An Investigation of the Sol-Gel Transition of Chitosan Lactate and Chitosan Chloride Solutions via Rheological and NMR Studies
Abstract
:1. Introduction
2. Results and Discussion
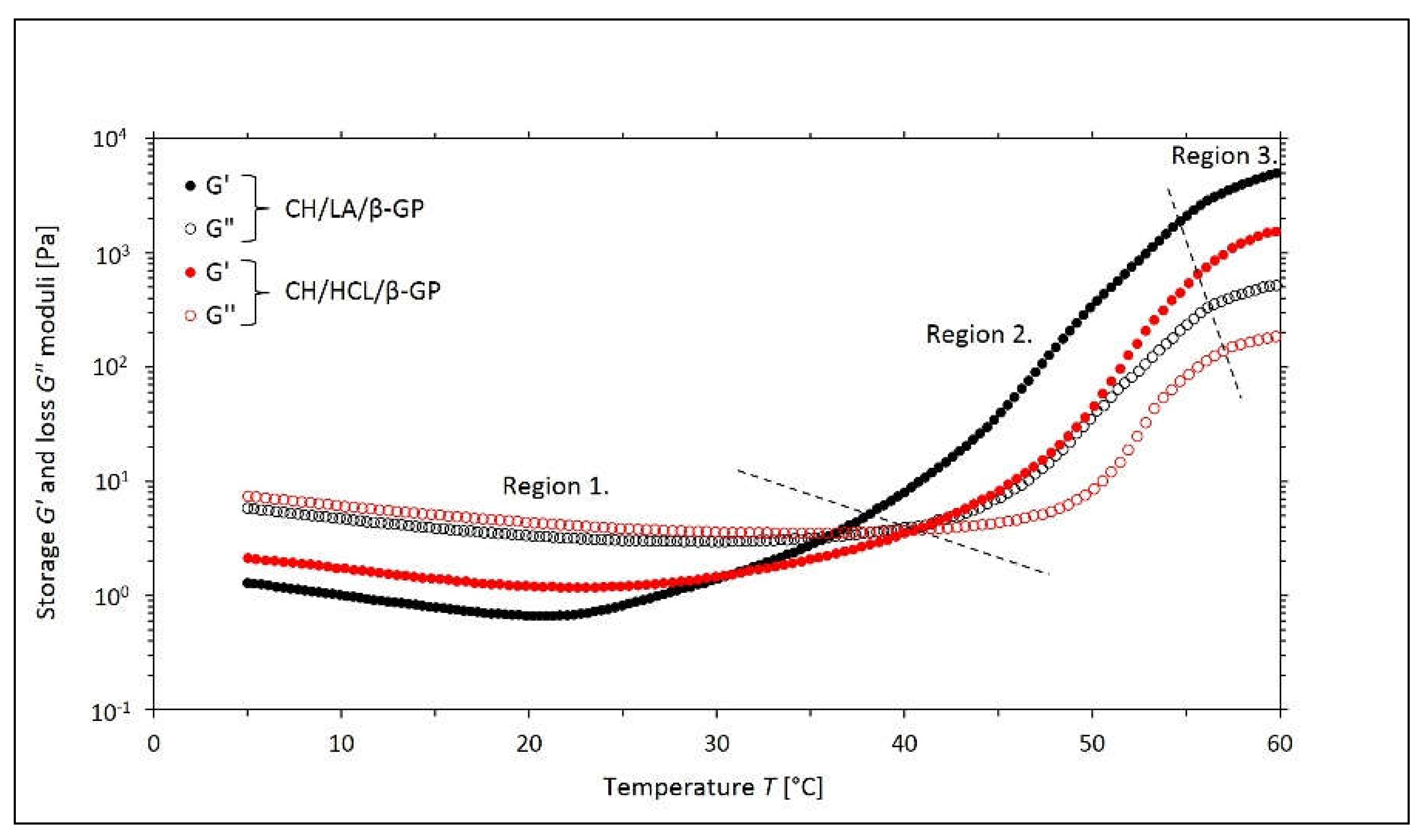
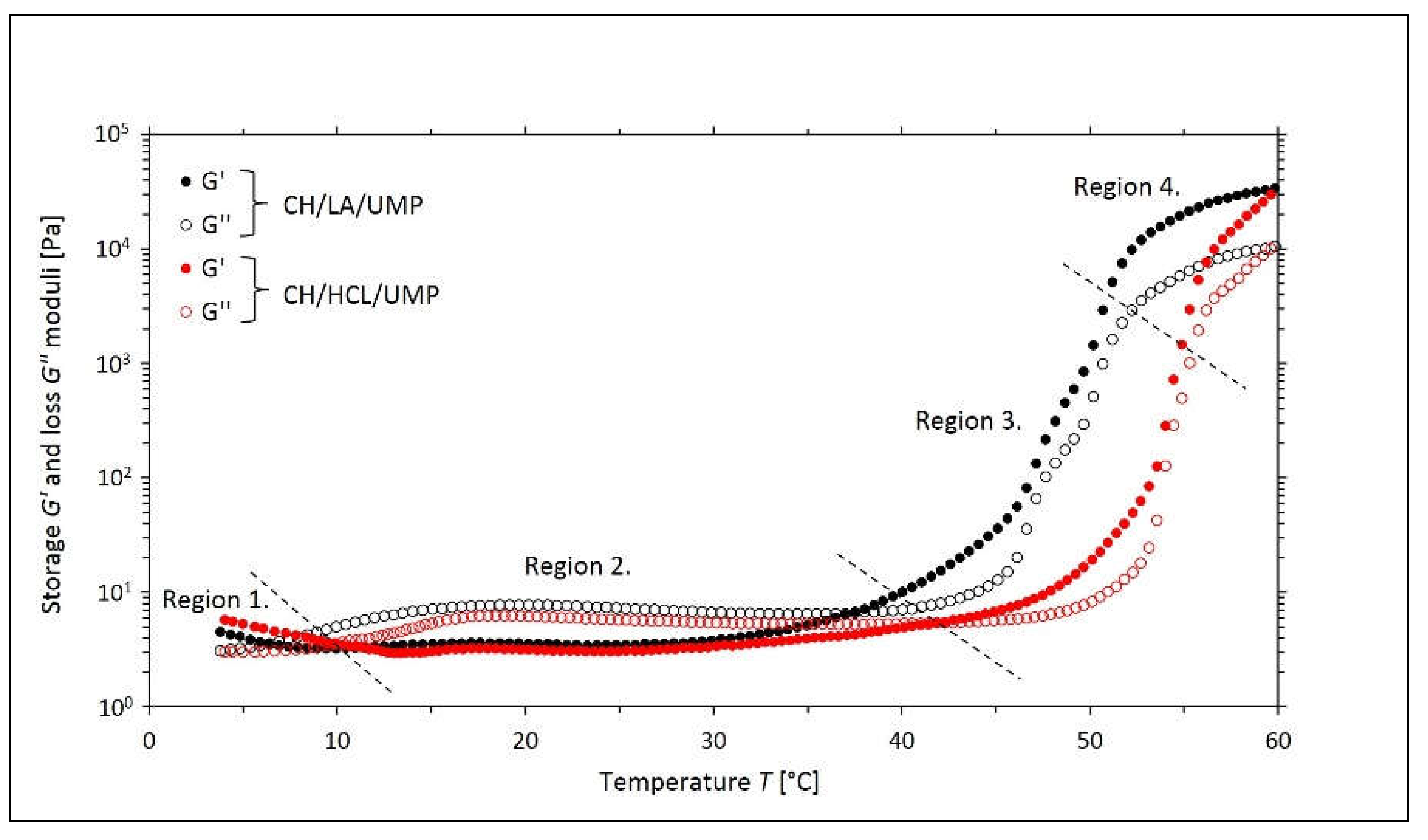
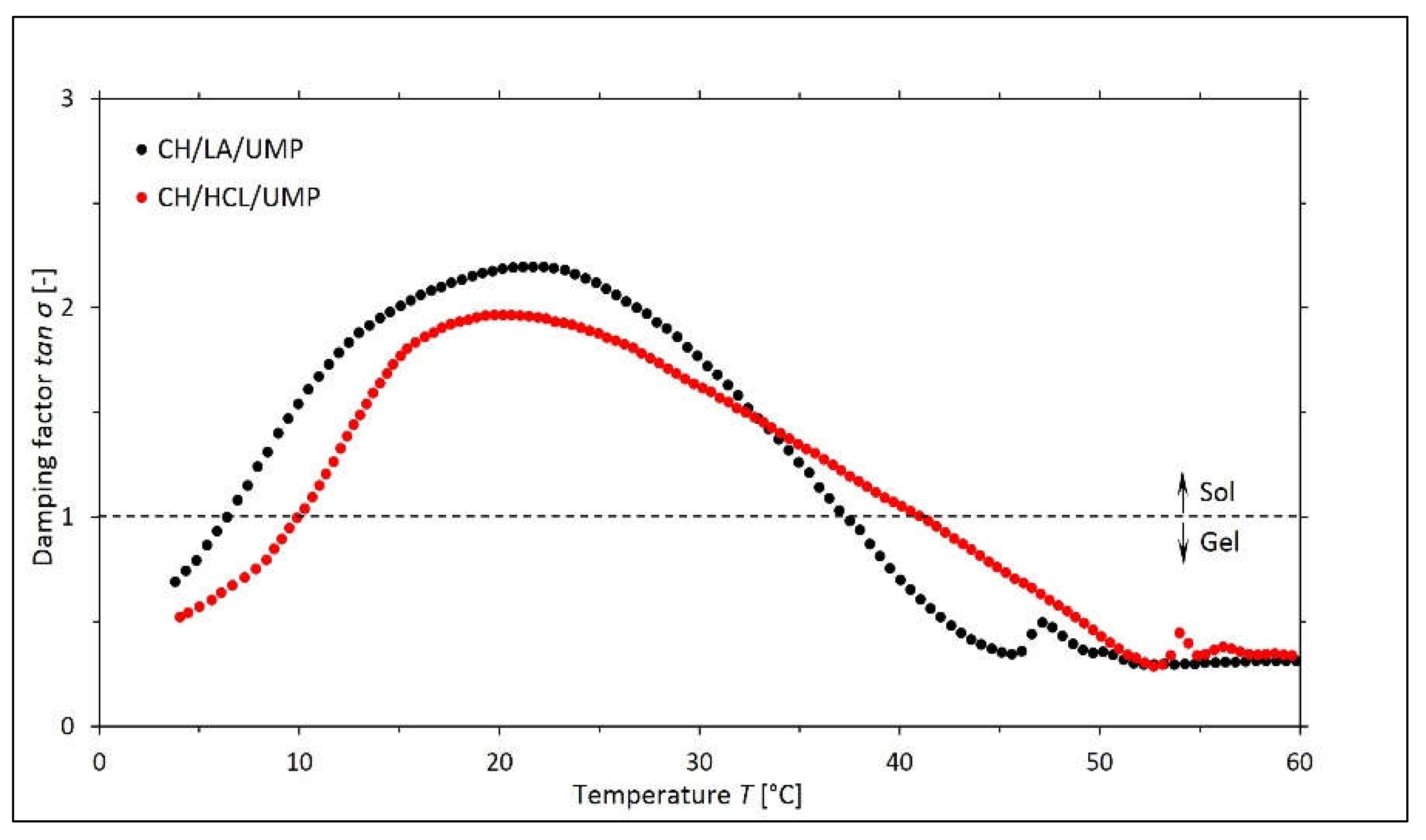
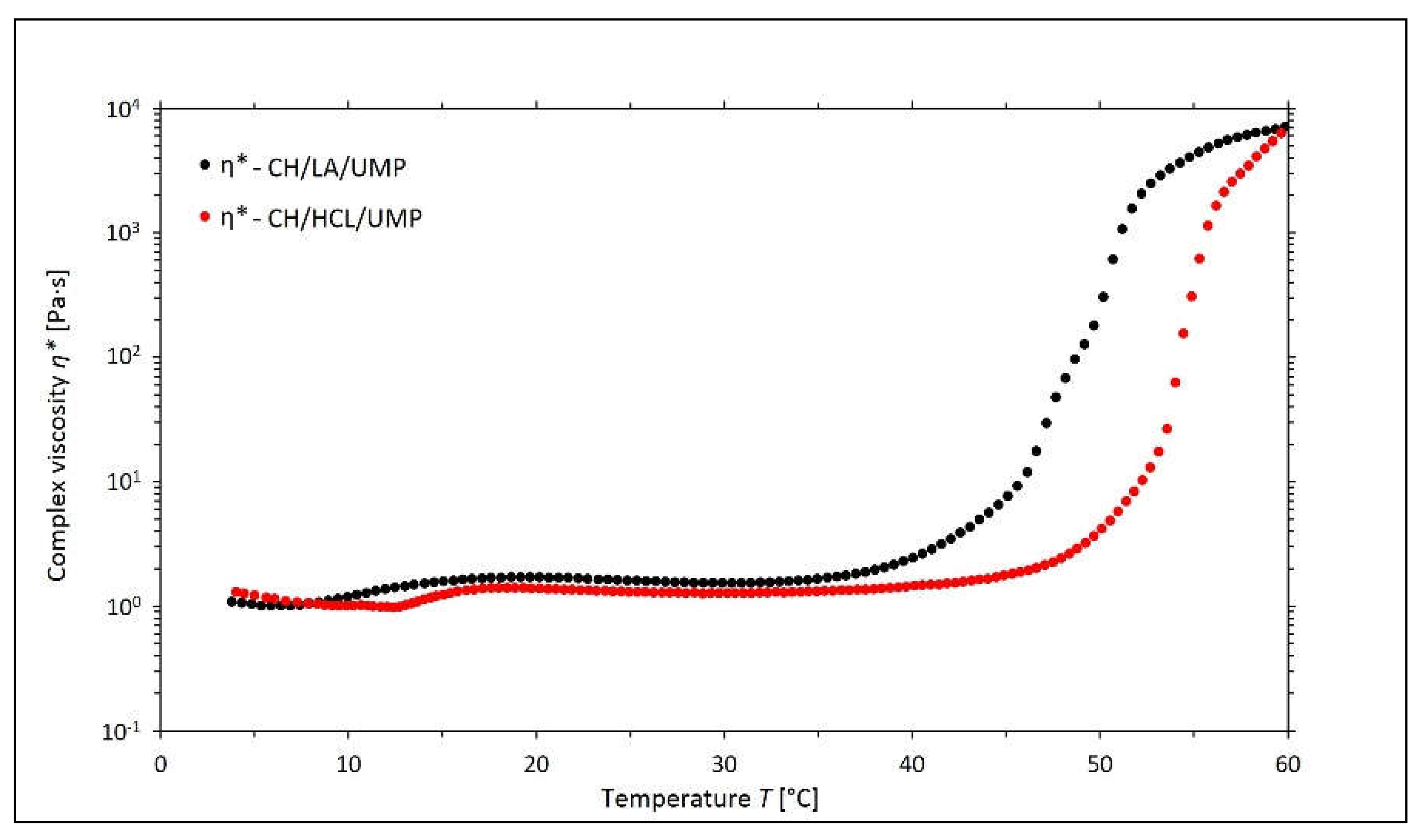
2.1. Non-Isothermal Sol-Gel Transition Process
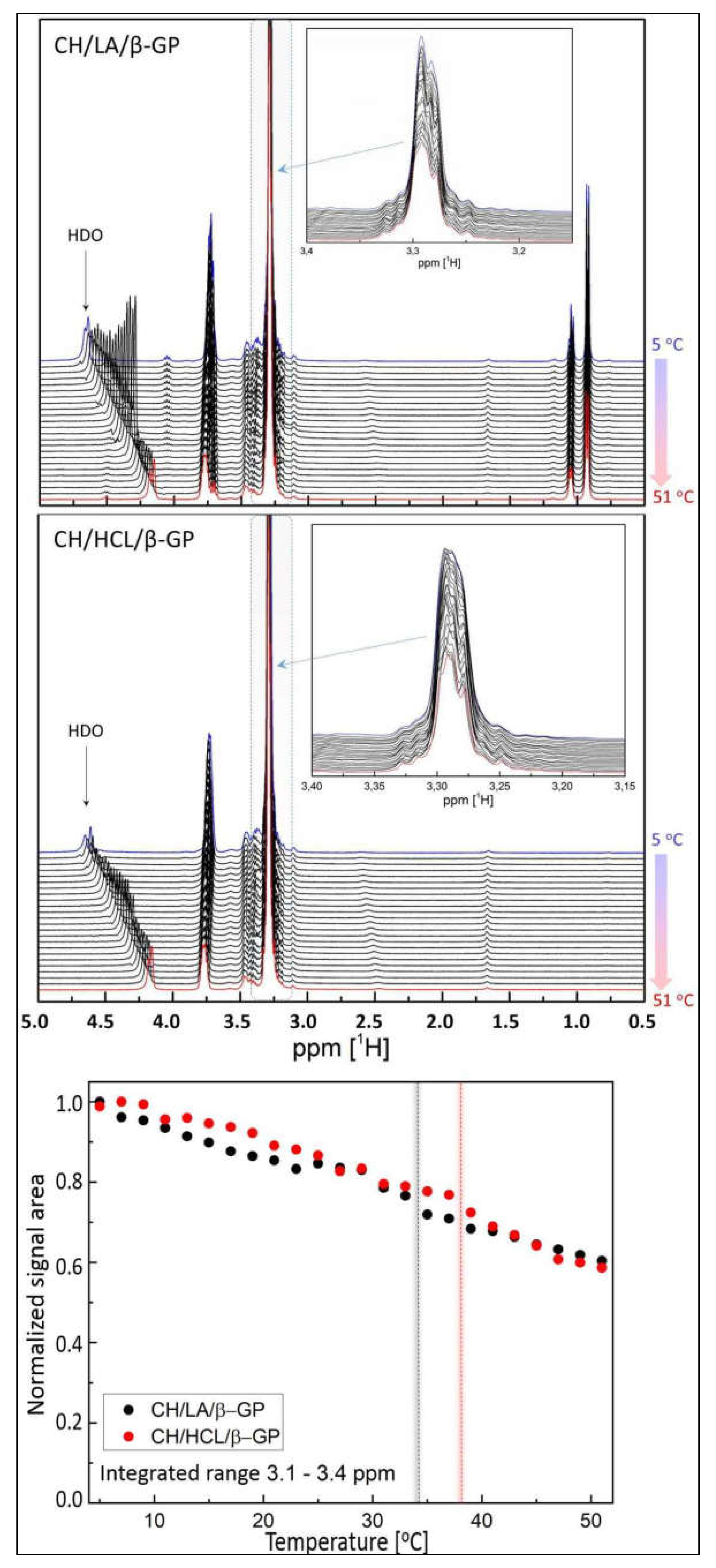
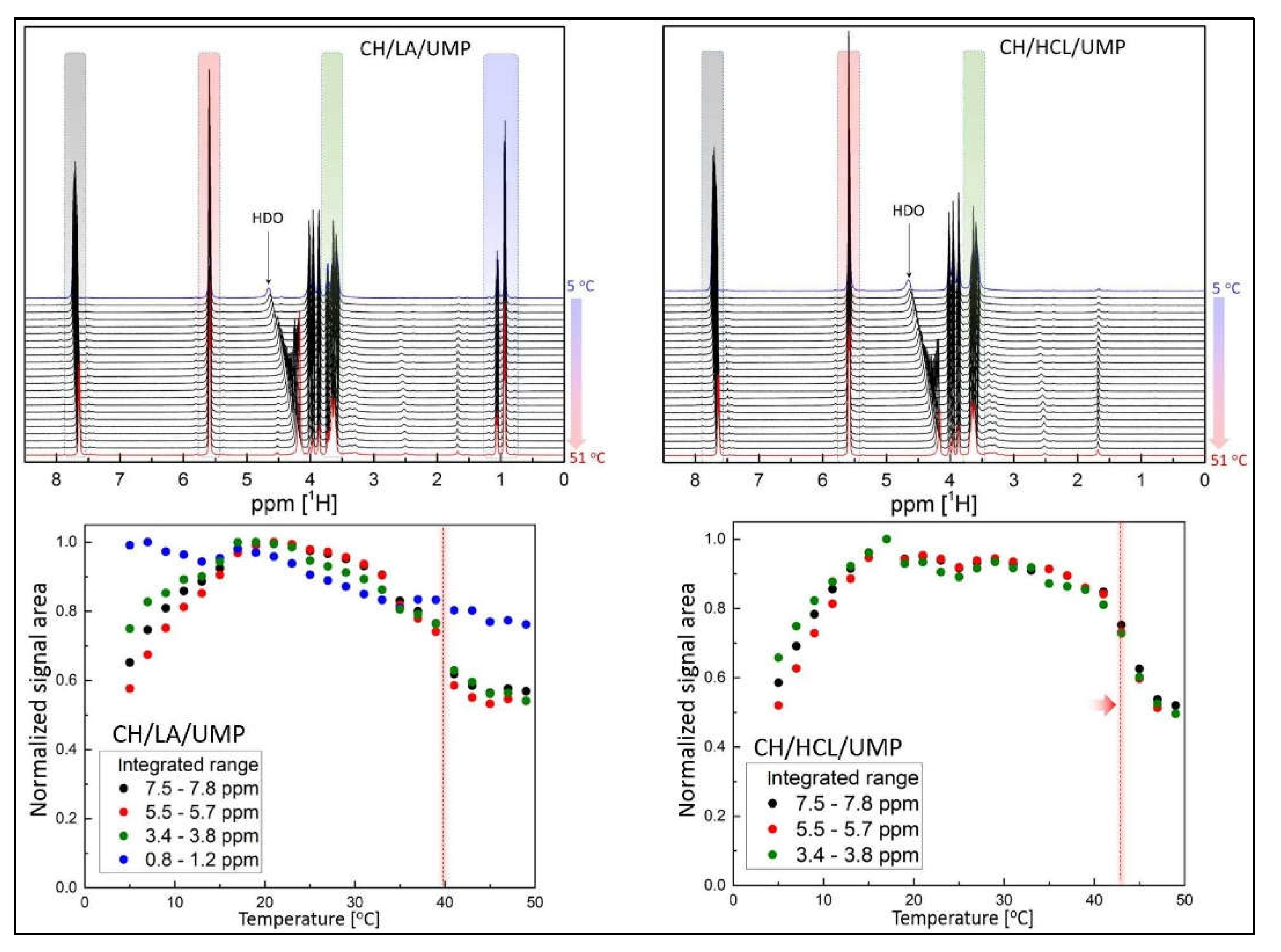
2.2. Nuclear Magnetic Resonance (NMR) Spectra
3. Conclusions
4. Materials and Methods
4.1. Materials and Preparation of Chitosan Salt Solutions
4.2. Rheological Studies
4.3. NMR Experiments
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pillai, C.K.S. Challenges for Natural Monomers and Polymers: Novel Design Strategies and Engineering to Develop Advanced Polymers. Des. Monomers Polym. 2010, 13, 87–121. [Google Scholar] [CrossRef]
- Ko, H.-F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Phil. Trans. R. Soc. A. 2010, 368, 1981–1997. [Google Scholar] [CrossRef] [PubMed]
- Benabid, F.Z.; Zouai, F. Natural polymers: Cellulose, chitin, chitosan, gelatin, starch, carrageenan, xylan and dextran. Alger. J. Nat. Prod. 2016, 4, 348–357. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2020, 138, 50376. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of Structures and Applications in Food Science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Hu, D.; Zeng, M.; Sun, Y.; Yuan, J.; Wei, Y. Cellulose-based hydrogels regulated by supramolecular chemistry. SusMat 2021, 1, 266–284. [Google Scholar] [CrossRef]
