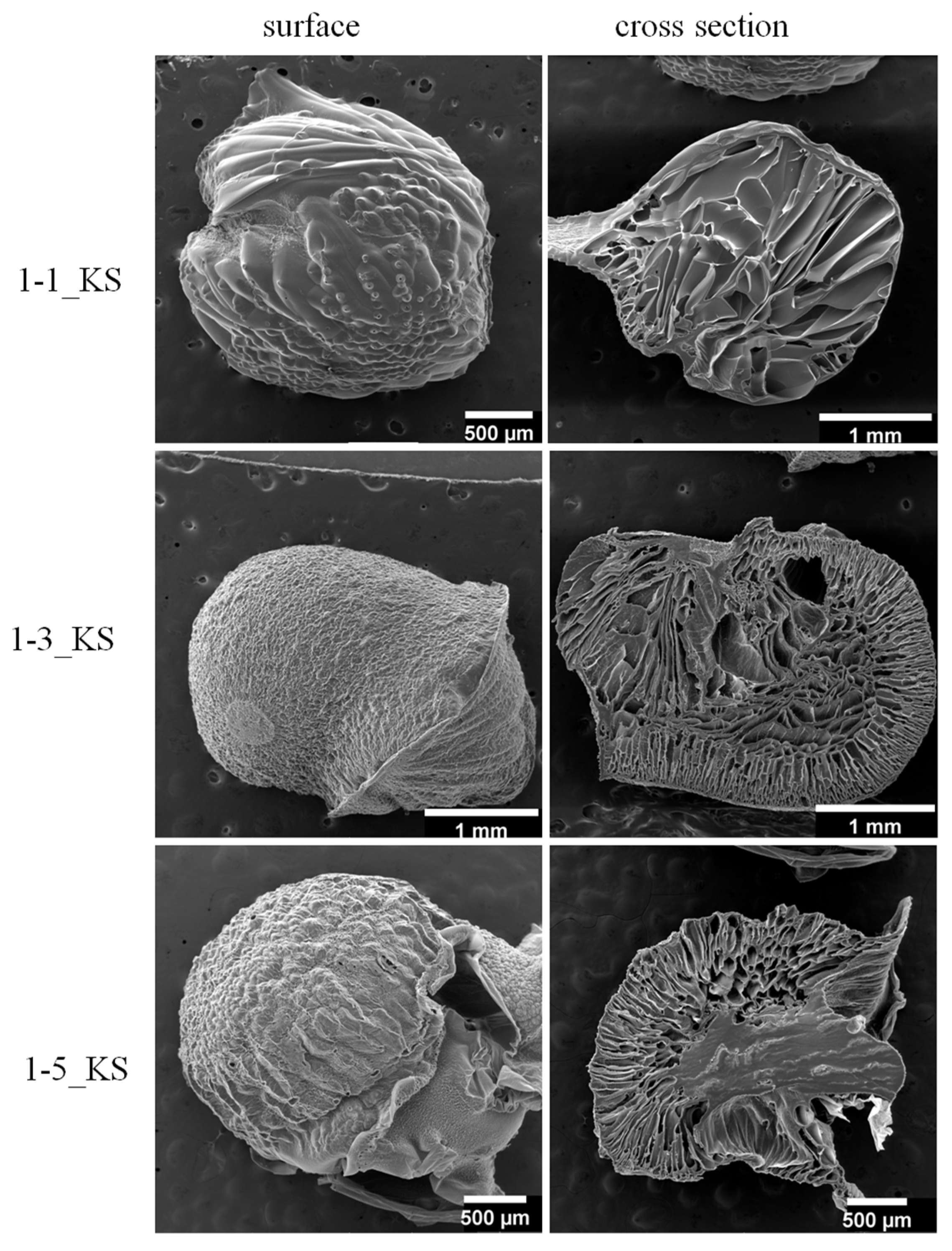
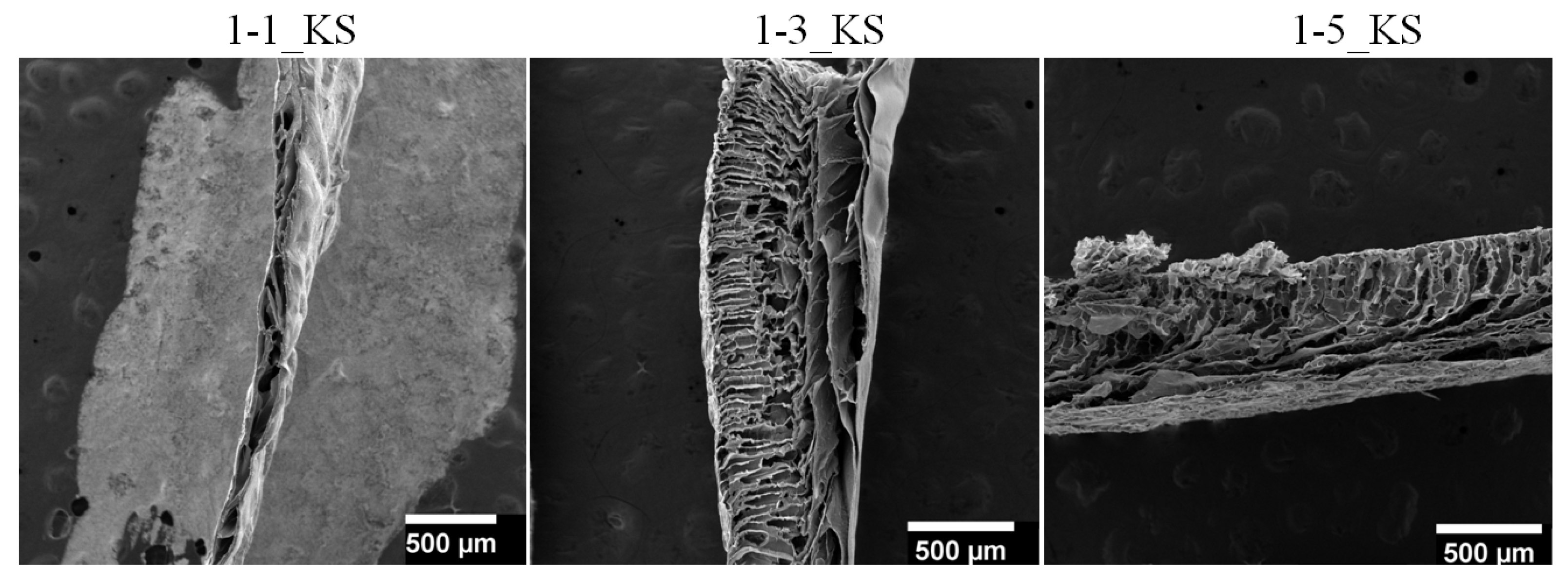
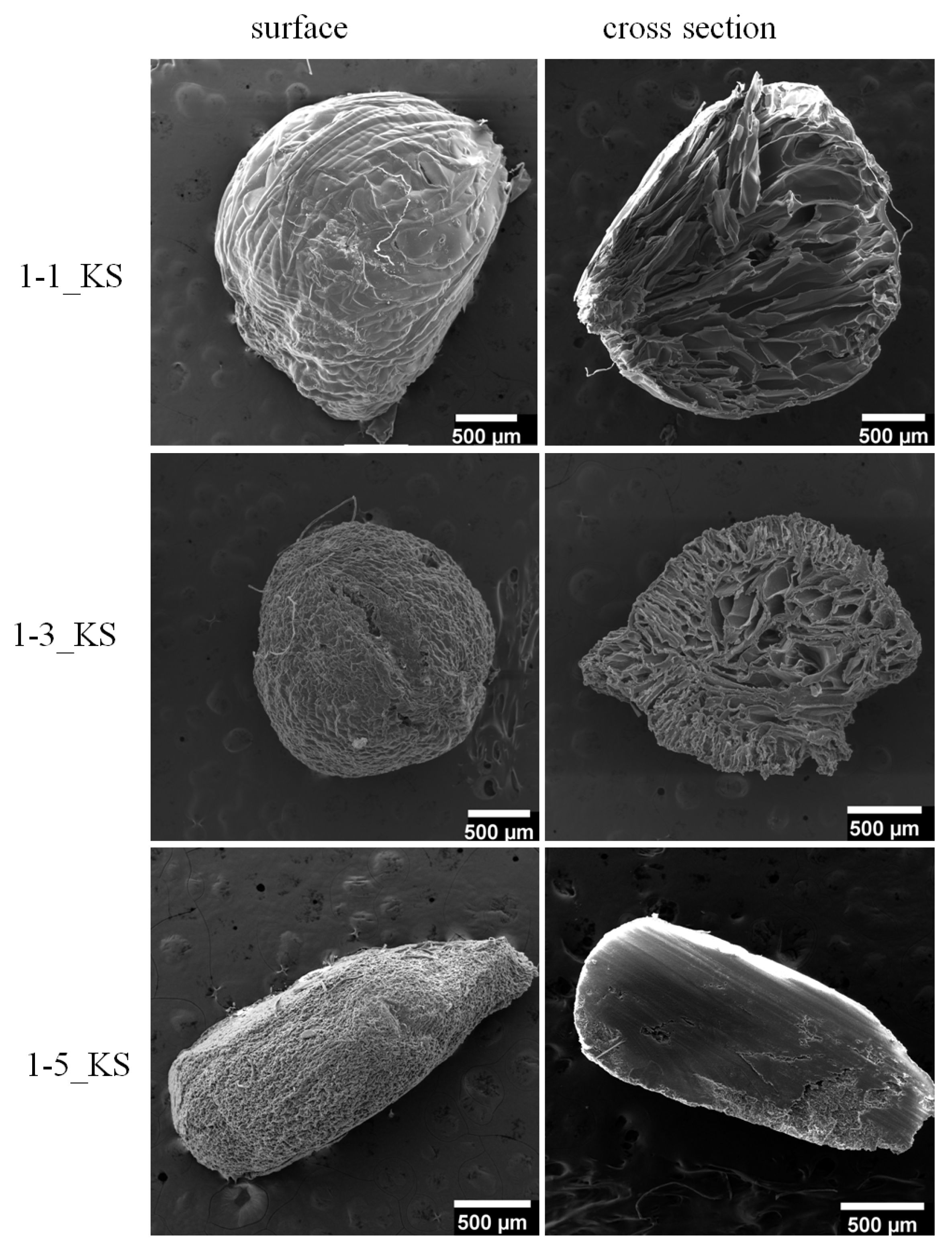
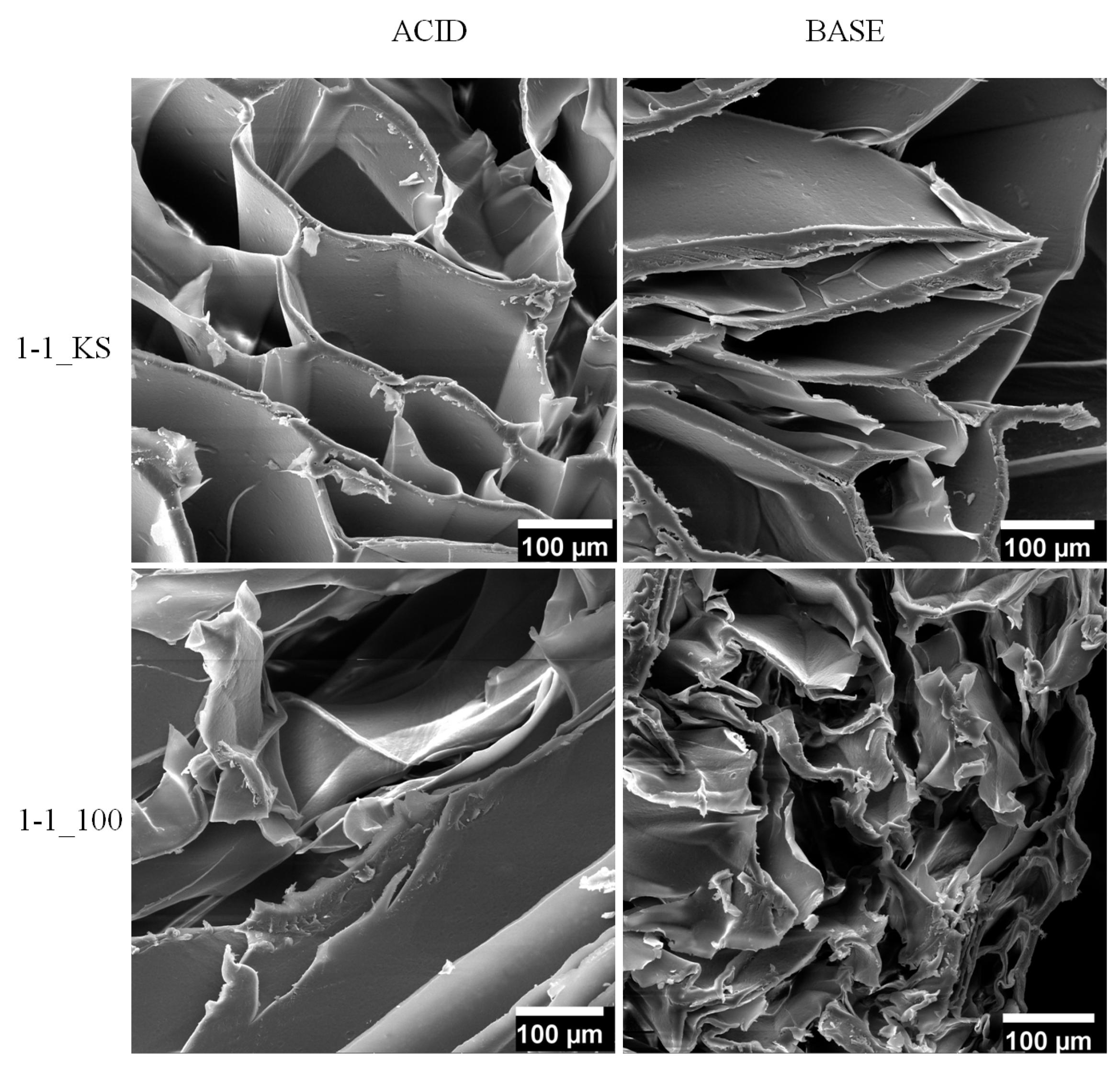
2.2.2. Scanning Electron Microscopy Analysis
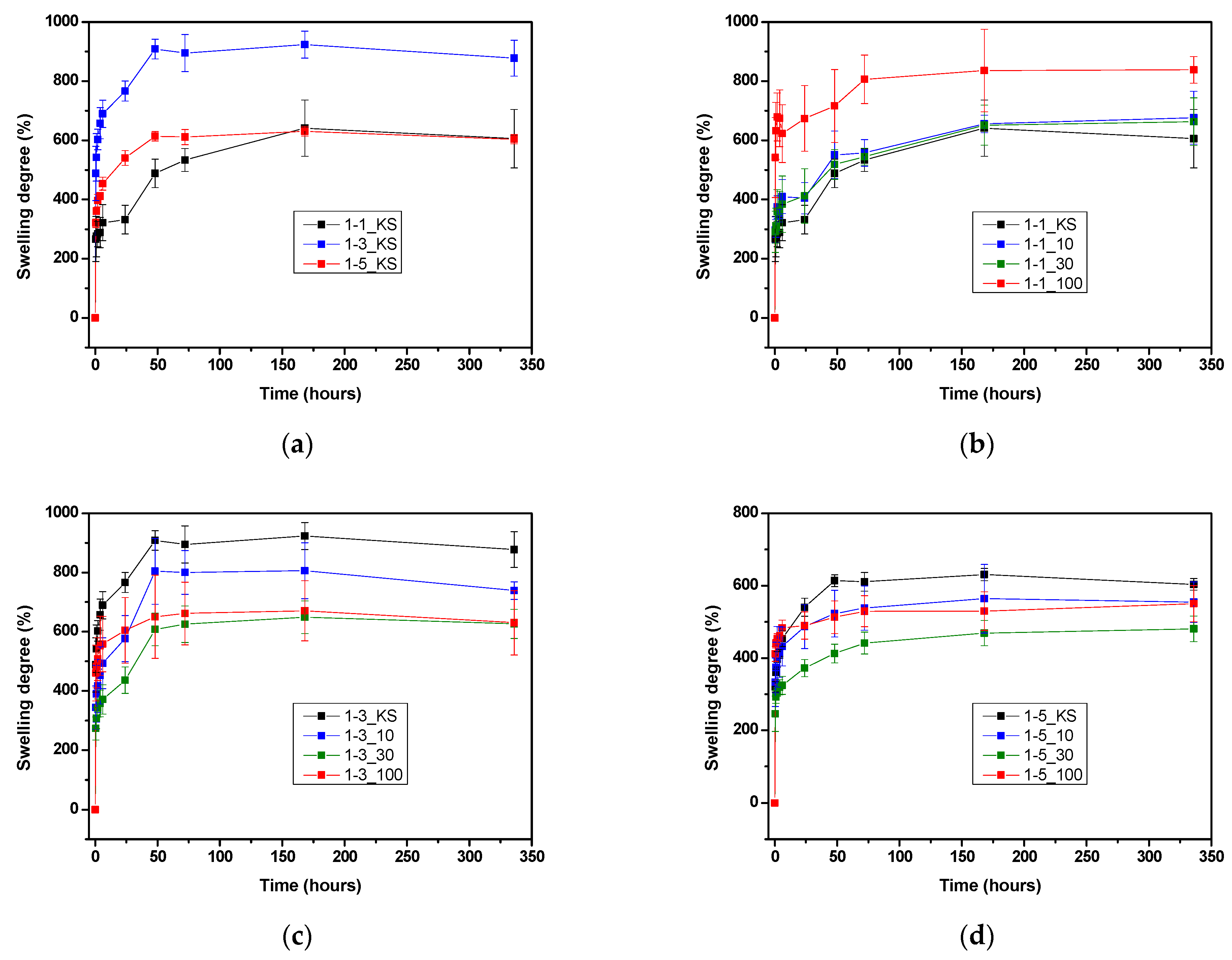
2.3. Swelling Study
2.3.1. Equilibrium Hydrogel Swelling at 20 °C and pH 5.5
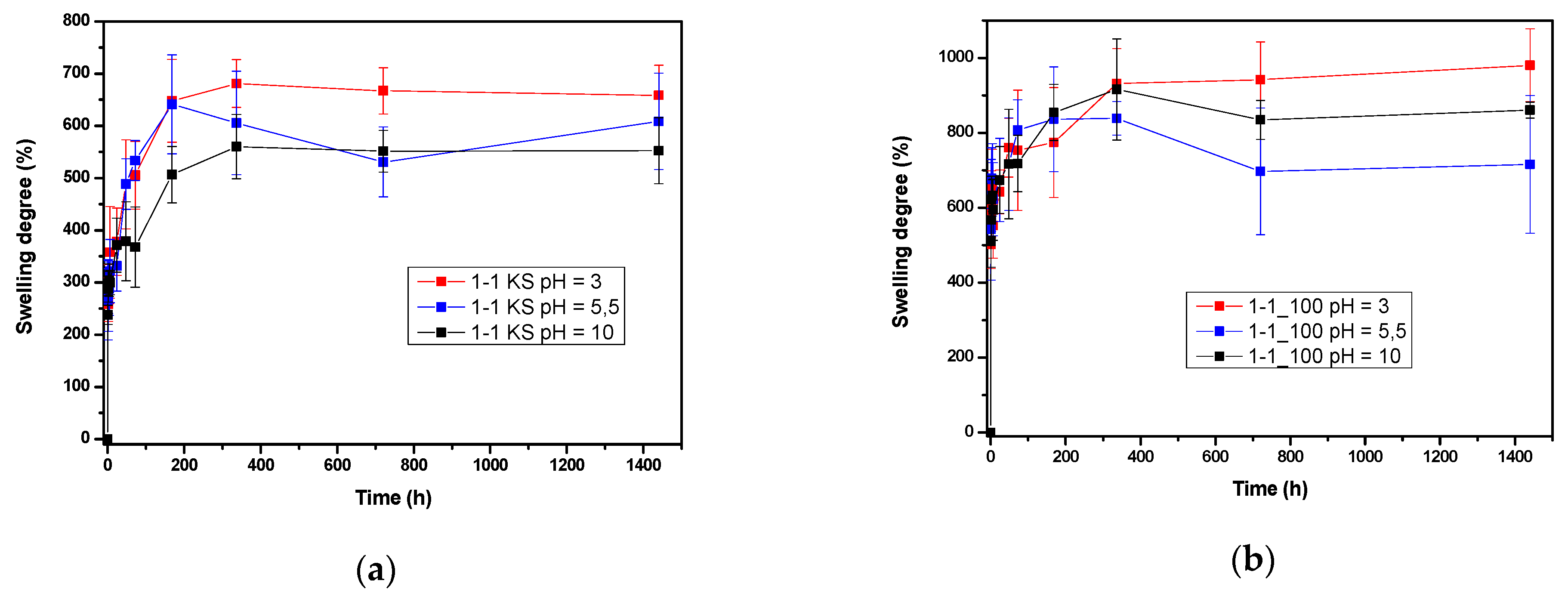
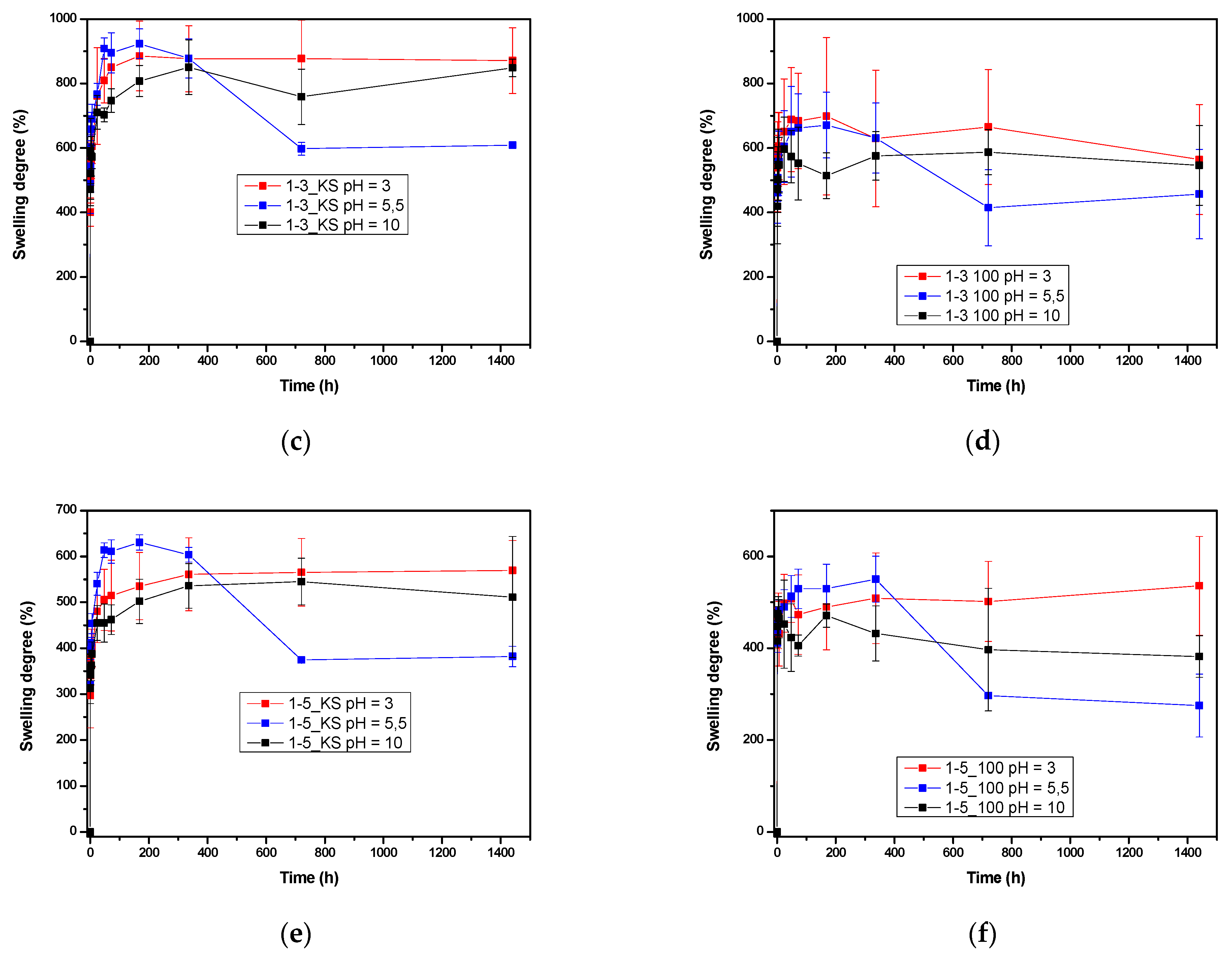
2.3.2. Comparison Equilibrium Hydrogel Swelling at 20 °C and pH = 3.0, pH 5.5 and pH 10
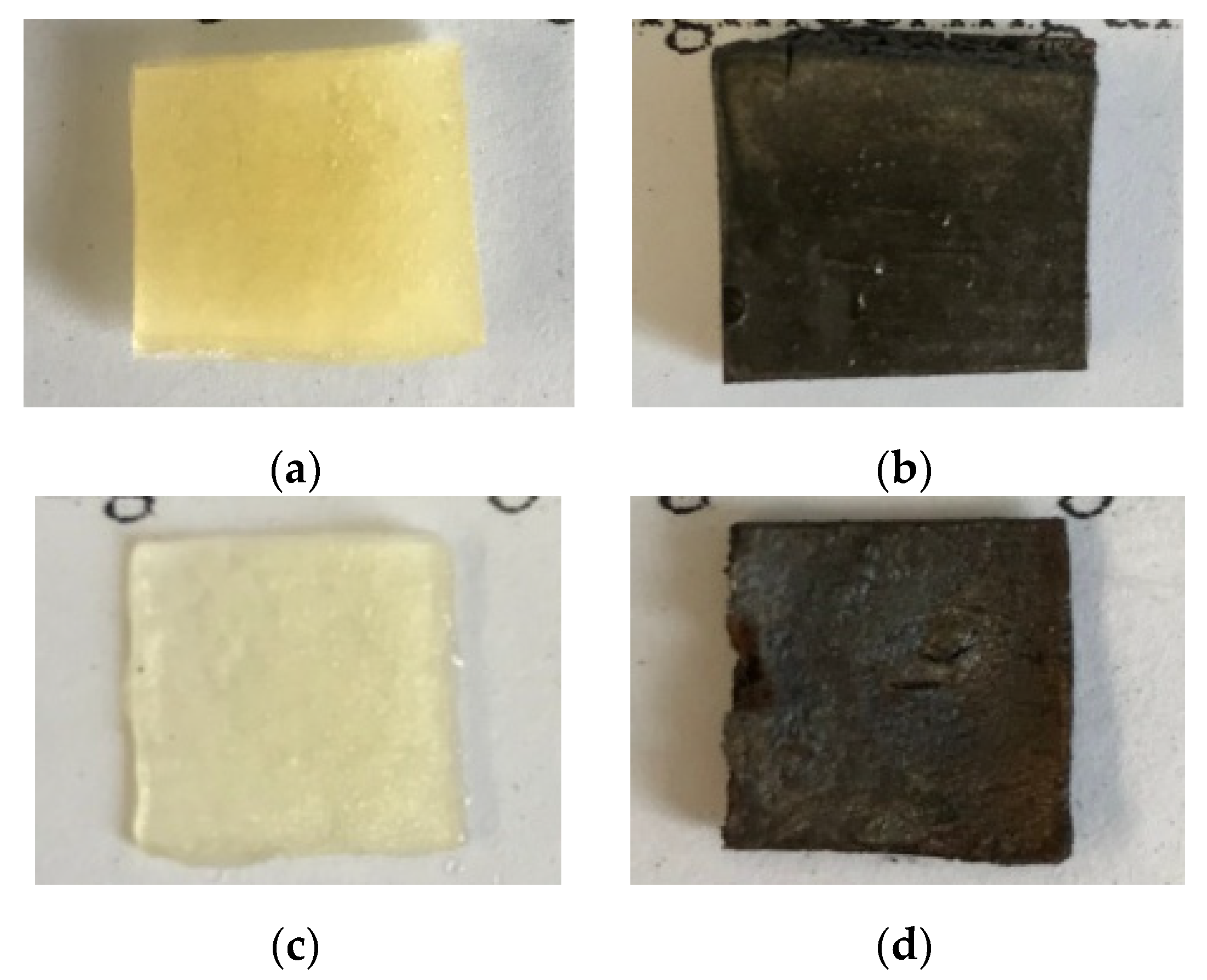
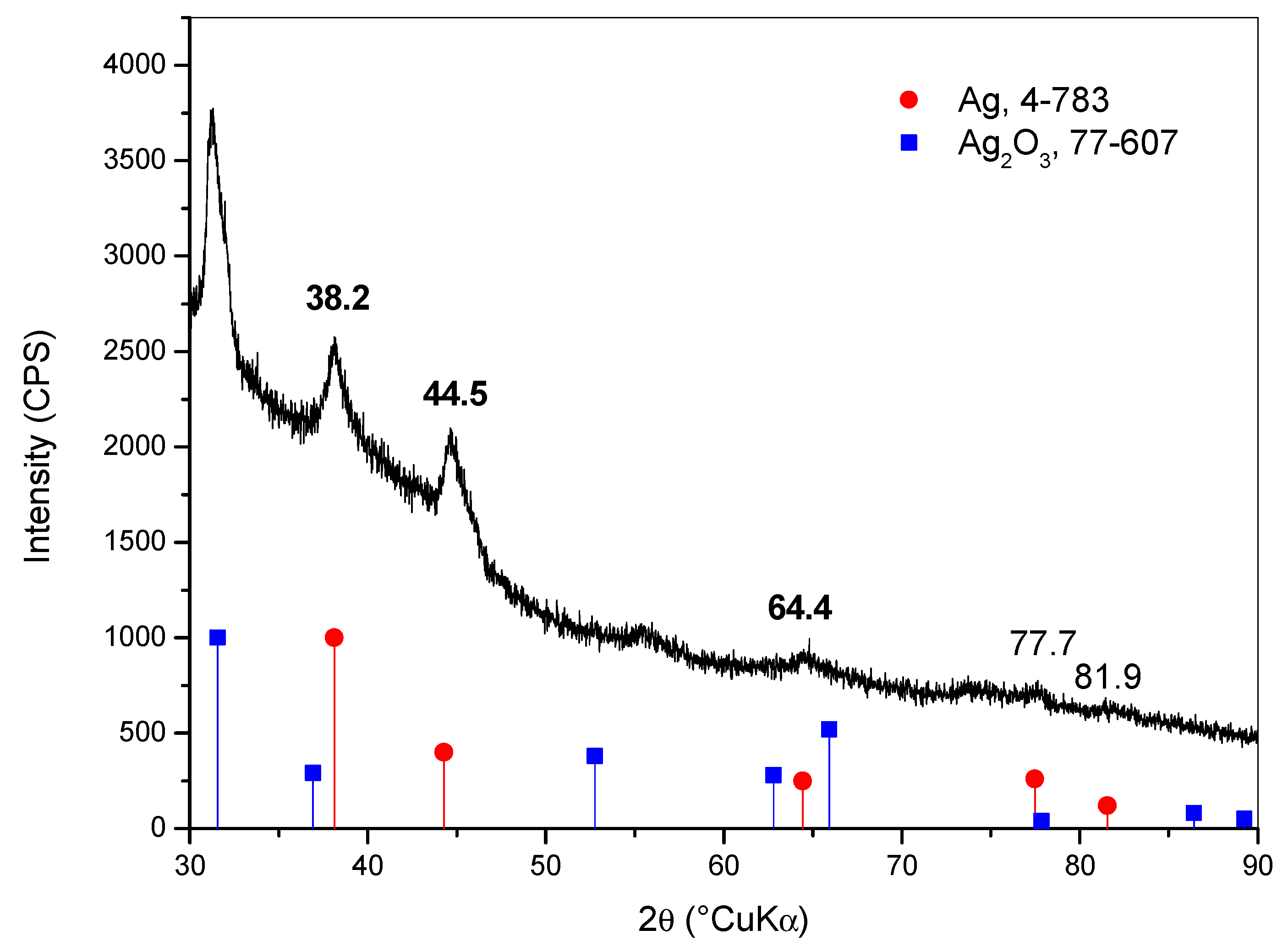
2.4. Decorating Hydrogel Films with Silver Particles
2.5. Antimicrobial Testing
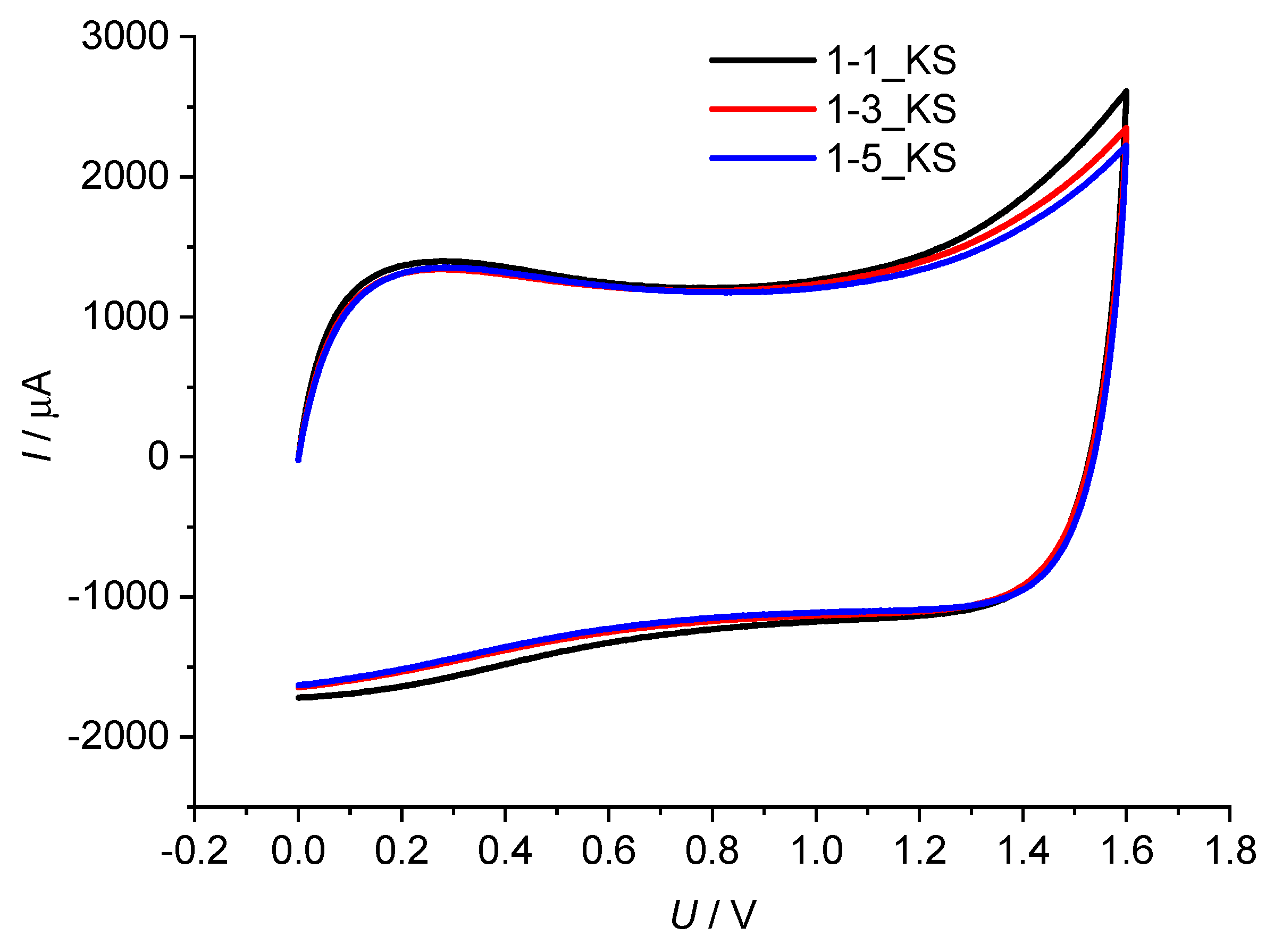
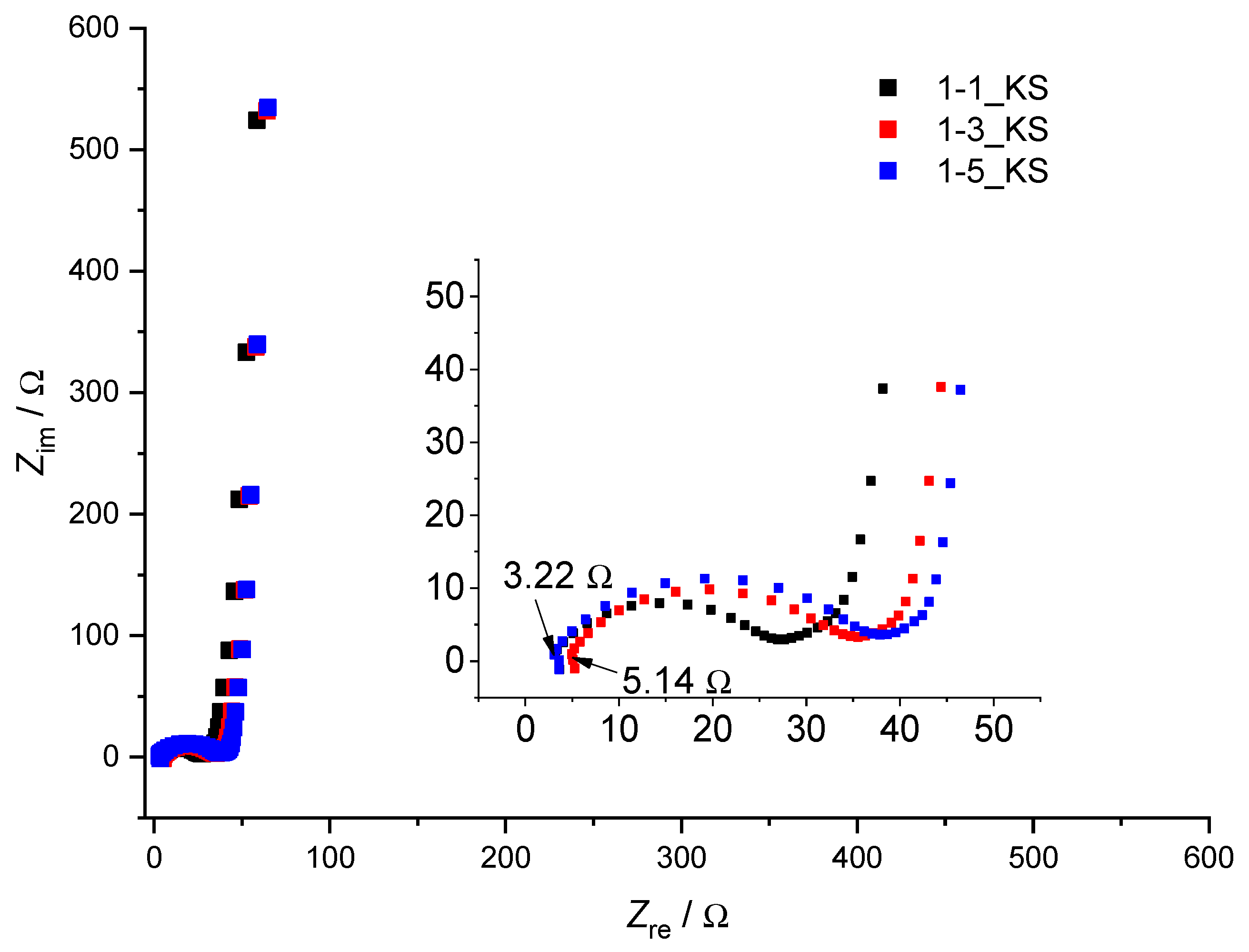
2.6. Electrochemical Testing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Method of Cellulose/Poly(dimethylaminoethyl methacrylate) Hydrogles (Cel-g-PDMAEMA) Synthesis
4.2.1. Gamma-Ray Irradiation of Hydrogel
4.2.2. Preparation of Hydrogel Films
4.2.3. Decorating Hydrogel Films with Silver Particles
4.3. Structural Confirmation of Cel-g-PDMAEMA Hydrogels
4.3.1. Fourier Transform Infrared Spectroscopy
4.3.2. Scanning Electron Microscopy Analysis
4.3.3. Swelling Study
4.4. X-ray Diffraction of Hydrogel Films Decorated with Silver Particles
4.5. Antibacterial Properties
4.6. Electrochemical Testing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent Advances in Smart Hydrogels for Biomedical Applications: From Self-Assembly to Functional Approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.S. Artificial Small-Diameter Blood Vessels: Materials, Fabrication, Surface Modification, Mechanical Properties, and Bioactive Functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef]
- Ruben, M. Soft Contact Lenses: Clinical and Appled Technology. Arch. Ophthalmol. 1979, 97, 989. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive Hydrogels for Bone Regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Tran, H.D.N.; Park, K.D.; Ching, Y.C.; Huynh, C.; Nguyen, D.H. A Comprehensive Review on Polymeric Hydrogel and Its Composite: Matrices of Choice for Bone and Cartilage Tissue Engineering. J. Ind. Eng. Chem. 2020, 89, 58–82. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef]
- Peppas, N.A.; Van Blarcom, D.S. Hydrogel-Based Biosensors and Sensing Devices for Drug Delivery. J. Control. Release 2016, 240, 142–150. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Ali, A.; Shabeer, M.; Shah, M.N.; Haq, F.; Iqbal, M.; Ullah, R.; Khan, F.U. Manufactures of Bio-Degradable and Bio-Based Polymers for Bio-Materials in the Pharmaceutical Field. J. Appl. Polym. Sci. 2022, 139, e52624. [Google Scholar] [CrossRef]
