Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
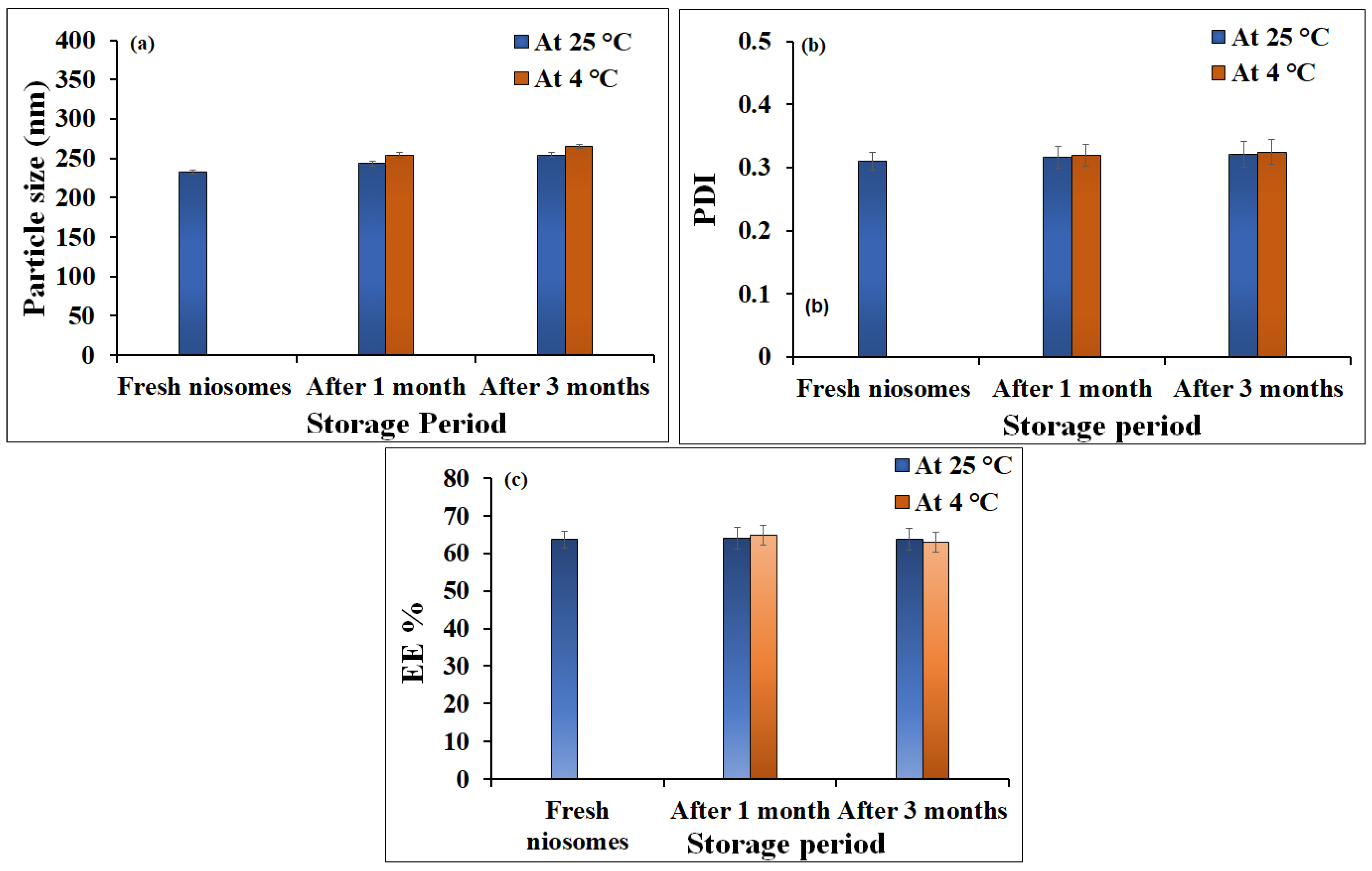
2.1. Characterization of Niosomes Loaded with Ginger Extract
2.2. Development of Ginger Extract Niosomal Emulgel
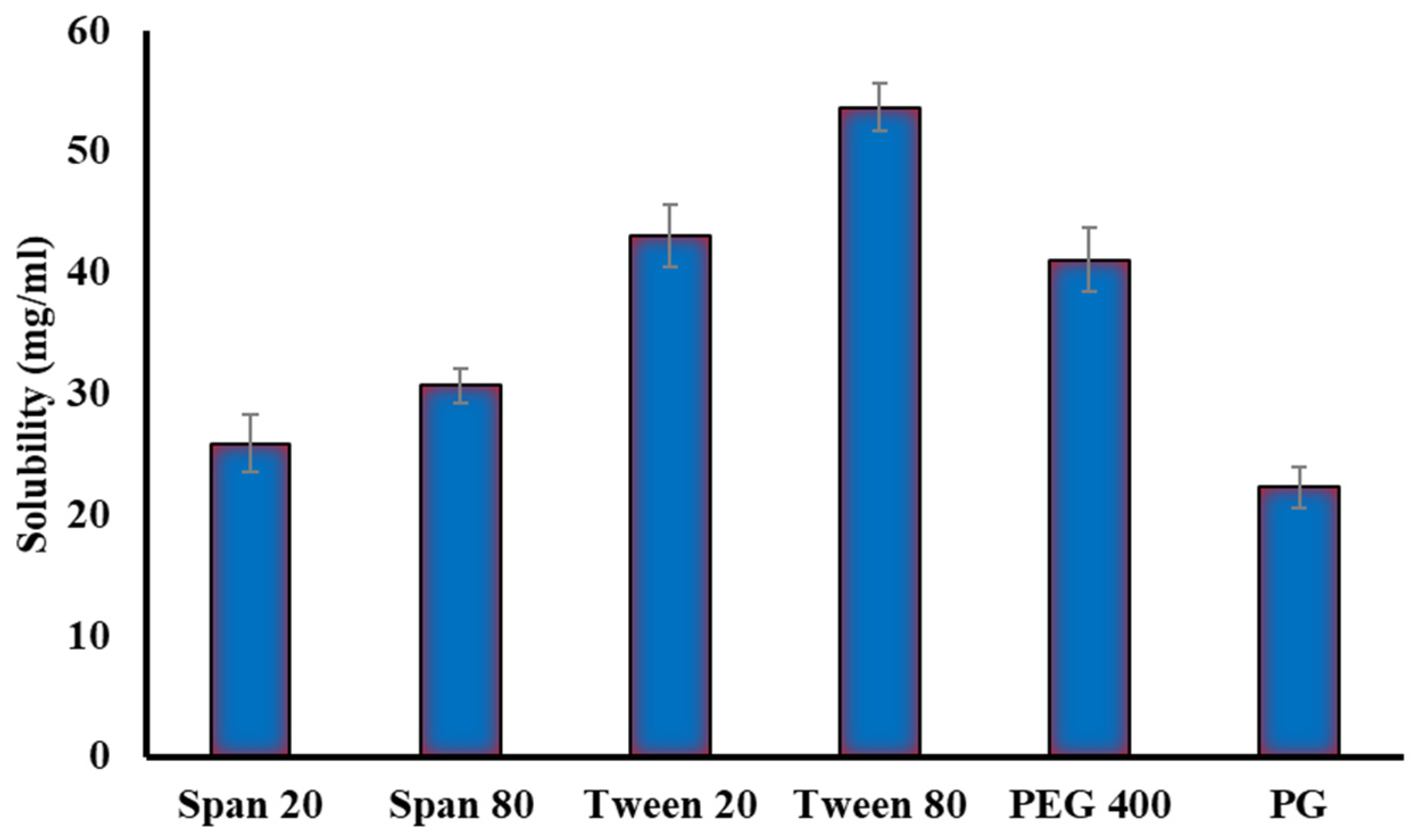
2.2.1. Solubility Studies
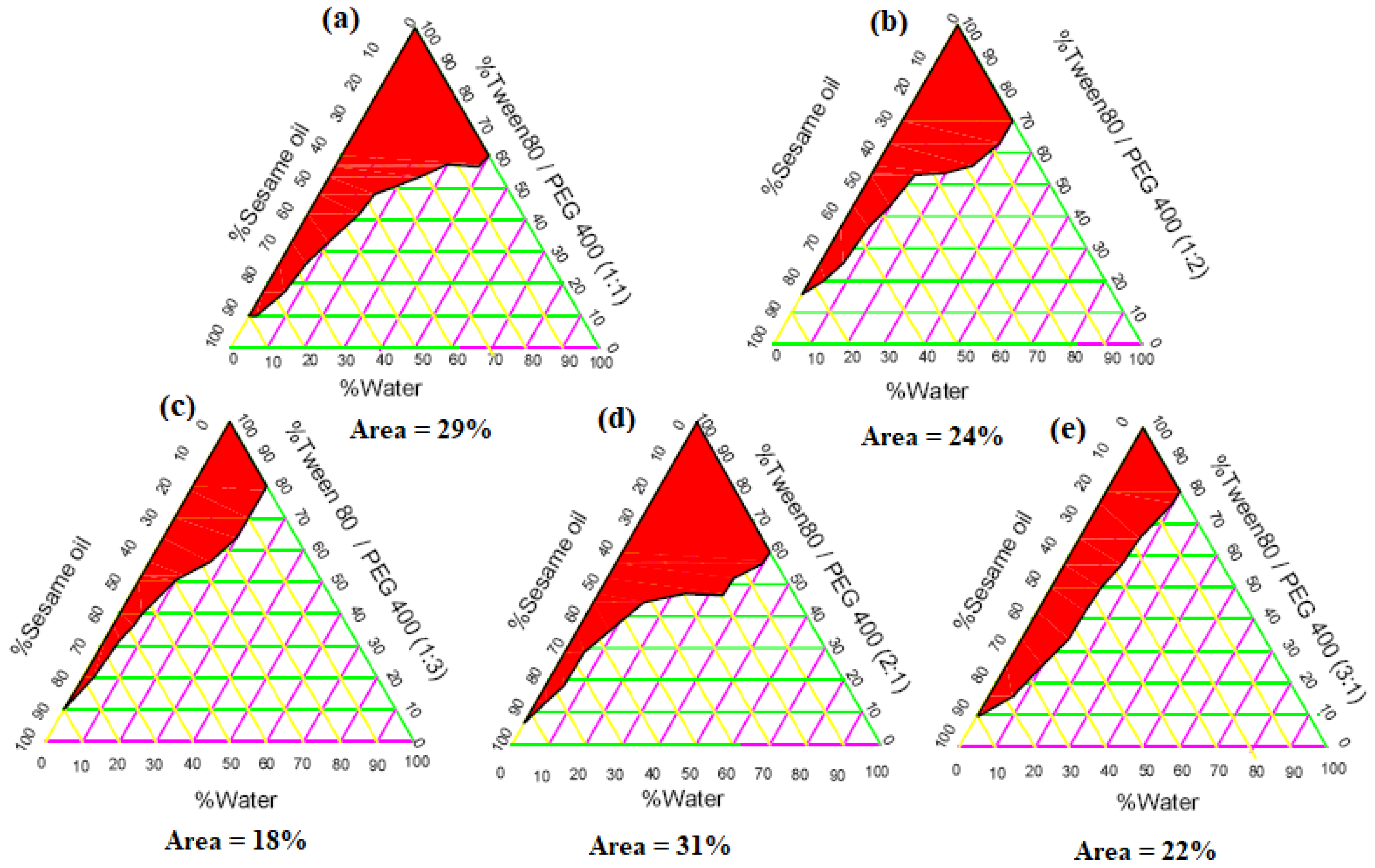
2.2.2. Construction of Phase Diagrams
2.3. Central Composite (CC) Design for Emulgel Optimization
2.3.1. Design Statistical Analysis
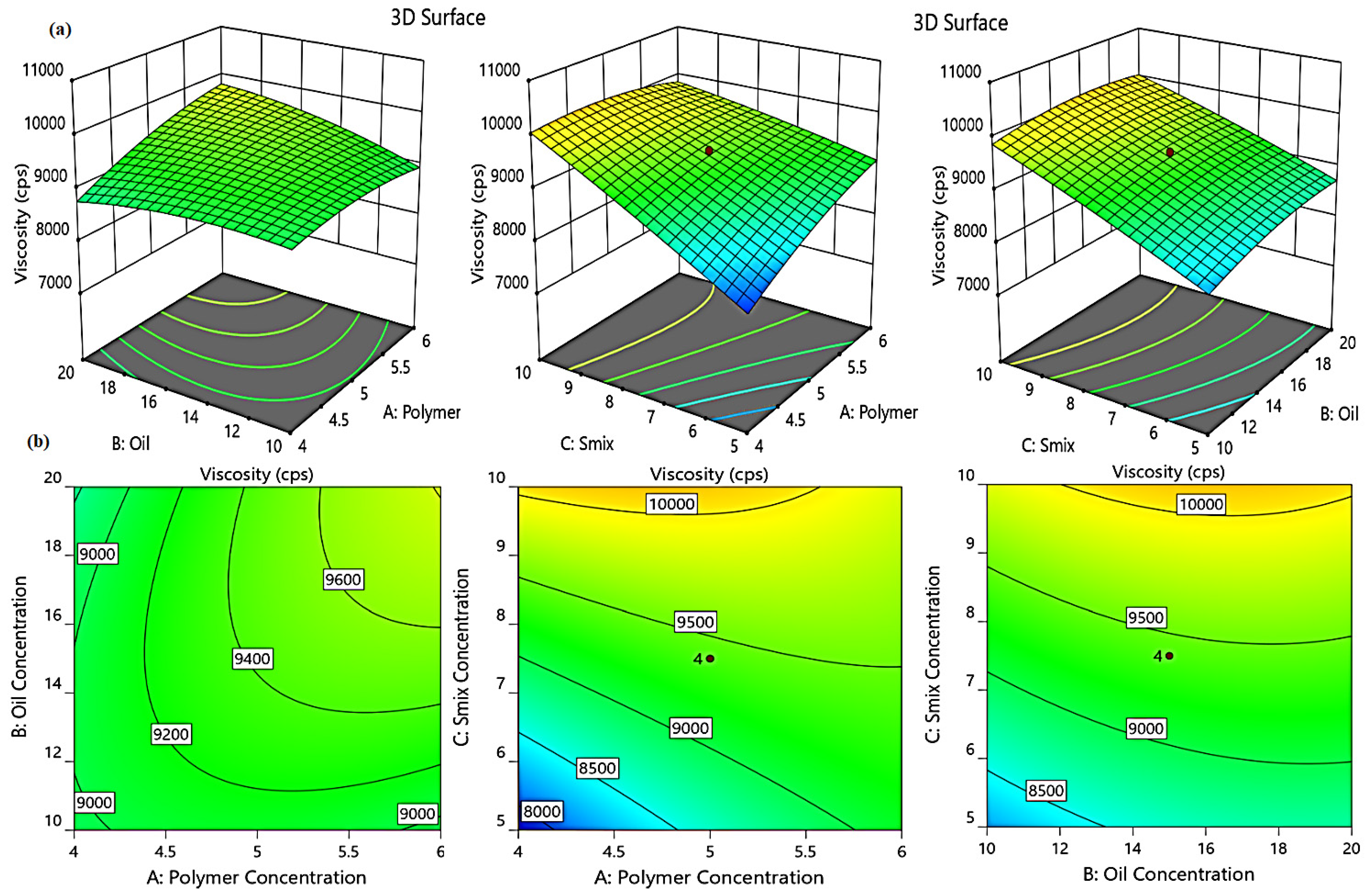
2.3.2. Effect of Independent Variables on Viscosity (Y1)
2.3.3. Effect of Independent Factors on the in Vitro Drug Release (Y2)
2.3.4. Effect of Independent Variables on the % of Drug Content (Y3)
2.3.5. Optimized Formulation of Niosomal Emulgel
2.4. Characterization of Optimized Niosomal Emulgel Formulation
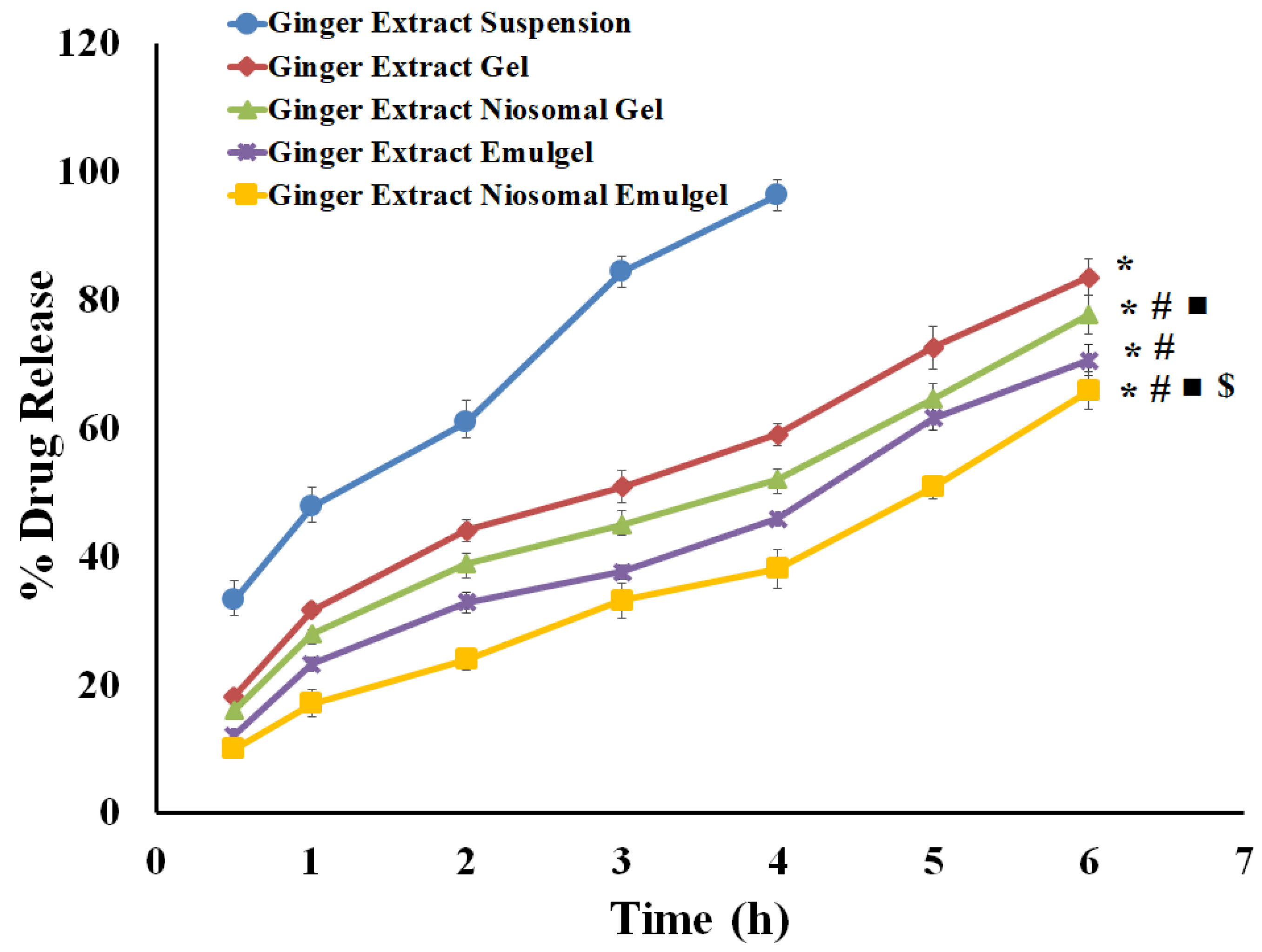
2.5. In Vitro Release Investigations
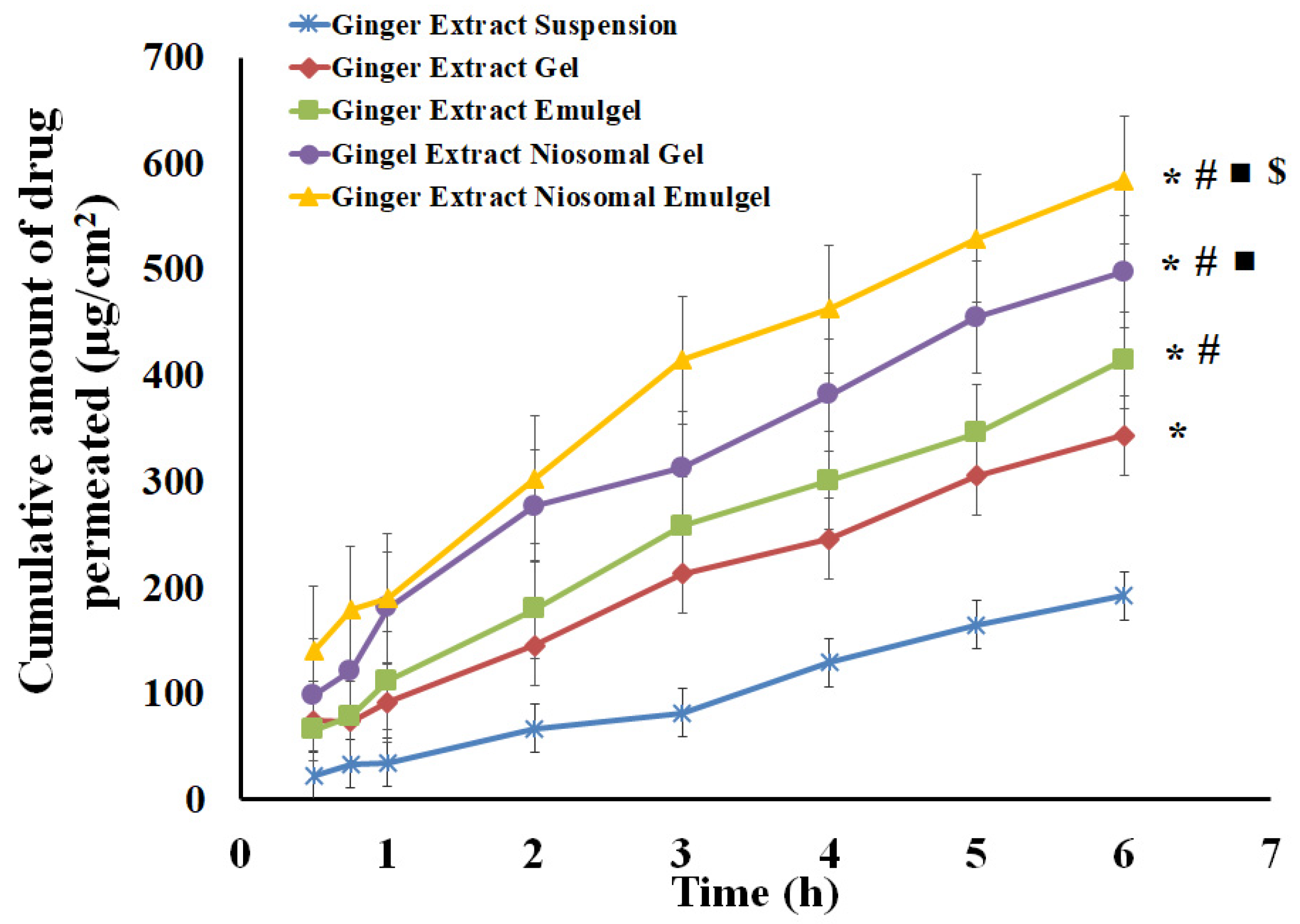
2.6. Skin Permeation Study of Ginger Extract from Different Formulations
2.7. Anti-Inflammatory Testing; Carrageenan-Induced Rat Paw Edema Test
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Ginger Extract
4.3. Preparation of Ginger Extract Loaded Niosomal Vesicles
4.4. Characterization of the Developed Niosomal Vesicle Loaded with Ginger Extract
4.4.1. Entrapment Efficiency Determination
4.4.2. Vesicular Size and Size Distribution
4.5. Stability Studies
4.6. Development of Ginger Extract Niosomal Emulgel
4.6.1. Solubility Studies
4.6.2. Construction of Phase Diagrams
4.6.3. Experimental Modelling Using Central Composite Design
4.6.4. Preparation of Niosomal Sesame Oil Based Emulgel
4.7. Characterization of the Investigated Ginger Loaded Niosomal Emulgel
4.7.1. Visual Inspection