- Chamkouri, H. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Rana, T.; Fatima, M.; Khan, A.Q.; Naeem, Z.; Javaid, S.; Sajid, N.; Habib, A. Hydrogels: A Novel Drug Delivery System. J. Biomed. Res. Environ. Sci. 2020, 1, 439–451. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, e2100062. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Hayano, K.; Yoshioka, H.; Yoshioka, H. A Novel Method of Dissolving Chitosan in Water for Industrial Application. Polym. J. 2001, 33, 640–642. [Google Scholar] [CrossRef] [Green Version]
- Hamdine, M.; Heuzey, M.-C.; Bégin, A. Viscoelastic properties of phosphoric and oxalic acid-based chitosan hydrogels. Rheol. Acta 2006, 45, 659–675. [Google Scholar] [CrossRef]
- Kjøniksen, A.-L.; Nyström, B.; Iversen, C.; Nakken, T.; Palmgren, O.; Tande, T. Viscosity of Dilute Aqueous Solutions of Hydrophobically Modified Chitosan and Its Unmodified Analogue at Different Conditions of Salt and Surfactant Concentrations. Langmuir 1997, 13, 4948–4952. [Google Scholar] [CrossRef]
- Filion, D.; Lavertu, A.; Buschmann, M.D. Ionization and Solubility of Chitosan Solutions Related to Thermosensitive Chitosan/Glycerol-Phosphate Systems. Biomacromolecules 2007, 8, 3224–3234. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, Y.; Wang, L.; Lu, S.; Shao, Z.; Chen, X. Synergistic interactions during thermosensitive chitosan-β-glycerophosphate hydrogel formation. RSC Adv. 2011, 1, 282–289. [Google Scholar] [CrossRef]