- Gil, C.J.; Li, L.; Hwang, B.; Cadena, M.; Theus, A.S.; Finamore, T.A.; Bauser-Heaton, H.; Mahmoudi, M.; Roeder, R.K.; Serpooshan, V. Tissue Engineered Drug Delivery Vehicles: Methods to Monitor and Regulate the Release Behavior. J. Control. Release 2022, 349, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Vega, M.A.; Rodríguez, V.; Esteban, P.P.; Martín del Valle, E.M. Biodegradable Gellan Gum Hydrogels Loaded with Paclitaxel for HER2+ Breast Cancer Local Therapy. Carbohydr. Polym. 2022, 294, 119732. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical Translation of Long-Acting Drug Delivery Formulations. Nat. Rev. Mater. 2022, 7, 406–420. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A Novel Hydrogel Based on Renewable Materials for Agricultural Application. Int. J. Polym. Sci. 2020, 2020, 8363418. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P. Starch-Chitosan Hydrogels for the Controlled-Release of Herbicide in Agricultural Applications: A Study on the Effect of the Concentration of Raw Materials and Crosslinkers. J. Polym. Environ. 2022, 30, 2448–2461. [Google Scholar] [CrossRef]
- Abobatta, W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Neethu, T.M.; Dubey, P.K.; Kaswala, A.R. Prospects and Applications of Hydrogel Technology in Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3155–3162. [Google Scholar] [CrossRef]
- Khan, A.; Othman, M.B.H.; Razak, K.A.; Akil, H.M. Synthesis and Physicochemical Investigation of Chitosan-PMAA-Based Dual-Responsive Hydrogels. J. Polym. Res. 2013, 20, 273. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and Their Medical Applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Yildiz, U.; Kemik, Ö.F.; Hazer, B. The Removal of Heavy Metal Ions from Aqueous Solutions by Novel PH-Sensitive Hydrogels. J. Hazard. Mater. 2010, 183, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.M.; Patel, R.G.; Patel, M.P. Nickel and Copper Removal Study from Aqueous Solution Using New Cationic Poly[Acrylamide/N,N-DAMB/N,N-DAPB] Super Absorbent Hydrogel. J. Appl. Polym. Sci. 2011, 119, 2485–2493. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, Y.; Yin, G.; Li, X. Adsorption of Copper (II) and Lead (II) Ions onto Cottonseed Protein-PAA Hydrogel Composite. Polym.-Plast. Technol. Eng. 2012, 51, 612–619. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Wu, W.; Jing, Y.; Dai, H.; Fang, G. Nanocellulose/Poly(2-(dimethylamino)ethyl Methacrylate)Interpenetrating Polymer Network Hydrogels for Removal of Pb(II) and Cu(II) Ions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 474–480. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Vimala, K.; Varsha, T.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.K.; Mohana Raju, K. Controlling of silver nanoparticles structure by hydrogel networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef]
- Rodríguez Nuñez, Y.A.; Castro, R.I.; Arenas, F.A.; López-Cabaña, Z.E.; Carreño, G.; Carrasco-Sánchez, V.; Marican, A.; Villaseñor, J.; Vargas, E.; Santos, L.S.; et al. Preparation of Hydrogel/Silver Nanohybrids Mediated by Tunable-Size Silver Nanoparticles for Potential Antibacterial Applications. Polymers 2019, 11, 716. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Urošević, M.; Nikolić, L.; Petrović, D.; Stanojević, J.; Najman, S.; Nikolić, V. Intelligent Poly(N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition. Polymers 2020, 12, 1112. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Feng, Z.; Wang, M.; Cai, C.; Wang, J.; Zhang, L. Poly(2-(diethylamino)ethyl Methacrylate)-Based, PH-Responsive, Copolymeric Mixed Micelles for Targeting Anticancer Drug Control Release. Int. J. Nanomed. 2017, 12, 6857–6870. [Google Scholar] [CrossRef]
- Goracci, G.; Arbe, A.; Alegría, A.; García Sakai, V.; Rudić, S.; Schneider, G.J.; Lohstroh, W.; Juranyi, F.; Colmenero, J. Influence of Solvent on Poly(2-(dimethylamino)ethyl Methacrylate) Dynamics in Polymer-Concentrated Mixtures: A Combined Neutron Scattering, Dielectric Spectroscopy, and Calorimetric Study. Macromolecules 2015, 48, 6724–6735. [Google Scholar] [CrossRef]
- Rawlinson, L.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.M.; Brayden, D.J. Antibacterial Effects of Poly(2-(dimethylamino ethyl)methacrylate) against Selected Gram-Positive and Gram-Negative Bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, P.; Cherng, J.-Y.; Talsma, H.; Crommelin, D.J. Hennink, W. 2-(dimethylamino)ethyl Methacrylate Based (Co)Polymers as Gene Transfer Agents. J. Control. Release 1998, 53, 145–153. [Google Scholar] [CrossRef]
- Van De Wetering, P.; Schuurmans-Nieuwenbroek, N.M.E.; Van Steenbergen, M.J.; Crommelin, D.J.A.; Hennink, W.E. Copolymers of 2-(dimethylamino)ethyl Methacrylate with Ethoxytriethylene Glycol Methacrylate or N-Vinyl-Pyrrolidone as Gene Transfer Agents. J. Control. Release 2000, 64, 193–203. [Google Scholar] [CrossRef]
