Water Desalination Using Polyelectrolyte Hydrogel: Gibbs Ensemble Modeling
Abstract
1. Introduction
1.1. Water Desalination Technologies
1.2. Hydrogels for Desalination
1.3. Physics behind the Desalination Process
2. Results and Discussion
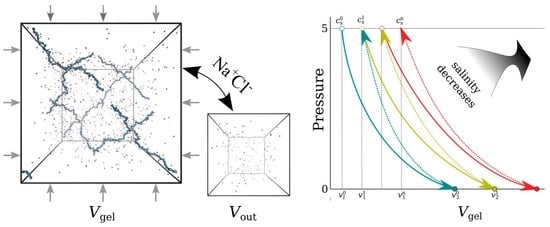
2.1. Open and Closed Systems
2.2. Compression in Open System
2.3. Compression in Closed System
2.4. Desalination Scheme
2.5. The Efficiency of Desalination
- 1.
- First, the gel equilibrates with the feed solution and is compressed in the closed system. The gel volume decreases by liters, and the salinity of the supernate decreases from to . The volume of the product solution is .
- 2.
- The squeezed gel is put back into the feed solution and is equilibrated there under pressure, so it does not swell.
- 3.
- After equilibration, the gel swells in the closed system, so the salinity of the external solution increases to the value .
- 4.
- Finally, the gel is taken out and compressed at 5 bar pressure in the open system in equilibrium with the brine bath. The change of the gel volume in this process is L/mol, which equals the volume of the produced brine.
2.6. Study Limitations
2.7. Implications and Future Perspectives
3. Conclusions
4. Materials and Methods
4.1. Molecular Dynamics
4.2. Monte Carlo Sampling in an Open System
- 1.
- The combinatorial entropy , which reflects the freedom of choice among the particles;
- 2.
- The mixing entropy , which reflects the freedom to place the chosen particle randomly within the simulation box.
- 1.
- Propose the new configuration of the system by insertion (or deletion) of an ion pair, ;
- 2.
- Accept the new configuration ifwhere is a uniformly distributed random number in the range between 0 and 1;
- 3.
- Then, collect the number of ions, and , to the samples array.
4.3. Monte Carlo Sampling in Closed System
4.4. Algorithm
- 1.
- Initiate the systems to simulate: the gel of volume and the external solution of volume ;
- 2.
- Equilibrate the system, interspersing the MD and MC stages;
- 3.
- Run the MD subsimulation and collect the observables: pressure in both volumes, , , and distances between the nodes of the gel network. The latter is needed to estimate the autocorrelation of the MD simulation;
- 4.
- Run the MC procedure, simulating ion exchange, and collect the number of ions in both boxes, and ;
- 5.
- Repeat the MD and MC subsimulations until the desired length of sample arrays is reached.
Author Contributions
Funding
Conflicts of Interest
References
- Guesmi, A.; Cherif, M.M.; Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Elfalleh, W.; Hamadi, N.B.; Khezami, L.; Assadi, A.A. Disinfection of corona and myriad viruses in water by non-thermal plasma: A review. Environ. Sci. Pollut. Res. 2022, 29, 55321–55335. [Google Scholar] [CrossRef] [PubMed]
- Baaloudj, O.; Badawi, A.K.; Kenfoud, H.; Benrighi, Y.; Hassan, R.; Nasrallah, N.; Assadi, A.A. Techno-economic studies for a pilot-scale Bi12TiO20 based photocatalytic system for pharmaceutical wastewater treatment: From laboratory studies to commercial-scale applications. J. Water Process. Eng. 2022, 48, 102847. [Google Scholar] [CrossRef]
- Shahzad, W.; Badawi, A.K.; Rehan, Z.A.; Khan, A.M.; Khan, R.A.; Shah, F.; Ali, S.; Ismail, B. Enhanced visible light photocatalytic performance of Sr0.3(Ba,Mn)0.7ZrO3 perovskites anchored on graphene oxide. Ceram. Int. 2022, 48, 24979–24988. [Google Scholar] [CrossRef]
- Miller, J. Review of Water Resources and Desalination Technologies; Technical Report; Sandia National Laboratories (SNL): Albuquerque, NM, USA, 2003. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, X.M. A critical review on draw solutes development for forward osmosis. Desalination 2016, 391, 16–29. [Google Scholar] [CrossRef]
- Wack, H.; Ulbricht, M. Effect of synthesis composition on the swelling pressure of polymeric hydrogels. Polymer 2009, 50, 2075–2080. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishio, I.; Sun, S.T.; Ueno-Nishio, S. Collapse of Gels in an Electric Field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef]
- Serizawa, T.; Wakita, K.; Akashi, M. Rapid Deswelling of Porous Poly(N-isopropylacrylamide) Hydrogels Prepared by Incorporation of Silica Particles. Macromolecules 2001, 35, 10–12. [Google Scholar] [CrossRef]