4.7.2. pH Value Estimation
4.7.3. Spreadability Test
4.7.4. Viscosity Measurement
4.7.5. Determination of Drug Content
4.8. In Vitro Drug Release Studies
4.9. Ex Vivo Drug Permeation Study
4.10. In Vivo Experimental Studies
4.10.1. Animals
4.10.2. Anti-Inflammatory Activity
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, M.; Holvey, C. Transdermal patches: The emerging mode of drug delivery system in psychiatry. Ther. Adv. Psychopharmacol. 2012, 2, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waqas, M.K.; Sadia, H.; Khan, M.I.; Omer, M.O.; Siddique, M.I.; Qamar, S.; Zaman, M.; Butt, M.H.; Mustafa, M.W.; Rasool, N. Development and characterization of niosomal gel of fusidic acid: In-vitro and ex-vivo approaches. Des. Monomers Polym. 2022, 25, 165–174. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Sabry, S.A.; Hasan, A.A. Enhancing Transdermal Delivery of Glimepiride Via Entrapment in Proniosomal Gel. J. Young Pharm. 2016, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Elsewedy, H.S.; Younis, N.S.; Shehata, T.M.; Mohamed, M.E.; Soliman, W.E. Enhancement of Anti-Inflammatory Activity of Optimized Niosomal Colchicine Loaded into Jojoba Oil-Based Emulgel Using Response Surface Methodology. Gels 2021, 8, 16. [Google Scholar] [CrossRef]
- Sah, S.K.; Badola, A.; Nayak, B.K. Emulgel: Magnifying the application of topical drug delivery. Indian J. Pharm. Biol. Res. 2017, 5, 25–33. [Google Scholar] [CrossRef]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A Promising Phase in Drug Delivery. Curr. Pharm. Des. 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Nastiti, C.M.