Polymer Gels Used in Oil–Gas Drilling and Production Engineering
Abstract
:1. Introduction
2. Different Types of Crosslinked Polymer Gels
2.1. In Situ Crosslinked Polymer Gels
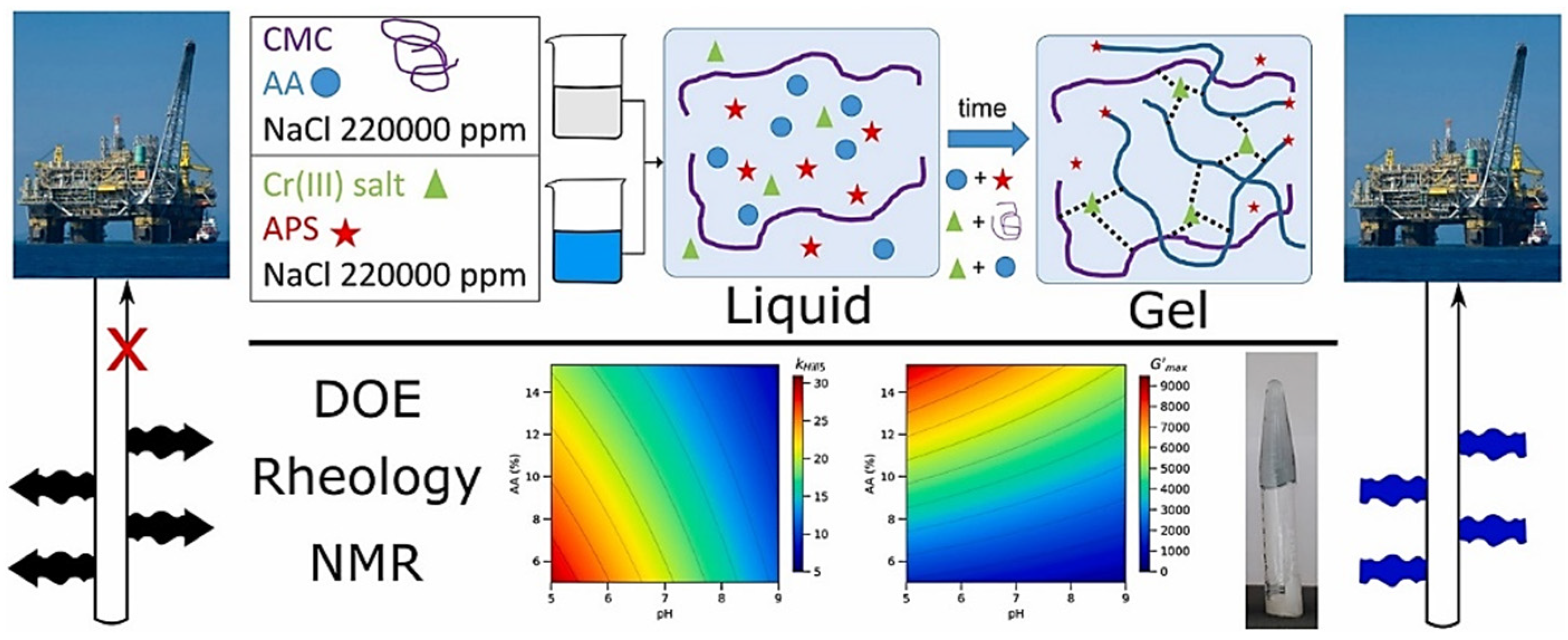
2.1.1. Free-Radical-Based Monomer Crosslinked Gels
2.1.2. Ionic-Bond-Based Metal Crosslinked Gels
2.1.3. Covalent-Bond-Based Organic Crosslinked Gels
2.2. Pre-Crosslinked Polymer Gels
2.2.1. Preformed Particle Gels
2.2.2. Pre-Crosslinked Polymer Gel Microspheres
2.3. Physically Crosslinked Polymer Gels
2.3.1. Hydrogen-Bonded Physically Crosslinked Gels
2.3.2. Hydrophobic-Association-Based Physically Crosslinked Gels
2.3.3. Physically Crosslinked Gels Based on Electrostatic Interaction
3. Polymer Gels for Oil–Gas Drilling
3.1. Polymer Gels as Plugging Agents
3.2. Polymer Gels as Lost Circulation Materials
4. Polymer Gels for Oil–Gas Production
4.1. Polymer Gels as Profile Control Agents
4.2. Polymer Gels as Water Shutoff Agents
4.3. Polymer Gel as Chemical Flooding Agents
4.4. Polymer Gels as Fracturing Fluids
5. Prospects of Polymer Gels for Oil–Gas Drilling and Production
5.1. Improving the Temperature Resistance
5.2. Improving the Salinity Resistance
5.3. Improving the Rheological Property
5.4. Improving the Gelation Strength
5.5. Improving the Environmental Friendliness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, D.; Roy, S.G.; De, P. Amino acid-based polymeric gel network and its application in different fields. J. Indian Chem. Soc. 2022, 99, 100366. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Q.; Liu, W.; Sepehrnoori, K. Modeling preformed particle gel surfactant combined flooding for enhanced oil recovery after polymer flooding. Fuel 2017, 194, 42–49. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.; Liao, B.; Bai, Y.; Shao, Z.; Huang, X.; Wang, Q.; Li, Y. Molecular Simulation of Interactions between High-Molecular-Polymer Flocculation Gel for Oil-Based Drilling Fluid and Clay Minerals. Gels 2022, 8, 442. [Google Scholar] [CrossRef]
- Hamza, A.; Shamlooh, M.; Hussein, I.A.; Nasser, M.; Salehi, S. Polymeric formulations used for loss circulation materials and wellbore strengthening applications in oil and gas wells: A review. J. Pet. Sci. Eng. 2019, 180, 197–214. [Google Scholar] [CrossRef]
- Yang, J.-b.; Sun, J.-s.; Bai, Y.-r.; Lv, K.-h.; Wang, Z.-y.; Xu, C.-y.; Dai, L.-y.; Wang, R. Review of the application of environmentally responsive gels in drilling and oil recovery engineering: Synthetic materials, mechanism, and application prospect. J. Pet. Sci. Eng. 2022, 215, 110581. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, W.; Khan, N.; Zheng, L. Effect of stress relaxation and creep recovery on transportation in porous medium and fracture of the millimeter-scale polymer gel particles for conformance control of heterogeneous oil reservoir. J. Pet. Sci. Eng. 2020, 185, 106648. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Iqbal, T.; Al Harthi, M.A.; Kamal, M.S. Synergistic effect of polymer and nanoparticles on shale hydration and swelling performance of drilling fluids. J. Pet. Sci. Eng. 2021, 205, 108763. [Google Scholar] [CrossRef]
- Karimi, S.; Esmaeilzadeh, F.; Mowla, D. Identification and selection of a stable gel polymer to control or reduce water production in gas condensate fields. J. Nat. Gas Sci. Eng. 2014, 21, 940–950. [Google Scholar] [CrossRef]
- Hasankhani, G.M.; Madani, M.; Esmaeilzadeh, F.; Mowla, D. Experimental investigation of asphaltene-augmented gel polymer performance for water shut-off and enhancing oil recovery in fractured oil reservoirs. J. Mol. Liq. 2019, 275, 654–666. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Chen, Z.; Chen, W. Synthesis and characterization of high-temperature self-crosslinking polymer latexes and their application in water-based drilling fluid. Powder Technol. 2021, 389, 392–405. [Google Scholar] [CrossRef]