- Lietor-Santos, J.J.; Sierra-Martin, B.; Vavrin, R.; Hu, Z.; Gasser, U.; Fernandez-Nieves, A. Deswelling Microgel Particles Using Hydrostatic Pressure. Macromolecules 2009, 42, 6225–6230. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Yao, J.; Simon, G.P.; Wang, H. Stimuli-responsive polymer hydrogels as a new class of draw agent for forward osmosis desalination. Chem. Commun. 2011, 47, 1710. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, J.; Simon, G.P. Response to Osmotic Pressure versus Swelling Pressure: Comment on “Bifunctional Polymer Hydrogel Layers As Forward Osmosis Draw Agents for Continuous Production of Fresh Water Using Solar Energy”. Environ. Sci. Technol. 2014, 48, 4214–4215. [Google Scholar] [CrossRef] [PubMed]
- Arens, L.; Albrecht, J.B.; Höpfner, J.; Schlag, K.; Habicht, A.; Seiffert, S.; Wilhelm, M. Energy Consumption for the Desalination of Salt Water Using Polyelectrolyte Hydrogels as the Separation Agent. Macromol. Chem. Phys. 2017, 218, 1700237. [Google Scholar] [CrossRef]
- Fengler, C.; Arens, L.; Horn, H.; Wilhelm, M. Desalination of Seawater Using Cationic Poly(acrylamide) Hydrogels and Mechanical Forces for Separation. Macromol. Mater. Eng. 2020, 305, 2000383. [Google Scholar] [CrossRef]
- Ali, W.; Gebert, B.; Hennecke, T.; Graf, K.; Ulbricht, M.; Gutmann, J.S. Design of Thermally Responsive Polymeric Hydrogels for Brackish Water Desalination: Effect of Architecture on Swelling, Deswelling, and Salt Rejection. ACS Appl. Mater. Interfaces 2015, 7, 15696–15706. [Google Scholar] [CrossRef]
- Rud, O.V.; Landsgesell, J.; Holm, C.; Košovan, P. Modeling of weak polyelectrolyte hydrogels under compression – Implications for water desalination. Desalination 2021, 506, 114995. [Google Scholar] [CrossRef]
- Rud, O.; Borisov, O.; Košovan, P. Thermodynamic model for a reversible desalination cycle using weak polyelectrolyte hydrogels. Desalination 2018, 442, 32–43. [Google Scholar] [CrossRef]
- Rud, O.V.; Kazakov, A.D.; Nova, L.; Uhlik, F. Polyelectrolyte Hydrogels as Draw Agents for Desalination of Solutions with Multivalent Ions. Macromolecules 2022, 55, 1763–1770. [Google Scholar] [CrossRef]
- Landsgesell, J.; Hebbeker, P.; Rud, O.; Lunkad, R.; Košovan, P.; Holm, C. Grand-Reaction Method for Simulations of Ionization Equilibria Coupled to Ion Partitioning. Macromolecules 2020, 53, 3007–3020. [Google Scholar] [CrossRef]
- Zhulina, E.; Klein Wolterink, J.; Borisov, O. Screening Effects in a Polyelectrolyte Brush: Self-Consistent-Field Theory. Macromolecules 2000, 33, 4945–4953. [Google Scholar] [CrossRef]
- Wang, L.; Violet, C.; DuChanois, R.M.; Elimelech, M. Derivation of the Theoretical Minimum Energy of Separation of Desalination Processes. J. Chem. Educ. 2020, 97, 4361–4369. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S. A novel single-pass reverse osmosis configuration for high-purity water production and low energy consumption in seawater desalination. Desalination 2018, 429, 142–154. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry, 9th ed.; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Prokacheva, V.M.; Rud, O.V.; Uhlík, F.; Borisov, O.V. Phase transition in hydrophobic weak polyelectrolyte gel utilized for water desalination. Desalination 2021, 511, 115092. [Google Scholar] [CrossRef]
- Jin, S.; Collins, L.R. Dynamics of dissolved polymer chains in isotropic turbulence. New J. Phys. 2007, 9, 360. [Google Scholar] [CrossRef]
- Grest, G.S.; Kremer, K. Molecular dynamics simulation for polymers in the presence of a heat bath. Phys. Rev. A 1986, 33, 3628–3631. [Google Scholar] [CrossRef]
- Nagle, J.F. Regarding the Entropy of Distinguishable Particles. J. Stat. Phys. 2004, 117, 1047–1062. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Panagiotopoulos, A.; Quirke, N.; Stapleton, M.; Tildesley, D. Phase equilibria by simulation in the Gibbs ensemble. Mol. Phys. 1988, 63, 527–545. [Google Scholar] [CrossRef]
- Erdos, M.; Galteland, O.; Bedeaux, D.; Kjelstrup, S.; Moultos, O.A.; Vlugt, T.J.H. Gibbs Ensemble Monte Carlo Simulation of Fluids in Confinement: Relation between the Differential and Integral Pressures. Nanomaterials 2020, 10, 293. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laktionov, M.; Nová, L.; Rud, O.V. Water Desalination Using Polyelectrolyte Hydrogel: Gibbs Ensemble Modeling. Gels 2022, 8, 656. https://doi.org/10.3390/gels8100656
Laktionov M, Nová L, Rud OV. Water Desalination Using Polyelectrolyte Hydrogel: Gibbs Ensemble Modeling. Gels. 2022; 8(10):656. https://doi.org/10.3390/gels8100656
Chicago/Turabian StyleLaktionov, Mikhail, Lucie Nová, and Oleg V. Rud. 2022. "Water Desalination Using Polyelectrolyte Hydrogel: Gibbs Ensemble Modeling" Gels 8, no. 10: 656. https://doi.org/10.3390/gels8100656
APA StyleLaktionov, M., Nová, L., & Rud, O. V. (2022). Water Desalination Using Polyelectrolyte Hydrogel: Gibbs Ensemble Modeling. Gels, 8(10), 656. https://doi.org/10.3390/gels8100656