- De Souza, V.V.; Carretero, G.P.B.; Vitale, P.A.M.; Todeschini, Í.; Kotani, P.O.; Saraiva, G.K.V.; Guzzo, C.R.; Chaimovich, H.; Florenzano, F.H.; Cuccovia, I.M. Stimuli-Responsive Polymersomes of Poly [2-(dimethylamino) ethyl methacrylate]-b-Polystyrene. Polym. Bull. 2022, 79, 785–805. [Google Scholar] [CrossRef]
- Diaz, I.L.; Sierra, C.A.; Jérôme, V.; Freitag, R.; Perez, L.D. Target Grafting of Poly(2-(dimethylamino)ethyl methacrylate) to Biodegradable Block Copolymers. J. Polym. Sci. 2020, 58, 2168–2180. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, S.; Lv, L.; Ji, H.; Li, G.; Jiang, Z.; Wu, Y. Effect of 2-(dimethylamino) ethyl Methacrylate on Nanostructure and Properties of PH and Temperature Sensitive Cellulose-Based Hydrogels. J. Nanosci. Nanotechnol. 2020, 20, 1799–1806. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, D.; Lyu, J.; Sigen, A.; Xu, Q.; Newland, B.; Matyjaszewski, K.; Tai, H.; Wang, W. Complex Polymer Architectures through Free-Radical Polymerization of Multivinyl Monomers. Nat. Rev. Chem. 2020, 4, 194–212. [Google Scholar] [CrossRef]
- Blažic, R.; Marušić, K.; Vidović, E. Swelling and viscoelastic properties of cellulose based hydrogels prepared by free radical polymerization of dimethylaminoethyl methacrylate in cellulose solution. 2022; in press. [Google Scholar]
- Chen, W.; He, H.; Zhu, H.; Cheng, M.; Li, Y.; Wang, S. Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation. Polymers 2018, 10, 592. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose Prepared by Acid Hydrolysis of Isolated Cellulose from Sugarcane Bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Ayu Laksanawati, T.; Novarita Trisanti, P. Sumarno Synthesis and Characterization of Composite Gels Starch-Graftacrylic Acid/Bentonite (St-g-AA/B) Using N′N-methylenebisacrylamide (MBA). IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 8–14. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.S.; Nikolić, L.B.; Nikolić, V.D.; Petrović, S.D. Smart Hydrogels for Pharmaceutical Applications. Polym. Bull. 2017, 46, 278–310. [Google Scholar]
- Blažic, R.; Lenac, K.; Vidović, E. Priprava celuloznih hidrogelova modificiranih 2-dimetilaminoetil-metakrilatom i srebrovim nanočesticama. Kem. Ind. 2020, 69, 269–279. [Google Scholar] [CrossRef]
- Angelini, A.; Fodor, C.; Yave, W.; Leva, L.; Car, A.; Meier, W. PH-Triggered Membrane in Pervaporation Process. ACS Omega 2018, 3, 18950–18957. [Google Scholar] [CrossRef] [PubMed]