- Pieklarz, K.; Tylman, M.; Modrzejewska, Z. Applications of chitosan-graphene oxide nanocomposites in medical science: A review. Prog. Chem. Appl. Chitin Deriv. 2018, XXIII, 5–24. [Google Scholar] [CrossRef]
- Pieklarz, K.; Tylman, M.; Modrzejewska, Z. Current Progress in Biomedical Applications of Chitosan-Carbon Nanotube Nanocomposites: A Review. Mini Rev. Med. Chem. 2020, 20, 1619–1632. [Google Scholar] [CrossRef]
- Pieklarz, K.; Faculty of Process and Environmental Engineering Lodz University of Technology; Modrzejewska, Z. SARS-COV-2—The latest global threat and the prospect of COVID-19 therapy with the use of chitosan. Prog. Chem. Appl. Chitin Deriv. 2021, 26, 41–60. [Google Scholar] [CrossRef]
- El-hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Chitosan Physical Forms: A Short Review. Aust. J. Basic Appl. Sci. 2011, 5, 670–677. [Google Scholar]
- Denkbaş, E.B.; Oztürk, E.; Ozdemir, N.; Keçeci, K.; Agalar, C. Norfloxacin-loaded Chitosan Sponges as Wound Dressing Material. J. Biomater. Appl. 2004, 18, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent advances of chitosan-based polymers in biomedical applications and environmental protection. J. Polym. Res. 2022, 29, 259. [Google Scholar] [CrossRef]
