Thermo-/pH-Dual-Sensitive PEG/PAMAM Nanogel: Reaction Dynamics and Plugging Application of CO2 Channeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermo-/pH-Dual-Sensitive Property
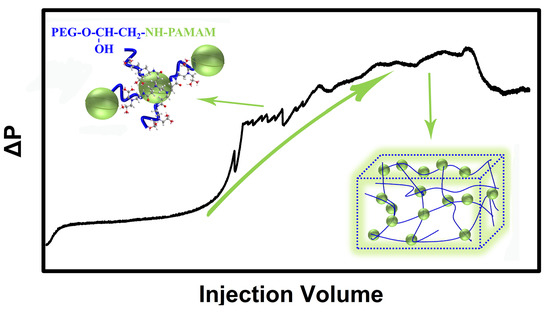
2.2. Reaction Dynamics
2.2.1. Reaction Dynamics Studies on the Temperature Sensitivity of PEG/PAMAM Hydrogels
2.2.2. Reaction Dynamics Studies on pH Sensitivity of PEG/PAMAM Hydrogels
2.2.3. Dickson’s Dynamics Density Dimension and Microstructure of PEG/PAMAM Hydrogels
2.3. Plugging Application of CO2 Channeling
3. Conclusions
- (1)
- PEG/PAMAM hydrogels present a secondary growth pattern from three to one dimension. The phase transition temperature is 50 °C, and the phase transition pH is 10.0.
- (2)
- PEG/PAMAM hydrogels exhibit a secondary growth pattern from three to one dimension. The primary growth activation energy of the gel system is 659.14 kJ/mol, and the secondary growth activation energy is 122.62 kJ/mol under the condition of 45–60 °C and pH 10.0.
- (3)
- High temperature and pH lead to an increase in the spherical aggregation of the hydrogel, along with an accelerated rate of gel reaction, leading to a scale transformation from micrometer to nanometer size and a morphology transformation from a sphere to an irregular ellipsoid or disk shape.
- (4)
- PEG/PAMAM hydrogels present great plugging performance (plugging efficiency > 99%) and have high residual resistance after being broken through by CO2 in low-permeability cores.
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Avrami Reaction Dynamics Model Based on Rheology
4.2.2. Dickinson Reaction Dynamics Model Based on Rheology
4.2.3. Plugging Experiment
4.3. Characterizations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; He, Y.; Wang, Z.; Liang, Q. An Insight into Skeletal Networks Analysis for Smart Hydrogels. Adv. Funct. Mater. 2022, 32, 2108489. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.F.; Xie, R.; Ju, X.J.; Liu, Z.; Chu, L.Y. Designable Micro-/Nano-Structured Smart Polymeric Materials. Adv. Mater. 2021, 2107877. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.L.; Bernards, M.T. Polyampholyte Hydrogels in Biomedical Applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Sun, J.; Lv, K.; Zhang, Q.; Yang, J. Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels 2022, 8, 386. [Google Scholar] [CrossRef]
- Bai, X.; Bao, Z.; Bi, S.; Li, Y.; Yu, X.; Hu, S.; Tian, M.; Zhang, X.; Cheng, X.; Chen, X. Chitosan-based Thermo/pH Double Sensitive Hydrogel for Controlled Drug delivery. Macromol. Biosci. 2018, 18, 1700305. [Google Scholar] [CrossRef]
- Jin, C.; Song, W.; Liu, T.; Xin, J.; Hiscox, W.C.; Zhang, J.; Liu, G.; Kong, Z. Temperature and pH Responsive Hydrogels Using Methacrylated Lignosulfonate Cross-linker: Synthesis, Characterization, and Properties. ACS Sustain. Chem. Eng. 2018, 6, 1763–1771. [Google Scholar] [CrossRef]
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of Polymer Gels for Conformance Control in Oilfield. Adv. Colloid. Interf. 2021, 289, 102363. [Google Scholar] [CrossRef] [PubMed]
- Merati, A.A.; Hemmatinejad, N.; Shakeri, M.; Bashari, A. Preparation, Classification, and Applications of Smart Hydrogels (Chapter 12). In Advanced Functional Textiles and Polymers: Fabrication, Processing and Applications, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Buwalda, S.J.; Bethry, A.; Hunger, S.; Kandoussi, S.; Coudane, J.; Nottelet, B. Ultrafast in Situ Forming Poly (ethylene glycol)-poly (amido amine) Hydrogels with Tunable Drug Release Properties via Controllable Degradation Rates. Eur. J. Pharm. Biopharm. 2019, 139, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, K.; Wang, J.; Yuan, Y.; Wu, H. High-tough Hydrogels Formed via Schiff Base Reaction between PAMAM Dendrimer and Tetra-PEG and Their Potential as Dual-function Delivery Systems. Mater. Today Commun. 2022, 30, 103019. [Google Scholar] [CrossRef]