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Akram, A.; Rasul, A.; Waqas, M.K.; Irfan, M.; Khalid, S.H.; Aamir, M.N.; Murtaza, G.; Ur Rehman, K.; Iqbal, M.; Khan, B.A. Development, characterization and evaluation of in-vitro anti-inflammatory activity of ginger extract based micro emulsion. Pak. J. Pharm. Sci. 2019, 32, 1327–1332. [Google Scholar] [PubMed]
- Habib, S.H.M.; Makpol, S.; Hamid, N.A.A.; Das, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Beltagi, H.S.; Maraei, R.W.; El-Ansary, A.E.; Rezk, A.A.; Mansour, A.T.; Aly, A.A. Characterizing the Bioactive Ingredients in Sesame Oil Affected by Multiple Roasting Methods. Foods 2022, 11, 2261. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.; Farraj, J.F.; Carr, R.I. Sesamum indicum (sesame) enhances NK anti-cancer activity, modulates Th1/Th2 balance, and suppresses macrophage inflammatory response. Asian Pac. J. Trop. Biomed. 2020, 10, 316–324. [Google Scholar] [CrossRef]
- Wichitsranoi, J.; Weerapreeyakul, N.; Boonsiri, P.; Settasatian, C.; Settasatian, N.; Komanasin, N.; Sirijaichingkul, S.; Teerajetgul, Y.; Rangkadilok, N.; Leelayuwat, N. Antihypertensive and antioxidant effects of dietary black sesame meal in pre-hypertensive humans. Nutr. J. 2011, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Hsu, E.; Parthasarathy, S. Anti-inflammatory and antioxidant effects of sesame oil on atherosclerosis: A descriptive literature review. Cureus 2017, 9, e1438. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, R.; Yadav, G.; Jadhav, V.; Jain, A. Formulation and evaluation of black sesame seed oil sunscreen emulgel using natural gelling agent. Int. Res. J. Pharm. 2018, 9, 197–201. [Google Scholar] [CrossRef]