- Takács, E.; Wojnárovits, L.; Borsa, J.; Földváry, C.; Hargittai, P.; Zöld, O. Effect of γ-Irradiation on Cotton-Cellulose. Radiat. Phys. Chem. 1999, 55, 663–666. [Google Scholar] [CrossRef]
- Almeida do Nascimento, H.; Didier Pedrosa Amorim, J.; José Galdino da Silva, C., Jr.; D’Lamare Maia de Medeiros, A.; Fernanda de Santana Costa, A.; Carla Napoleão, D.; Maria Vinhas, G.; Asfora Sarubbo, L. Influence of Gamma Irradiation on the Properties of Bacterial Cellulose Produced with Concord Grape and Red Cabbage Extracts. Curr. Res. Biotechnol. 2022, 4, 119–128. [Google Scholar] [CrossRef]
- Driscoll, M.; Stipanovic, A.; Winter, W.; Cheng, K.; Manning, M.; Spiese, J.; Galloway, R.A.; Cleland, M.R. Electron Beam Irradiation of Cellulose. Radiat. Phys. Chem. 2009, 78, 539–542. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable Porous Carbonaceous Materials from Renewable Resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef]
- Henryk, K.K.; Kinga, W.; Sławomir, B. The Effect of Gamma Radiation on the Supramolecular Structure of Pine Wood Cellulose in Situ Revealed by X-ray Diffraction. Electron. J. Polish Agric. Univ. 2004, 7. Available online: http://www.ejpau.media.pl/volume7/issue1/wood/art-06.html (accessed on 27 July 2022).
- Salama, A.; Shukry, N.; El-Sakhawy, M. Carboxymethyl Cellulose-g-Poly(2-(dimethylamino) ethyl Methacrylate) Hydrogel as Adsorbent for Dye Removal. Int. J. Biol. Macromol. 2015, 73, 72–75. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Antimicrobial Activity against Representative Microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W. Nanosilver as a New Generation of Silver Catalysts in Organic Transformations for Efficient Synthesis of Fine Chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 165257. [Google Scholar] [CrossRef]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green Synthesis of Silver Nanoparticles and Their Characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 012050. [Google Scholar] [CrossRef]
- Anwar, Y.; Ul-Islam, M.; Mohammed Ali, H.S.H.; Ullah, I.; Khalil, A.; Kamal, T. Silver Impregnated Bacterial Cellulose-Chitosan Composite Hydrogels for Antibacterial and Catalytic Applications. J. Mater. Res. Technol. 2022, 18, 2037–2047. [Google Scholar] [CrossRef]
- Dos Santos, O.A.L.; de Araujo, I.; Dias da Silva, F.; Sales, M.N.; Christoffolete, M.A.; Backx, B.P. Surface Modification of Textiles by Green Nanotechnology against Pathogenic Microorganisms. Curr. Res. Green Sustain. Chem. 2021, 4, 100206. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Huang, X.; Zhao, L.; Zhang, X.; Wang, L.; Tang, B.; Ma, S.; Chu, P.K. The Effects of Titania Nanotubes with Embedded Silver Oxide Nanoparticles on Bacteria and Osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]