- Wu, Q.; Ge, J.; Ding, L.; Wei, K.; Deng, X.; Liu, Y. Identification and characterization of a proper gel system for large-volume conformance control treatments in a fractured tight reservoir: From lab to field. J. Pet. Sci. Eng. 2021, 198, 108199. [Google Scholar] [CrossRef]
- Cozens, E.J.; Roohpour, N.; Gautrot, J.E. Comparative adhesion of chemically and physically crosslinked poly(acrylic acid)-based hydrogels to soft tissues. Eur. Polym. J. 2021, 146, 110250. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Bai, Y.; Lv, K.; Zhang, G.; Li, Y. Status and Prospect of Drilling Fluid Loss and Lost Circulation Control Technology in Fractured Formation. Gels 2022, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, Y.; Sun, J.; Lv, K.; Han, J.; Dai, L. Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control. Gels 2022, 8, 229. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; Chen, A.; Yan, Z.; Zhao, M. Experimental study and application of gels formed by nonionic polyacrylamide and phenolic resin for in-depth profile control. J. Pet. Sci. Eng. 2015, 135, 552–560. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Al-Assi, A.A.; Willhite, G.P.; Green, D.W.; McCool, C.S. Formation and Propagation of Gel Aggregates Using Partially Hydrolyzed Polyacrylamide and Aluminum Citrate. SPE J. 2009, 14, 450–461. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, D.; Gong, Y.; Qin, J.; Liu, X.; Pi, Y.; Zhao, Q.; Luo, R.; Wang, W.; Zhi, K.; et al. Degradable preformed particle gel as temporary plugging agent for low-temperature unconventional petroleum reservoirs: Effect of molecular weight of the cross-linking agent. Pet. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Varel, F.T.; Dai, C.; Shaikh, A.; Li, J.; Sun, N.; Yang, N.; Zhao, G. Chromatography and oil displacement mechanism of a dispersed particle gel strengthened Alkali/Surfactant/Polymer combination flooding system for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125642. [Google Scholar] [CrossRef]
- Kuru, E.A.; Orakdogen, N.; Okay, O. Preparation of homogeneous polyacrylamide hydrogels by free-radical crosslinking copolymerization. Eur. Polym. J. 2007, 43, 2913–2921. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, H.; Li, J.; Cheng, R.; Wang, M.; Bai, X.; Liu, Y.; Sun, Y.; Dai, C. Experimental study of acrylamide monomer polymer gel for water plugging in low temperature and high salinity reservoir. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2948–2959. [Google Scholar] [CrossRef]
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of polymer gels for conformance control in oilfield. Adv. Colloid Interface Sci. 2021, 289, 102363. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Leng, J.; Wei, M. A comprehensive review of in-situ polymer gel simulation for conformance control. Pet. Sci. 2022, 19, 189–202. [Google Scholar] [CrossRef]
- Pereira, F.C.; Clinckspoor, K.J.; Moreno, R.B.Z.L. Optimization of an in-situ polymerized and crosslinked hydrogel formulation for lost circulation control. J. Pet. Sci. Eng. 2022, 208, 109687. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, D.; Lyu, J.; A, S.; Xu, Q.; Newland, B.; Matyjaszewski, K.; Tai, H.; Wang, W. Complex polymer architectures through free-radical polymerization of multivinyl monomers. Nat. Rev. Chem. 2020, 4, 194–212. [Google Scholar] [CrossRef]