- Mohd Hilmi, A.B.; Halim, A.S.; Jaafar, H.; Asiah, A.B.; Hassan, A. Chitosan Dermal Substitute and Chitosan Skin Substitute Contribute to Accelerated Full-Thickness Wound Healing in Irradiated Rats. BioMed Res. Int. 2013, 2013, 795458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef]
- Stępniewski, M.; Martynkiewicz, J.; Gosk, J. Chitosan and its composites: Properties for use in bone substitution. Polym. Med. 2017, 47, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.E.G.; Lardy, B.; Bossard, F.; Martínez, F.A.S.; Rinaudo, M. Chitosan based biomaterials for cartilage tissue engineering: Chondrocyte adhesion and proliferation. Food Hydrocoll. Health 2021, 1, 100018. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.D.; Geuna, S.; Maurício, A.C. Challenges for Nerve Repair Using Chitosan-Siloxane Hybrid Porous Scaffolds. BioMed Res. Int. 2014, 2014, 153808. [Google Scholar] [CrossRef] [Green Version]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Sanabria-Espinoza, P.; Murillo-Castillo, B.; Montes de Oca-Vásquez, G.; Batista-Menezes, D.; Calvo-Guzmán, B.; Ramírez-Arguedas, N.; Vega-Baudrit, J. Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics 2021, 13, 2100. [Google Scholar] [CrossRef]
- Bokura, H.; Kobayashi, S. Chitosan decreases total cholesterol in women: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Pieklarz, K.; Tylman, M.; Modrzejewska, Z. Preparation and characterization of a new generation of chitosan hydrogels containing pyrimidine ribonucleotides. Prog. Chem. Appl. Chitin Deriv. 2020, XXV, 192–200. [Google Scholar] [CrossRef]