- Ando, S.; Hioki, T.; Yamada, T.; Watanabe, N.; Higashitani, A. Ag2O3 Clathrate is a Novel and Effective Antimicrobial Agent. J. Mater. Sci. 2012, 47, 2928–2931. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Grigor’Eva, A.; Saranina, I.; Tikunova, N.; Safonov, A.; Timoshenko, N.; Rebrov, A.; Ryabchikova, E. Fine Mechanisms of the Interaction of Silver Nanoparticles with the Cells of Salmonella Typhimurium and Staphylococcus Aureus. BioMetals 2013, 26, 479–488. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.M.S.; Kashef, M.T.; Rasmy, S.A.; Aboul-Magd, D.S.; El-Bazza, Z.E. Antimicrobial Activity of Silver Nanoparticles Synthesized Using Honey and Gamma Radiation against Silver-Resistant Bacteria from Wounds and Burns. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045009. [Google Scholar] [CrossRef]
- Šálek, P.; Trousil, J.; Nováčková, J.; Hromádková, J.; Mahun, A.; Kobera, L. Poly[2-(dimethylamino)ethyl Methacrylate-co-Ethylene Dimethacrylate]Nanogel by Dispersion Polymerization for Inhibition of Pathogenic Bacteria. RSC Adv. 2021, 11, 33461–33470. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic Silver Nanoparticles: Synthesis, Mechanism and Applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of Silver Nanoparticles-Nanoparticle or Silver Ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ruano, G.; Iribarren, J.I.; Pérez-Madrigal, M.M.; Torras, J.; Alemán, C. Electrical and Capacitive Response of Hydrogel Solid-like Electrolytes for Supercapacitors. Polymers 2021, 13, 1337. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, C.; Sun, N.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent Progress in Multifunctional Hydrogel-Based Supercapacitors. J. Sci. Adv. Mater. Devices 2021, 6, 338–350. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Lian, K. Alkaline Quaternary Ammonium Hydroxides and Their Polymer Electrolytes for Electrochemical Capacitors. RSC Adv. 2014, 4, 21332–21339. [Google Scholar] [CrossRef]