- Bai, B.; Li, L.; Liu, Y.; Liu, H.; Wang, Z.; You, C. Preformed Particle Gel for Conformance Control: Factors Affecting Its Properties and Applications. SPE Reserv. Eval. Eng. 2007, 10, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yang, H.; Sarsenbekuly, B.; Zhang, M.; Jiang, H.; Kang, W.; Aidarova, S. The advances of organic chromium based polymer gels and their application in improved oil recovery. Adv. Colloid Interface Sci. 2020, 282, 102214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, F.; Pu, C. Mechanism of formation of chromium acetate(Cr3+)/ phenol-formaldehyde resin prepolymer(PRP) complex and its compound cross-linking reaction with polymer for conformance control. J. Pet. Sci. Eng. 2019, 179, 675–683. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.; Gu, Y.; Zhao, Q.; Yang, R.; Yang, Y. Polyacrylamide gel formed by Cr(III) and phenolic resin for water control in high-temperature reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Yang, H.; Kang, W.; Zhang, H.; Zhou, B.; Li, X.; Wang, F. The New Development of Amphiphilic Polymer Profile Control Agent in High Temperature and High Salinity Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11 November 2019; p. D031S081R002. [Google Scholar]
- Dai, C.; Zhao, G.; You, Q.; Zhao, M. A study on environment-friendly polymer gel for water shut-off treatments in low-temperature reservoirs. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, N.A.; Pu, C. Influence of salinity on the properties of the different HPAM/Al3+systems. Oil Gas Sci. Technol.-Rev. de l IFP 2019, 74, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Hou, J.; Wei, Q.; Chen, Y.; Peng, K. Development of a High-Temperature Resistant Polymer Gel System for Conformance Control in Jidong Oilfield. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017; p. D012S036R008. [Google Scholar]
- Zhang, S.; Wei, F.; Liu, P.; Shao, L.; Li, W. Plugging mechanisms of polymer gel used for hydraulic fracture water shutoff. e-Polymers 2020, 20, 411–422. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Lin, Y.; Han, S. A Novel Thermal-Resistance and Salt-Tolerance Gel with Low-Concentration Crosslinkers for Water Shutoff in Tahe Oilfield. In Proceedings of the SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. [Google Scholar]
- Sengupta, B.; Sharma, V.P.; Udayabhanu, G. Gelation studies of an organically cross-linked polyacrylamide water shut-off gel system at different temperatures and pH. J. Pet. Sci. Eng. 2012, 81, 145–150. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Qi, Z.; Luo, T.; Yan, W.; Xu, H.; Cheng, K.; Li, H. Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel. Energies 2019, 12, 3648. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, S.; Lane, R.H. Delayed Crosslink Polymer Gel System for Water Shutoff in Conventional and Unconventional Oil and Gas Reservoirs. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar]
- Al-Muntasheri, G.A.; Hussein, I.A.; Nasr-El-Din, H.A.; Amin, M.B. Viscoelastic properties of a high temperature cross-linked water shut-off polymeric gel. J. Pet. Sci. Eng. 2007, 55, 56–66. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Wang, K.; Zhao, M.; Gao, M.; Yang, Z.; Fang, J.; Wu, Y. Investigation on Preparation and Profile Control Mechanisms of the Dispersed Particle Gels (DPG) Formed from Phenol–Formaldehyde Cross-linked Polymer Gel. Ind. Eng. Chem. Res. 2016, 55, 6284–6292. [Google Scholar] [CrossRef]
- Bai, B.; Sun, X. Development of Swelling-Rate Controllable Particle Gels to Control the Conformance of CO2 Flooding. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020; p. D031S045R001. [Google Scholar]
- Zhou, B.; Kang, W.; Yang, H.; Zhu, T.; Zhang, H.; Li, X.; Sarsenbekuly, B.; Sarsenbek, T. Preparation and properties of an acid-resistant preformed particle gel for conformance control. J. Pet. Sci. Eng. 2021, 197, 107964. [Google Scholar] [CrossRef]