- Nazari, M.; Mehrnia, M.A.; Jooyandeh, H.; Barzegar, H. Preparation and characterization of water in sesame oil microemulsion by spontaneous method. J. Food Process Eng. 2019, 42, e13032. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Upgrading of dissolution and anti-hypertensive effect of Carvedilol via two combined approaches: Self-emulsification and liquisolid techniques. Drug Dev. Ind. Pharm. 2018, 44, 873–885. [Google Scholar] [CrossRef]
- Rao, M.; Sukre, G.; Aghav, S.; Kumar, M. Optimization of metronidazole emulgel. J. Pharm. 2013, 2013, 501082. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abdelnabi, D.M.; Elghamry, H.A. Response Surface Methodology for Optimization of Buspirone Hydrochloride-Loaded In Situ Gel for Pediatric Anxiety. Gels 2022, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagdale, S.; Pawar, S. Gellified emulsion of ofloxacin for transdermal drug delivery system. Adv. Pharm. Bull. 2017, 7, 229. [Google Scholar] [CrossRef] [Green Version]
- Shehata, T.M.; Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Alhaider, I.A.; Attimarad, M.; Elsewedy, H.S.; Ibrahim, M.M. Vesicular emulgel based system for transdermal delivery of insulin: Factorial design and in vivo evaluation. Appl. Sci. 2020, 10, 5341. [Google Scholar] [CrossRef]
- Daood, N.M.; Jassim, Z.E.; Gareeb, M.M.; Zeki, H. Studying the effect of different gelling agent on the preparation and characterization of metronidazole as topical emulgel. Asian J. Pharm. Clin. Res. 2019, 12, 571–577. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of anti-hypertensive effect of carvedilol. J. Liposome Res. 2019, 29, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Katare, O.; Vyas, S. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Kasliwal, N. Development, characterization, and evaluation of liposomes and niosomes of bacitracin zinc. J. Dispers. Sci. Technol. 2012, 33, 1267–1273. [Google Scholar] [CrossRef]