- Taleghani, A.S.; Ebrahimnejad, P.; Heydarinasab, A.; Akbarzadeh, A. Adsorption and Controlled Release of Iron-chelating Drug from the Amino-terminated PAMAM/ordered Mesoporous Silica Hybrid Materials. J. Drug Deliv. Sci. Tec. 2020, 56, 101579. [Google Scholar]
- Shi, J.; Yu, L.; Ding, J. PEG-based Thermosensitive and Biodegradable Hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Terech, P.; Raghavan, S.R.; Weiss, R.G. Kinetics of 5α-cholestan-3β-yl N-(2-naphthyl) Carbamate/n-alkane Organogel Formation and Its Influence on the Fibrillar Networks. J. Am. Chem. Soc. 2005, 127, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Nasr, P.; Leung, H.; Auzanneau, F.I.; Rogers, M.A. Supramolecular Fractal Growth of Self-Assembled Fibrillar Networks. Gels 2021, 7, 46. [Google Scholar] [CrossRef]
- Katashima, T. Rheological Studies on Polymer Networks with Static and Dynamic Crosslinks. Polym. J. 2021, 53, 1073–1082. [Google Scholar] [CrossRef]
- Wagner, C.E.; Turner, B.S.; Rubinstein, M.; McKinley, G.H.; Ribbeck, K. A Rheological Study of the Association and Dynamics of MUC5AC Gels. Biomacromolecules 2017, 18, 3654–3664. [Google Scholar] [CrossRef]
- Bai, B.; Leng, J.; Wei, M. A Comprehensive Review of in-situ Polymer Gel Simulation for Conformance Control. Petrol. Sci. 2022, 9, 189–202. [Google Scholar] [CrossRef]
- Ghriga, M.A.; Grassl, B.; Gareche, M.; Khodja, M.; Lebouachera, S.E.I.; Andreu, N.; Drouiche, N. Review of Recent Advances in Polyethylenimine Crosslinked Polymer Gels Used for Conformance Control Applications. Polym. Bull. 2019, 76, 6001–6029. [Google Scholar] [CrossRef]
- Shen, H.; Yang, Z.; Li, X.; Peng, Y.; Lin, M.; Zhang, J.; Dong, Z. CO2-responsive Agent for Restraining Gas Channeling during CO2 Flooding in Low Permeability Reservoirs. Fuel 2021, 292, 120306. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Liu, D.; Cao, X.P.; Yuan, B.; Zhao, M. CO2 Responsive Expansion Hydrogels with Programmable Swelling for in-depth CO2 Conformance Control in Porous Media. Fuel 2023, 332, 126047. [Google Scholar] [CrossRef]
- Durucan, S.; Korre, A.; Shi, J.Q.; Govindan, R.; Mosleh, M.H.; Syed, A. The Use of Polymer-gel Solutions for CO2 Flow Diversion and Mobility Control within Storage Sites. Energy Procedia 2016, 86, 450–459. [Google Scholar] [CrossRef]
- Wiese, B.U.; Fleury, M.; Basic, I.; Abdollahi, J.; Patrnogic, A.; Hofstee, C.; Karas, D. Near Well-bore Sealing in the Bečej CO2 Reservoir: Field Tests of a Silicate Based Sealant. Int. J. Greenh. Gas Con. 2019, 83, 156–165. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, M.; Wang, J.; Zong, C. Performance and Gas Breakthrough During CO2 Immiscible Flooding in Ultra-low Permeability Reservoirs. Petrol. Explor. Dev. 2014, 41, 88–95. [Google Scholar] [CrossRef]
- Liu, X.; Qu, G.; Yu, Q.; Zhang, N.; Wang, L.; Wang, J. Synthesis of Poly (ethylene glycol) Grafted Polyamidoamine Dendrimer Hydrogels and Their Temperature and pH Sensitive Properties. Polym. Sci. Ser. B 2020, 62, 400–410. [Google Scholar]
- Whittle, M.; Dickinson, E. Large Deformation Rheological Behaviour of A Model Particle Gel. J. Chem. Soc. Faraday Trans. 1998, 94, 2453–2462. [Google Scholar] [CrossRef]