- Abdulfarraj, M.; Imqam, A. The potential of using micro-sized crosslinked polymer gel to remediate water leakage in cement sheaths. J. Pet. Explor. Prod. Technol. 2020, 10, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Wei, M.; Geng, J.; Wu, F. Successful Field Application of Microgel Treatment in High Temperature High Salinity Reservoir in China. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Salunkhe, B.; Schuman, T.; Al Brahim, A.; Bai, B. Ultra-high temperature resistant preformed particle gels for enhanced oil recovery. Chem. Eng. J. 2021, 426, 130712. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, N.; Pu, C. A New Method of Plugging the Fracture to Enhance Oil Production for Fractured Oil Reservoir using Gel Particles and the HPAM/Cr3+ System. Polymers 2019, 11, 446. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Tang, X.; Yang, H.; Gao, Y.; Lashari, Z.A.; Cao, C.; Kang, W. Preparation of a micron-size silica-reinforced polymer microsphere and evaluation of its properties as a plugging agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 8–18. [Google Scholar] [CrossRef]
- Dai, S.; Chen, Z.; Huang, Z. Synthesis and characterization of water-sensitive core-shell type microspheres for water shut-off in the oil field. Russ. J. Appl. Chem. 2017, 90, 310–323. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Kadam, Y.; Pochat-Bohatier, C.; Sanchez, J.; El Ghzaoui, A. Modulating Viscoelastic Properties of Physically Crosslinked Self-Assembled Gelatin Hydrogels through Optimized Solvent Conditions. J. Dispers. Sci. Technol. 2015, 36, 1349–1356. [Google Scholar] [CrossRef]
- Chen, S.; Geng, Z.; Zheng, X.; Xu, J.; Binder, W.H.; Zhu, J. Engineering the morphology of hydrogen-bonded comb-shaped supramolecular polymers: From solution self-assembly to confined assembly. Polym. Chem. 2020, 11, 4022–4028. [Google Scholar] [CrossRef]
- Shi, X.; Yue, X.a. Migration and plugging mechanisms of self-aggregated microspheres as a novel profile control. J. Pet. Sci. Eng. 2020, 184, 106458. [Google Scholar] [CrossRef]
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S.S. Weak Hydrogen Bonding Enables Hard, Strong, Tough, and Elastic Hydrogels. Adv. Mater. 2015, 27, 6899–6905. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.N.; Zheng, S.Y.; Song, Y.; Wu, Z.L.; Zheng, Q. Hydrogen bond reinforced poly(1-vinylimidazole-co-acrylic acid) hydrogels with high toughness, fast self-recovery, and dual pH-responsiveness. Polymer 2017, 131, 95–103. [Google Scholar] [CrossRef]
- Liang, X.; Ding, H.; Wang, Q.; Sun, G. Tough physical hydrogels reinforced by hydrophobic association with remarkable mechanical property, rapid stimuli-responsiveness and fast self-recovery capability. Eur. Polym. J. 2019, 120, 109278. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, C.; Liu, X.; Zhang, G.; Yang, M.; Liu, F. Construction and Properties of Hydrophobic Association Hydrogels with High Mechanical Strength and Reforming Capability. Macromol. Mater. Eng. 2009, 294, 815–820. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Staropoli, M.; Appavou, M.-S.; Wyss, H.M.; Pyckhout-Hintzen, W.; Sijbesma, R.P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules 2017, 50, 3333–3346. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; An, H.; Lu, C.; Tan, Y.; Li, P.; Wang, P. Facile fabrication method of hydrophobic-associating cross-linking hydrogel with outstanding mechanical performance and self-healing property in the absence of surfactants. Polymer 2013, 54, 5665–5672. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Yuan, N.; Xu, L.; Wang, H.; Fu, Y.; Zhang, Z.; Liu, L.; Wang, C.; Zhao, J.; Rong, J. Dual Physically Cross-Linked Double Network Hydrogels with High Mechanical Strength, Fatigue Resistance, Notch-Insensitivity, and Self-Healing Properties. ACS Appl. Mater. Interfaces 2016, 8, 34034–34044. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Thareja, P. Hydrogels differentiated by length scales: A review of biopolymer-based hydrogel preparation methods, characterization techniques, and targeted applications. Eur. Polym. J. 2022, 163, 110935. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, P. Underwater Communication and Optical Camouflage Ionogels. Adv. Mater. 2021, 33, 2008479. [Google Scholar] [CrossRef]
- Catanzano, O.; Soriente, A.; La Gatta, A.; Cammarota, M.; Ricci, G.; Fasolino, I.; Schiraldi, C.; Ambrosio, L.; Malinconico, M.; Laurienzo, P.; et al. Macroporous alginate foams crosslinked with strontium for bone tissue engineering. Carbohydr. Polym. 2018, 202, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; An, R.; Han, L.; Wang, X.; Shi, Y.; Ran, R. Preparation of high strength double physically cross-linked hydrogels by immersion method—How to avoid uneven soaking. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 74–82. [Google Scholar] [CrossRef]
- Shao, C.; Meng, L.; Cui, C.; Yang, J. An integrated self-healable and robust conductive hydrogel for dynamically self-adhesive and highly conformable electronic skin. J. Mater. Chem. C 2019, 7, 15208–15218. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, B.; Zhou, E.; Pu, J.; Schuman, T. Experimental Evaluation of Oxidizing Breakers for a Polyacrylamide-Based Re-Crosslinkable Preformed Particle Gel. Energy Fuels 2019, 33, 5001–5010. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Fang, X.-Y.; Sun, R.-X.; Xu, Z.-H.; Liu, Y.; Liu, J.-Y. Development of degradable pre-formed particle gel (DPPG) as temporary plugging agent for petroleum drilling and production. Pet. Sci. 2021, 18, 479–494. [Google Scholar] [CrossRef]
- Li, H.; Lv, K.; Huang, X.; Lu, Z.; Dong, X. The Synthesis of Polymeric Nanospheres and the Application as High-Temperature Nano-Plugging Agent in Water Based Drilling Fluid. Front. Chem. 2020, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-g.; Qiu, Z.; Zhao, X.; Mou, T.; Zhong, H.; Huang, W. A polymer microsphere emulsion as a high-performance shale stabilizer for water-based drilling fluids. RSC Adv. 2018, 8, 20852–20861. [Google Scholar] [CrossRef] [Green Version]
- Azimi Dijvejin, Z.; Ghaffarkhah, A.; Sadeghnejad, S.; Vafaie Sefti, M. Effect of silica nanoparticle size on the mechanical strength and wellbore plugging performance of SPAM/chromium (III) acetate nanocomposite gels. Polym. J. 2019, 51, 693–707. [Google Scholar] [CrossRef]
- Azimi Dijvejin, Z.; Ghaffarkhah, A.; Vafaie Sefti, M.; Moraveji, M.K. Synthesis, structure and mechanical properties of nanocomposites based on exfoliated nano magnesium silicate crystal and poly(acrylamide). J. Dispers. Sci. Technol. 2018, 40, 276–286. [Google Scholar] [CrossRef]
- Mansour, A.; Dahi Taleghani, A.; Salehi, S.; Li, G.; Ezeakacha, C. Smart lost circulation materials for productive zones. J. Pet. Explor. Prod. Technol. 2019, 9, 281–296. [Google Scholar] [CrossRef] [Green Version]
- Magzoub, M.I.; Shamlooh, M.; Salehi, S.; Hussein, I.; Nasser, M.S. Gelation kinetics of PAM/PEI based drilling mud for lost circulation applications. J. Pet. Sci. Eng. 2021, 200, 108383. [Google Scholar] [CrossRef]
- Kang, W.; Wang, J.; Ye, Z.; Gu, G.; Li, W.; Yang, H.; Li, Z.; Xu, H.; Lv, Z.; Sarsenbekuly, B. Study on preparation and plugging effect of sawdust gel particle in fractured reservoir. J. Pet. Sci. Eng. 2022, 212, 110358. [Google Scholar] [CrossRef]