- Magnusson, B.M.; Walters, K.A.; Roberts, M.S. Veterinary drug delivery: Potential for skin penetration enhancement. Adv. Drug Deliv. Rev. 2001, 50, 205–227. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Bharadia, P.; Al-Dhubiab, B.E. Proniosomal gel for transdermal delivery of lornoxicam: Optimization using factorial design and in vivo evaluation in rats. DARU J. Pharm. Sci. 2019, 27, 59–70. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Shehata, T.M. The enhancement of transdermal permeability of water soluble drug by niosome-emulgel combination. J. Drug Deliv. Sci. Technol. 2012, 22, 353–359. [Google Scholar] [CrossRef]
- Monteiro, É.M.H.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.S.; Vilela, F.M.P.; Rodarte, M.P.; De Oliveira Pinto, M.A.; Da Penha Henriques do Amaral, M.; Silvério, M.S.; De Matos Araújo, A.L.S.; et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 2014, 6, 1931–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessai, P.; Mhaskar, G.M. Formulation and Evaluation of Ginger officinale Emulgel. Res. J. Pharm. Technol. 2019, 12, 1559–1565. [Google Scholar] [CrossRef]
- Abdelnabi, D.M.; Abdallah, M.H.; Elghamry, H.A. Buspirone hydrochloride loaded in situ nanovesicular gel as an anxiolytic nasal drug delivery system: In vitro and animal studies. AAPS PharmSciTech 2019, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef]