- Verkoyen, P.; Frey, H. Amino-functional Polyethers: Versatile, Stimuli-responsive Polymers. Polym. Chem. 2020, 11, 3940–3950. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer Hydrogels: A Review. Polym.-Plast. Techn. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Rogers, M.A. Experimental Validation of the Modified Avrami Model for Non-isothermal Crystallization Conditions. Cryst. Eng. Comm. 2011, 13, 866–875. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.J.; Li, J.L.; Wang, R.Y. Distinct Kinetics of Molecular Gelation in a Confined Space and Its Relation to the Structure and Property of Thin Gel Films. Phys. Chem. Chem. Phys. 2015, 17, 8258–8265. [Google Scholar] [CrossRef]
- Wang, R.Y.; Liu, X.Y.; Narayanan, J.; Xiong, J.Y.; Li, J.L. Architecture of Fiber Network: From Understanding to Engineering of Molecular Gels. J. Phys. Chem. B 2006, 110, 25797–25802. [Google Scholar] [CrossRef]
- Xiao, P.; Yang, Z.; Wang, X.; Xiao, H.; Wang, X. Experimental Investigation on CO2 Injection in The Daqing Extra/ultra-low Permeability Reservoir. J. Pet. Sci. Eng. 2017, 149, 765–771. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Wang, J.; Su, J. Pore-Scale Simulation of Particle Flooding for Enhancing Oil Recovery. Energies 2021, 14, 2305. [Google Scholar] [CrossRef]
- Boutahar, K.; Carrot, C.; Guillet, J. Crystallization of Polyolefins from Rheological Measurements Relation between the Transformed Fraction and the Dynamic Moduli. Macromolecules 1998, 31, 1921–1929. [Google Scholar] [CrossRef]
- Wu, H.; Morbidelli, M. A Model Relating Structure of Colloidal Gels to Their Elastic Properties. Langmuir 2001, 17, 1030–1036. [Google Scholar] [CrossRef]







| Temperature (°C) | n1 | Growth Pattern | n2 | Growth Pattern | k1 | k2 | Ea1 (kJ/mol) | Ea2 (kJ/mol) |
|---|---|---|---|---|---|---|---|---|
| 45 | 4.00 | 3D | 1.58 | 1D | 1.00 × 10−11 | 2.44 × 10−5 | 659.14 | 122.62 |
| 50 | 4.57 | 3D | 1.39 | 1D | 2.00 × 10−11 | 6.15 × 10−5 | ||
| 55 | 2.96 | 3D | 1.34 | 1D | 1.74 × 10−8 | 3.68 × 10−4 | ||
| 60 | 2.85 | 2D–3D | 1.32 | 1D | 2.71 × 10−8 | 4.54 × 10−4 |
| pH | n1 | Growth Pattern | n2 | Growth Pattern | k1 | k2 |
|---|---|---|---|---|---|---|
| 10.0 | 4.00 | 3D | 1.58 | 1D | 1 × 10−11 | 2.44 × 10−5 |
| 11.0 | 3.65 | 3D | 1.47 | 1D | 3.8 × 10−10 | 1.67 × 10−4 |
| 12.0 | 2.82 | 2D–3D | 1.43 | 1D | 7.58 × 10−8 | 2.76 × 10−4 |
| Core | Matrix Permeability (mD) | Fracture Permeability (mD) | Plugging Efficiency (%) | Breakthrough Pressure (MPa) | Residual Pressure (MPa) |
|---|---|---|---|---|---|
| 1 | 10.378 mD | 0.0637 | 99.386 | 19.93 | 17.64 |
| 2 | 20.247 mD | 0.1164 | 99.425 | 16.79 | 15.91 |
| 3 | 40.183 mD | 0.2981 | 99.258 | 15.45 | 14.34 |
| Core | Permeability (mD) | Porosity (%) | Length (cm) | Diameter (cm) | Fracture Width (mm) |
|---|---|---|---|---|---|
| 1 | 10.378 mD | 7.93 | 10.0 | 2.5 | 0.1 |
| 2 | 20.247 mD | 9.79 | 10.0 | 2.5 | 0.1 |
| 3 | 40.183 mD | 12.45 | 10.0 | 2.5 | 0.1 |
| Ion Type | K+ + Na+ | Ca2+ | Mg2+ | SO42− | Cl− | HCO3− | Salinity |
|---|---|---|---|---|---|---|---|
| 2036.5 | 316.2 | 119.5 | 159.8 | 809.2 | 1641.4 | 5103.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, S.; Shi, W.; Liu, H. Thermo-/pH-Dual-Sensitive PEG/PAMAM Nanogel: Reaction Dynamics and Plugging Application of CO2 Channeling. Gels 2022, 8, 683. https://doi.org/10.3390/gels8100683
Liu X, Wang S, Shi W, Liu H. Thermo-/pH-Dual-Sensitive PEG/PAMAM Nanogel: Reaction Dynamics and Plugging Application of CO2 Channeling. Gels. 2022; 8(10):683. https://doi.org/10.3390/gels8100683
Chicago/Turabian StyleLiu, Xiangbin, Suling Wang, Weiguang Shi, and He Liu. 2022. "Thermo-/pH-Dual-Sensitive PEG/PAMAM Nanogel: Reaction Dynamics and Plugging Application of CO2 Channeling" Gels 8, no. 10: 683. https://doi.org/10.3390/gels8100683