- Dai, C.; Chen, W.; You, Q.; Wang, H.; Yang, Z.; He, L.; Jiao, B.; Wu, Y. A novel strengthened dispersed particle gel for enhanced oil recovery application. J. Ind. Eng. Chem. 2016, 41, 175–182. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2021, 202, 108416. [Google Scholar] [CrossRef]
- Li, C.; Yang, S.; Wen, Z.; Pan, Y.; Zeng, F.; Zhao, C.; Abouleilah, H.M.A. The research of new type gel plugging agent for deep well. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Wang, P.; Nie, X.; Zhang, X.; Luo, P.; Bai, Y. Study on a Special Association Polymer Gel Applied in Vicious Lost Circulation in Crushed Zones. Adv. Mater. Res. 2012, 518–523, 102–107. [Google Scholar] [CrossRef]
- He, Y.; Xiong, S.; Yang, Z.; Ruan, X.; Gong, Y. The Research on Cross-linking Polymer Gel as In-depth Profile Control Agent. Pet. Sci. Technol. 2009, 27, 1300–1311. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; Zhang, Y.; Chen, A.; Yan, Z.; Zhao, M. Enhanced foam stability by adding comb polymer gel for in-depth profile control in high temperature reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 115–124. [Google Scholar] [CrossRef]
- Jia, H.; Ren, Q.; Zhao, J. Swelling Mechanism Investigation of Microgel with Double-Cross-Linking Structures. Energy Fuels 2014, 28, 6735–6744. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, E.; Cui, X. Study on Profile Control and Water Shut-Off Performance of Interpenetrating Network Polymer Gel Composite System in Shallow Low Temperature Fractured Oil Layer. ChemistrySelect 2019, 4, 8158–8164. [Google Scholar] [CrossRef]
- Du, D.-j.; Pu, W.-f.; Tan, X.; Liu, R. Experimental study of secondary crosslinking core-shell hyperbranched associative polymer gel and its profile control performance in low-temperature fractured conglomerate reservoir. J. Pet. Sci. Eng. 2019, 179, 912–920. [Google Scholar] [CrossRef]
- Zhao, G.; You, Q.; Tao, J.; Gu, C.; Aziz, H.; Ma, L.; Dai, C. Preparation and application of a novel phenolic resin dispersed particle gel for in-depth profile control in low permeability reservoirs. J. Pet. Sci. Eng. 2018, 161, 703–714. [Google Scholar] [CrossRef]
- Cao, W.; Xie, K.; Wang, X.; Lu, X.; He, X.; Xu, G.; Li, X. Starch graft copolymer and polymer gel applied in Bohai oilfield for water plugging and profile control and their mechanisms. Geosystem Eng. 2020, 23, 197–204. [Google Scholar] [CrossRef]
- Bai, Y.; Shang, X.; Wang, Z.; Zhao, X. Experimental study of low molecular weight polymer/nanoparticle dispersed gel for water plugging in fractures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 95–107. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Y.; Chen, A.; You, Q.; Dai, C.; Cheng, R.; Liu, Y. Gelation Behavior Study of a Resorcinol–Hexamethyleneteramine Crosslinked Polymer Gel for Water Shut-Off Treatment in Low Temperature and High Salinity Reservoirs. Energies 2017, 10, 913. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.; Tang, X.; Kang, W. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.; Zhou, Q.; Fu, C.; He, S. Preparation and Experimental Study of a Low-Initial-Viscosity Gel Plugging Agent. ACS Omega 2020, 5, 15715–15727. [Google Scholar] [CrossRef]
- Alhuraishawy, A.K.; Bai, B.; Imqam, A.; Wei, M. Experimental study of combining low salinity water flooding and preformed particle gel to enhance oil recovery for fractured carbonate reservoirs. Fuel 2018, 214, 342–350. [Google Scholar] [CrossRef]
- Cao, W.; Xie, K.; Lu, X.; Liu, Y.; Zhang, Y. Effect of profile-control oil-displacement agent on increasing oil recovery and its mechanism. Fuel 2019, 237, 1151–1160. [Google Scholar] [CrossRef]