- Tawde, S.; Gawde, K.; Mahind, D.; Mhaprolkar, C.; Sawant, K.; Surve, C. GINGEL: Development and Evaluation of Anti-Arthritic Gel Containing Ginger (Zingiber officinale). Int. J. Innov. Sci. Res. Technol. 2020, 5, 7. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Elsewedy, H.S.; AbuLila, A.S.; Almansour, K.; Unissa, R.; Elghamry, H.A.; Soliman, M.S. Quality by Design for Optimizing a Novel Liposomal Jojoba Oil-Based Emulgel to Ameliorate the Anti-Inflammatory Effect of Brucine. Gels 2021, 7, 219. [Google Scholar] [CrossRef]
- Abdallah, M.H. Box-behnken design for development and optimization of acetazolamide microspheres. India 2014, 5, 1228–1239. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Lila, A.S.A.; Anwer, M.K.; Khafagy, E.-S.; Mohammad, M.; Soliman, M.S. Formulation, development and evaluation of ibuprofen loaded nano-transferosomal gel for the treatment of psoriasis. J. Pharm. Res 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Thorat, Y.S.; Kote, N.S.; Patil, V.V.; Hosmani, A.H. Formulation and evaluation of liposomal gel containing extract of piprine. Int. J. Curr. Pharm. Res. 2020, 12, 126–129. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and anti-Inflammatory effects of Zingiber officinale roscoe and Allium subhirsutum: In silico, biochemical and histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]

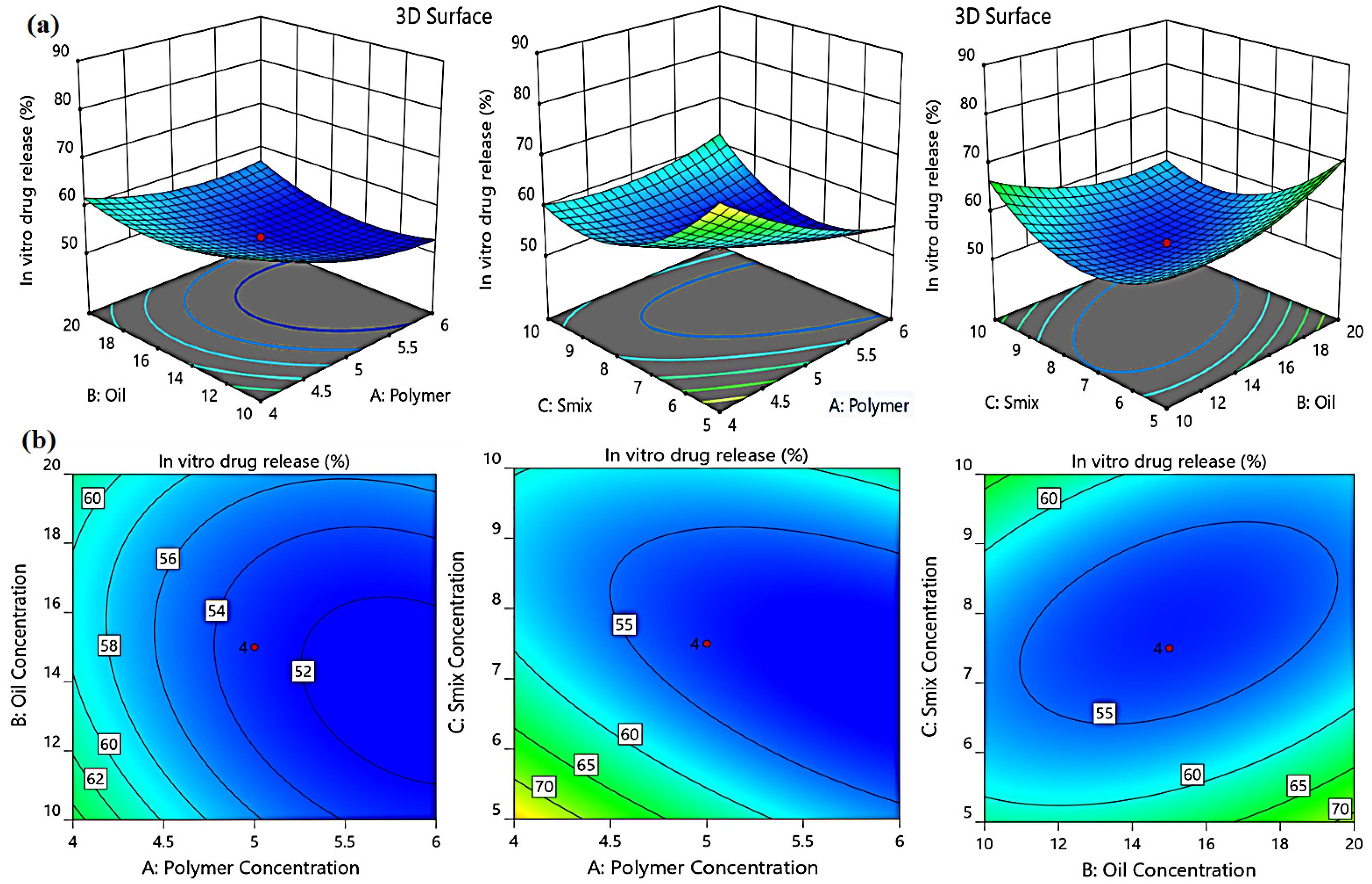
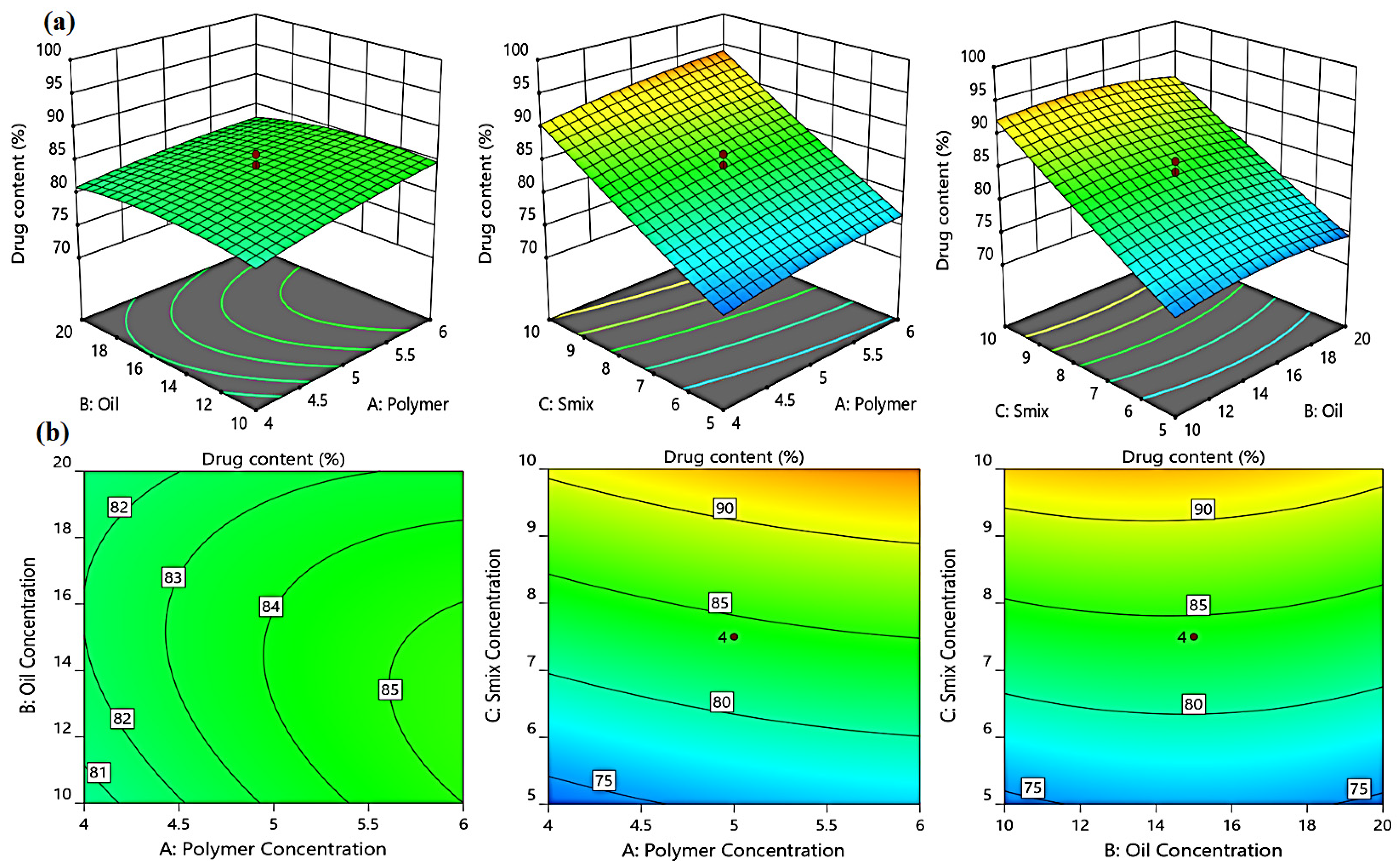

| Formulation | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| A | B | C | Y1 (cP) | Y2 (%) | Y3 (%) | |
| F1 | 5 | 15 | 7.5 | 9301.23 ± 114.31 | 53.45 ± 0.69 | 82.80 ± 1.15 |
| F2 | 4 | 10 | 10 | 9899.34 ± 127.23 | 68.86 ± 1.09 | 91.37 ± 0.24 |
| F3 | 6 | 10 | 5 | 8388.45 ± 175.22 | 53.43 ± 1.79 | 75.62 ± 0.88 |
| F4 | 5 | 15 | 7.5 | 9378.47 ± 114.10 | 52.12 ± 0.81 | 82.27 ± 0.63 |
| F5 | 6 | 20 | 10 | 9845.65 ± 234.52 | 62.76 ± 0.74 | 92.03 ± 1.36 |