- Wu, M.; Yang, C.; Xia, H.; Xu, J. Comparative Analysis of Different Separators for the Electrochemical Performances and Long-Term Stability of High-Power Lithium-Ion Batteries. Ionics 2021, 27, 1551–1558. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, Heat-Resistant and Flame-Retardant Cellulose-Based Composite Separator for High-Performance Lithium Ion Battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Qin, B.; Liu, Z.; Zhang, J.; Zhang, B.; Hu, P.; Zhang, C.; Xu, G.; Yao, J.; Cui, G. A Polyborate Coated Cellulose Composite Separator for High Performance Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A834–A838. [Google Scholar] [CrossRef]
- Zhao, C.; Niu, J.; Xiao, C.; Qin, Z.; Jin, X.; Wang, W.; Zhu, Z. Separator with High Ionic Conductivity and Good Stability Prepared from Keratin Fibers for Supercapacitor Applications. Chem. Eng. J. 2022, 444, 136537. [Google Scholar] [CrossRef]
- Radić, G.; Šajnović, I.; Petrović, Ž.; Roković, M.K. Reduced Graphene Oxide/α-Fe2O3 Fibres as Active Material for Supercapacitor Application. Croat. Chem. Acta 2018, 91, 481–490. [Google Scholar] [CrossRef]
- Sopčić, S.; Šešelj, N.; Kraljić Roković, M. Influence of Supporting Electrolyte on the Pseudocapacitive Properties of MnO2/Carbon Nanotubes. J. Solid State Electrochem. 2019, 23, 205–214. [Google Scholar] [CrossRef]
- Sopčić, S.; Antonić, D.; Mandić, Z. Effects of the Composition of Active Carbon Electrodes on the Impedance Performance of the AC/AC supercapacitors. J. Solid State Electrochem. 2022, 26, 591–605. [Google Scholar] [CrossRef]
- Ljubek, G.; Roković, M.K. Aktivni materijali koji se upotrebljavaju u superkondenzatorima. Kem. Ind. 2019, 68, 507–520. [Google Scholar] [CrossRef]
- Majer, M.; Roguljić, M.; Knežević, Ž.; Starodumov, A.; Ferenček, D.; Brigljević, V.; Mihaljević, B. Dose Mapping of the Panoramic 60Co Gamma Irradiation Facility at the Ruđer Bošković Institute–Geant4 Simulation and Measurements. Appl. Radiat. Isot. 2019, 154, 108824. [Google Scholar] [CrossRef]
- Teper, P.; Sotirova, A.; Mitova, V.; Oleszko-Torbus, N.; Utrata-Wesołek, A.; Koseva, N.; Kowalczuk, A.; Mendrek, B. Antimicrobial Activity of Hybrid Nanomaterials Based on Star and Linear Polymers of N, N′-Dimethylaminoethyl Methacrylate with in Situ Produced Silver Nanoparticles. Materials 2020, 13, 3037. [Google Scholar] [CrossRef] [PubMed]
- Vidović, E.; Klee, D.; Höcker, H. Evaluation of Water Uptake and Mechanical Properties of Biomedical Polymers. J. Appl. Polym. Sci. 2013, 130, 3682–3688. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed.; CLSI Document M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]

| Sample | 2940/ cm−1 (2925/ cm−1) | 2850 / cm−1 (2821/ cm−1) | 2770/ cm−1 | 1724/ cm−1 | 1156/ cm−1 | 1019/ cm−1 | 897/ cm−1 | 851/ cm−1 | 780/ cm−1 | 749/ cm−1 | I1724/I897 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1_KS | 8.0 | 6.3 | / | 3.1 | 7.9 | 26.0 | 6.2 | / | / | / | 0.5 |