- Goudarzi, A.; Almohsin, A.; Varavei, A.; Delshad, M.; Bai, B.; Sepehrnoori, K. New Experiments and Models for Conformance Control Microgels. In Proceeding of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Wang, W.; Gu, Y.; Liu, Y. Applications of Weak Gel for In-Depth Profile Modification and Oil Displacement. J. Can. Pet. Technol. 2003, 42, 1–6. [Google Scholar] [CrossRef]
- Cui, C.; Zhou, Z.; He, Z. Enhance oil recovery in low permeability reservoirs: Optimization and evaluation of ultra-high molecular weight HPAM / phenolic weak gel system. J. Pet. Sci. Eng. 2020, 195, 107908. [Google Scholar] [CrossRef]
- Hua, Z.; Lin, M.; Dong, Z.; Li, M.; Zhang, G.; Yang, J. Study of deep profile control and oil displacement technologies with nanoscale polymer microspheres. J. Colloid Interface Sci. 2014, 424, 67–74. [Google Scholar] [CrossRef]
- Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef] [Green Version]
- Roussennac, B.; Toschi, C. Brightwater® Trial in Salema Field (Campos Basin, Brazil). In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010. [Google Scholar]
- Wang, J.Y.; Holditch, S.A.; McVay, D.A. Effect of gel damage on fracture fluid cleanup and long-term recovery in tight gas reservoirs. J. Nat. Gas Sci. Eng. 2012, 9, 108–118. [Google Scholar] [CrossRef]
- Liu, D.; Fan, M.; Yao, L.; Zhao, X.; Wang, Y. A new fracturing fluid with combination of single phase microemulsion and gelable polymer system. J. Pet. Sci. Eng. 2010, 73, 267–271. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Guo, J.; Lai, J.; Wang, D.; He, L.; Qin, Y. A study of relation between suspension behavior and microstructure and viscoelastic property of guar gum fracturing fluid. J. Pet. Sci. Eng. 2014, 124, 432–435. [Google Scholar] [CrossRef]
- Song, J.; Fan, W.; Long, X.; Zhou, L.; Wang, C.; Li, G. Rheological behaviors of fluorinated hydrophobically associating cationic guar gum fracturing gel. J. Pet. Sci. Eng. 2016, 146, 999–1005. [Google Scholar] [CrossRef]
- Plank, J. Behavior of Titania Nanoparticles in Crosslinking Hydroxypropyl Guar Used in Hydraulic Fracturing Fluids For Oil Recovery. Energy Fuels 2015, 29, 150507182220006. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, G.; Wang, C.; Yang, L.; Ren, Y.; Liu, P.; Shi, Y. The influence of fiber on the rheological properties, microstructure and suspension behavior of the supramolecular viscoelastic fracturing fluid. J. Nat. Gas Sci. Eng. 2016, 35, 1207–1215. [Google Scholar] [CrossRef]
- Amir, Z.; Said, I.M.; Jan, B.M. In situ organically cross-linked polymer gel for high-temperature reservoir conformance control: A review. Polym. Adv. Technol. 2019, 30, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, S.; Li, L.; Wu, R.; Liu, D.; Wu, J.; Wu, W. High-temperature-resistant polymer gel system with metal–organic mixed cross-linking agents. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- El-Karsani, K.S.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer Systems for Water Shutoff and Profile Modification: A Review Over the Last Decade. SPE J. 2013, 19, 135–149. [Google Scholar] [CrossRef]
- Jia, H.; Pu, W.-F.; Zhao, J.-Z.; Liao, R. Experimental Investigation of the Novel Phenol−Formaldehyde Cross-Linking HPAM Gel System: Based on the Secondary Cross-Linking Method of Organic Cross-Linkers and Its Gelation Performance Study after Flowing through Porous Media. Energy Fuels 2011, 25, 727–736. [Google Scholar] [CrossRef]
- Han, X.; Liguo, Z.; Liu, Y.; Wang, Q.; Wang, C. Experimental study of polyacrylamide polymer gel for profile controlling in high-temperature offshore reservoirs. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Lashari, Z.A.; Yang, H.; Zhu, Z.; Tang, X.; Cao, C.; Iqbal, M.W.; Kang, W. Experimental research of high strength thermally stable organic composite polymer gel. J. Mol. Liq. 2018, 263, 118–124. [Google Scholar] [CrossRef]