| F6 | 4 | 10 | 5 | 7685.87 ± 128.51 | 76.40 ± 1.87 | 70.96 ± 0.46 |
| F7 | 6 | 10 | 10 | 9287.12 ± 165.32 | 69.75 ± 1.65 | 93.81 ± 0.90 |
| F8 | 5 | 15 | 7.5 | 9465.47 ± 188.24 | 52.91 ± 1.17 | 84.38 ± 0.62 |
| F9 | 5 | 15 | 7.5 | 9345.65 ± 129.65 | 53.34 ± 0.75 | 86.04 ± 0.38 |
| F10 | 4 | 20 | 5 | 7678.57 ± 137.21 | 80.25 ± 1.92 | 73.18 ± 0.53 |
| F11 | 6 | 20 | 5 | 9578.33 ± 145.45 | 67.28 ± 1.67 | 72.41 ± 2.32 |
| F12 | 5 | 23.41 | 7.5 | 9198.10 ± 152.32 | 64.15 ± 0.83 | 81.06 ± 1.40 |
| F13 | 5 | 15 | 3.29 | 7885.34 ± 134.01 | 78.25 ± 0.97 | 72.25 ± 1.53 |
| F14 | 6.68 | 15 | 7.5 | 9387.38 ± 117.08 | 52.14 ± 0.92 | 86.82 ± 0.57 |
| F15 | 5 | 6.59 | 7.5 | 8752.28 ± 115.32 | 61.82 ± 1.11 | 80.50 ± 0.73 |
| F16 | 4 | 20 | 10 | 9645.87 ± 128.24 | 58.07 ± 1.10 | 89.37 ± 1.00 |
| F17 | 3.32 | 15 | 7.5 | 8545.06 ± 136.36 | 68.05 ± 1.22 | 78.95 ± 1.53 |
| F18 | 5 | 15 | 11.70 | 10,698.20 ± 201.18 | 72.07 ± 1.05 | 97.58 ± 2.88 |
| Source | Y1 | Y2 | Y3 | |||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 65.72 | <0.0001 * | 122.50 | <0.0001 * | 31.10 | < 0.0001 * |
| A | 51.23 | <0.0001 * | 171.32 | <0.0001 * | 9.49 | 0.0151 * |
| B | 19.72 | 0.0014 * | 0.7694 | 0.4060 | 0.2827 | 0.6094 |
| C | 400.03 | <0.0001 * | 42.02 | 0.0002 * | 263.00 | < 0.0001 * |
| AB | 27.15 | 0.0006 * | 17.03 | 0.0033 * | 0.8908 | 0.3729 |
| AC | 61.12 | <0.0001 * | 154.35 | <0.0001 * | 0.0488 | 0.8307 |
| BC | 5.18 | 0.1026 | 112.76 | <0.0001 * | 0.2520 | 0.6292 |
| A² | 16.65 | 0.0040 * | 59.78 | <0.0001 * | 0.6749 | 0.4351 |
| B² | 15.98 | 0.0040 * | 117.11 | <0.0001 * | 4.75 | 0.0610 |
| C2 | 1.16 | 0.2681 | 565.32 | <0.000 *1 | 0.2354 | 0.6406 |
| Lack of Fit | 5.56 | 0.0943 | 5.60 | 0.0935 | 1.52 | 0.3871 |