| 1-1_100 | 11.7 | 9.3 | / | 5.6 | 13.7 | 43.8 | 10.5 | / | / | / | 0.5 |
| 1-3_KS | 11.3 | 8.7 | 5.7 | 16.8 | 19.5 | 34.6 | 3.8 | 1.9 | 1.6 | 2.1 | 4.5 |
| 1-3_100 | 6.6 | 4.9 | 5.5 | 17.9 | 19.7 | 22.7 | 1.7 | 2.5 | 2.5 | 3.3 | 10.5 |
| 1-5_KS | 9.0 | / | 8.9 | 27.9 | 30.5 | 32.9 | 2.1 | 4.4 | 4.5 | 4.7 | 13.3 |
| 1-5_100 | 7.5 | / | 6.9 | 21.5 | 23.3 | 23.7 | 0.7 | 3.4 | 3.7 | 4.2 | 32.1 |
| Sample | d (Inhibition Zone–E. coli)/cm | d (Inhibition Zone–P. aeruginosa)/cm | d (Inhibition Zone–B. subtilis)/cm |
|---|---|---|---|
| 1-1 KS | 0 | 0.05 | 0.1 |
| 1-1_KS + AgNO3 | 0.1 | 0.15 | 0.5 |
| 1-1_100 + AgNO3 | 0.2 | 0.25 | 0.7 |
| 1-3_KS | 0 | 0 | 0 |
| 1-3_KS + AgNO3 | 0.7 | 0.4 | 0.5 |
| 1-3_100 + AgNO3 | 1 | 0.6 | 0.7 |
| 1-5_KS | 0 | 0 | 0 |
| 1-5_KS + AgNO3 | 0.1 | 0.2 | 0.8 |
| 1-5_100 + AgNO3 | 0.6 | 0.4 | 1 |
| Sample | n(cel)/n(DMAEMA) | n(DMAEMA)/n(MBA) | Irradiation Dose (kGy) | AgNO3 |
|---|---|---|---|---|
| 1-1_KS | 1:1 | 15:1 | 0 | - |
| 1-1_KS + AgNO3 | 0 | + | ||
| 1-1_10 | 10 | - | ||
| 1-1_30 | 30 | - | ||
| 1-1_100 | 100 | - | ||
| 1-1_100 + AgNO3 | 100 | + | ||
| 1-3_KS | 1:3 | 15:1 | 0 | - |
| 1-3_KS + AgNO3 | 0 | + | ||
| 1-3_10 | 10 | - | ||
| 1-3_30 | 30 | - | ||
| 1-3_100 | 100 | - | ||
| 1-3_100 + AgNO3 | 100 | + | ||
| 1-5_KS | 1:5 | 15:1 | 0 | - |
| 1-5_KS + AgNO3 | 0 | + | ||
| 1-5_10 | 10 | - | ||
| 1-5_30 | 30 | - | ||
| 1-5_100 | 100 | - | ||
| 1-5_100 + AgNO3 | 100 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blažic, R.; Kučić Grgić, D.; Kraljić Roković, M.; Vidović, E. Cellulose-g-poly(2-(dimethylamino)ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels 2022, 8, 636. https://doi.org/10.3390/gels8100636
Blažic R, Kučić Grgić D, Kraljić Roković M, Vidović E. Cellulose-g-poly(2-(dimethylamino)ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels. 2022; 8(10):636. https://doi.org/10.3390/gels8100636
Chicago/Turabian StyleBlažic, Roko, Dajana Kučić Grgić, Marijana Kraljić Roković, and Elvira Vidović. 2022. "Cellulose-g-poly(2-(dimethylamino)ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application" Gels 8, no. 10: 636. https://doi.org/10.3390/gels8100636
APA StyleBlažic, R., Kučić Grgić, D., Kraljić Roković, M., & Vidović, E. (2022). Cellulose-g-poly(2-(dimethylamino)ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels, 8(10), 636. https://doi.org/10.3390/gels8100636