- Mehrabianfar, P.; Malmir, P.; Soulgani, B.S.; Hashemi, A. Study on the optimization of the performance of preformed particle gel (PPG) on the isolation of high permeable zone. J. Pet. Sci. Eng. 2020, 195, 107530. [Google Scholar] [CrossRef]
- Yin, H.; Yin, X.; Cao, R.; Zeng, P.; Wang, J.; Wu, D.; Luo, X.; Zhu, Y.; Zheng, Z.; Feng, Y. In situ crosslinked weak gels with ultralong and tunable gelation times for improving oil recovery. Chem. Eng. J. 2022, 432, 134350. [Google Scholar] [CrossRef]
- Al-saba, M.T.; Al Fadhli, A.; Marafi, A.; Hussain, A.; Bander, F.; Al Dushaishi, M.F. Application of Nanoparticles in Improving Rheological Properties of Water Based Drilling Fluids. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23 April 2018. [Google Scholar]
- Aqcheli, F.; Salehi, M.B.; Pahlevani, H.; Taghikhani, V. Rheological properties and the micromodel investigation of nanosilica gel-reinforced preformed particle gels developed for improved oil recovery. J. Pet. Sci. Eng. 2020, 192, 107258. [Google Scholar] [CrossRef]
- Gu, C.; Lv, Y.; Fan, X.; Zhao, C.; Dai, C.; Zhao, G. Study on rheology and microstructure of phenolic resin cross-linked nonionic polyacrylamide (NPAM) gel for profile control and water shutoff treatments. J. Pet. Sci. Eng. 2018, 169, 546–552. [Google Scholar] [CrossRef]
- Aldhaheri, M.; Wei, M.; Zhang, N.; Bai, B. Field design guidelines for gel strengths of profile-control gel treatments based on reservoir type. J. Pet. Sci. Eng. 2020, 194, 107482. [Google Scholar] [CrossRef]
- Tongwa, P.; Bai, B. Degradable nanocomposite preformed particle gel for chemical enhanced oil recovery applications. J. Pet. Sci. Eng. 2014, 124, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Yang, X.; Li, S.; Yu, P.; Zhang, J. Nanocomposite gel of high-strength and degradability for temporary plugging in ultralow-pressure fracture reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124108. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, B.; Zhu, T.; Wang, P.; Zhang, X.; Wang, T.; Wu, F.; Zhang, L.; Kang, W.; Ketova, Y.A.; et al. Conformance control mechanism of low elastic polymer microspheres in porous medium. J. Pet. Sci. Eng. 2021, 196, 107708. [Google Scholar] [CrossRef]





















| No. | Name | Molecular Formula | Structural Formula |
|---|---|---|---|
| 1# | Acrylamide | C3H5NO |  |
| 2# | Acrylate | CH2 = CHCOOR |  |
| 3# | Acrylic acid | C3H4O2 |  |
| 4# | 2-Acrylamido-2-methylpropane sulfonic acid (AMPS) | C7H13NO4S |  |
| 5# | Bromoacetic acid (MBAA) | C2H3O2Br |  |
| 6# | Tert-butyl acrylate (PAtBA) | C7H12O2 |  |
| No. | Name | Molecular Formula | Structural Formula |
|---|---|---|---|
| 1# | Phenol | C6H5OH |  |
| 2# | Formaldehyde | HCHO |  |
| 3# | Resorcinol | C6H6O2 |  |
| 4# | Hexamethylenetetramine (HMTA) | C6H12N4 |  |
| 5# | Water-soluble phenolic resin | C7H6O2 |  |
| 6# | Polyvinyl imine | (CH2CH2NH) n |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Sun, J.; Lv, K.; Yang, J.; Li, Y. Polymer Gels Used in Oil–Gas Drilling and Production Engineering. Gels 2022, 8, 637. https://doi.org/10.3390/gels8100637
Han J, Sun J, Lv K, Yang J, Li Y. Polymer Gels Used in Oil–Gas Drilling and Production Engineering. Gels. 2022; 8(10):637. https://doi.org/10.3390/gels8100637
Chicago/Turabian StyleHan, Jinliang, Jinsheng Sun, Kaihe Lv, Jingbin Yang, and Yuhong Li. 2022. "Polymer Gels Used in Oil–Gas Drilling and Production Engineering" Gels 8, no. 10: 637. https://doi.org/10.3390/gels8100637
APA StyleHan, J., Sun, J., Lv, K., Yang, J., & Li, Y. (2022). Polymer Gels Used in Oil–Gas Drilling and Production Engineering. Gels, 8(10), 637. https://doi.org/10.3390/gels8100637