| R2 analysis | ||||||
| R² | 0.9867 | 0.9928 | 0.9722 | |||
| Adjusted R² | 0.9716 | 0.9847 | 0.9410 | |||
| Predicted R² | 0.9036 | 0.9444 | 0.8289 | |||
| Adequate precision | 28.3038 | 32.3642 | 19.8035 | |||
| Model | Quadratic | Quadratic | Quadratic | |||
| Properties | Ginger Niosomal Gel | Ginger Niosomal Emulgel |
|---|---|---|
| Visual inspection | Smooth and homogenous | Smooth and homogenous |
| pH | 6.47± 0.31 | 6.62 ± 0.45 |
| Spreadability (mm) | 47.25± 2.4 | 36.54 ± 3.03 * |
| Viscosity (cP) | 8686.67 ± 210.49 | 9510.33 ± 162.79 * |
| Independent Variable | Symbol | Level of Variation | |
|---|---|---|---|
| −1 | +1 | ||
| Polymer concentration (%w/w) Oil concentration (%v/v) | A B | 4 10 | 6 20 |
| Smix concentration (%v/v) | C | 5 | 10 |
| Independent Variable | Symbol | Constrains | |
| Viscosity (cP) | Y1 | In range | |
| In vitro drug release (%) | Y2 | Maximize | |
| Drug content (%) | Y3 | Maximize | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.A.; Khafagy, E.-S.; Lila, A.S.A.; El-Horany, H.E.-S.; El-Housiny, S. Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity. Gels 2022, 8, 737. https://doi.org/10.3390/gels8110737
Abdallah MH, Elghamry HA, Khalifa NE, Khojali WMA, Khafagy E-S, Lila ASA, El-Horany HE-S, El-Housiny S. Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity. Gels. 2022; 8(11):737. https://doi.org/10.3390/gels8110737
Chicago/Turabian StyleAbdallah, Marwa H., Hanaa A. Elghamry, Nasrin E. Khalifa, Weam M. A. Khojali, El-Sayed Khafagy, Amr S. Abu Lila, Hemat El-Sayed El-Horany, and Shaimaa El-Housiny. 2022. "Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity" Gels 8, no. 11: 737. https://doi.org/10.3390/gels8110737