- Pieklarz, K.; Galita, G.; Tylman, M.; Maniukiewicz, W.; Kucharska, E.; Majsterek, I.; Modrzejewska, Z. Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. J. Funct. Biomater. 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Owczarz, P.; Ziółkowski, P.; Modrzejewska, Z.; Dziubiński, M. Phase transition of chitosan chloride solutions as potential material for application in biomedical engineering. Eng. Biomater. 2017, 20, 18–25. [Google Scholar]
- Owczarz, P.; Rył, A.; Modrzejewska, Z.; Dziubiński, M. The influence of the addition of collagen on the rheological properties of chitosan chloride solutions. Prog. Chem. Appl. Chitin Deriv. 2017, XXII, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kong, X.; Wang, X.; Shi, S.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. Gel-sol-gel thermo-gelation behavior study of chitosan–inorganic phosphate solutions. Eur. J. Pharm. Biopharm. 2010, 75, 388–392. [Google Scholar] [CrossRef]
- Lewinski, P.; Sosnowski, S.; Kazmierski, S.; Penczek, S. L-Lactide polymerization studied by 1H NMR with diffusion-ordered spectroscopy (DOSY): A “One NMR Tube Experiment” providing data on monomer conversion, polymer structure, Mn and Mw. Polym. Chem. 2015, 6, 4353–4357. [Google Scholar] [CrossRef]
- Kärger, J.; Avramovska, M.; Freude, D.; Haase, J.; Hwang, S.; Valiullin, R. Pulsed field gradient NMR diffusion measurement in nanoporous materials. Adsorption 2021, 27, 453–484. [Google Scholar] [CrossRef]
- Rössler, E.A.; Stapf, S.; Fatkullin, N. Recent NMR investigations on molecular dynamics of polymer melts in bulk and in confinement. Curr. Opin. Colloid Interface Sci. 2013, 18, 173–182. [Google Scholar] [CrossRef]
- Kiwilsza, A.; Milanowski, B.; Drużbicki, K.; Jenczyk, J.; Jarek, M.; Mielcarek, J.; Lulek, J.; Pajzderska, A.; Wąsicki, J. Molecular dynamics and the dissolution rate of nifedipine encapsulated in mesoporous silica. Microporous Mesoporous Mater. 2017, 250, 186–194. [Google Scholar] [CrossRef]
- Martinez-Moro, M.; Jenczyk, J.; Giussi, J.M.; Jurga, S.; Moya, S.E. Kinetics of the thermal response of poly(N-isopropylacrylamide co methacrylic acid) hydrogel microparticles under different environmental stimuli: A time-lapse NMR study. J. Colloid Interface Sci. 2020, 580, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.A.; Gayathri, C.; Gil, R.R.; Kowalewski, T.; Matyjaszewski, K. Comparison of the Thermoresponsive Deswelling Kinetics of Poly(2-(2-methoxyethoxy)ethyl methacrylate) Hydrogels Prepared by ATRP and FRP. Macromolecules 2010, 43, 4791–4797. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P.; Zhang, W.; Zhang, W.; Zhu, X. Effect of structural constraint on dynamic self-assembly behavior of PNIPAM-based nonlinear multihydrophilic block copolymers. Soft Matter 2013, 9, 1807–1816. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, W.; Lin, J.; Fang, C.; Deng, J.; Zhang, P.; Zhou, M.; Liu, P.; Weng, J.; Yu, F.; et al. Magnesium Oxide Nanoparticle Coordinated Phosphate-Functionalized Chitosan Injectable Hydrogel for Osteogenesis and Angiogenesis in Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 7592–7608. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Shishodia, S.; Zhang, D.; El-Sagheer, A.H.; Brown, T.; Claridge, T.D.W.; Schofield, C.J.; Hopkinson, R.J. NMR analyses on N-hydroxymethylated nucleobases—Implications for formaldehyde toxicity and nucleic acid demethylases. Org. Biomol. Chem. 2018, 16, 4021–4032. [Google Scholar] [CrossRef] [Green Version]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Filion, D.; Buschmann, M.D. Chitosan–glycerol-phosphate (GP) gels release freely diffusible GP and possess titratable fixed charge. Carbohydr. Polym. 2013, 98, 813–819. [Google Scholar] [CrossRef]
- Skwarczynska, A.; Kaminska, M.; Owczarz, P.; Bartoszek, N.; Walkowiak, B.; Modrzejewska, Z. The structural (FTIR, XRD, and XPS) and biological studies of thermosensitive chitosan chloride gels with β-glycerophosphate disodium. J. Appl. Polym. Sci. 2018, 135, 46459. [Google Scholar] [CrossRef]
- Owczarz, P.; Ziółkowski, P.; Modrzejewska, Z.; Kuberski, S.; Dziubiński, M. Rheo-Kinetic Study of Sol-Gel Phase Transition of Chitosan Colloidal Systems. Polymers 2018, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, G.M.; Davis, J.T. Supramolecular gels made from nucleobase, nucleoside and nucleotide analogs. Chem. Soc. Rev. 2016, 45, 3188–3206. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Tikhonov, V.E.; Dubovik, A.S.; Moskalets, A.P.; Khokhlov, A.R. Thermodynamic insight into the thermoresponsive behavior of chitosan in aqueous solutions: A differential scanning calorimetry study. Carbohydr. Polym. 2020, 229, 115558. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieklarz, K.; Jenczyk, J.; Modrzejewska, Z.; Owczarz, P.; Jurga, S. An Investigation of the Sol-Gel Transition of Chitosan Lactate and Chitosan Chloride Solutions via Rheological and NMR Studies. Gels 2022, 8, 670. https://doi.org/10.3390/gels8100670
Pieklarz K, Jenczyk J, Modrzejewska Z, Owczarz P, Jurga S. An Investigation of the Sol-Gel Transition of Chitosan Lactate and Chitosan Chloride Solutions via Rheological and NMR Studies. Gels. 2022; 8(10):670. https://doi.org/10.3390/gels8100670
Chicago/Turabian StylePieklarz, Katarzyna, Jacek Jenczyk, Zofia Modrzejewska, Piotr Owczarz, and Stefan Jurga. 2022. "An Investigation of the Sol-Gel Transition of Chitosan Lactate and Chitosan Chloride Solutions via Rheological and NMR Studies" Gels 8, no. 10: 670. https://doi.org/10.3390/gels8100670
APA StylePieklarz, K., Jenczyk, J., Modrzejewska, Z., Owczarz, P., & Jurga, S. (2022). An Investigation of the Sol-Gel Transition of Chitosan Lactate and Chitosan Chloride Solutions via Rheological and NMR Studies. Gels, 8(10), 670. https://doi.org/10.3390/gels8100670






